Galectin-3 is located in the cytoplasm and nucleus,
and it is transported to the cell surface, extracellular space and
circulation without the secretory signal sequence (9). It has been reported that galectin-3
binds with substrates in cells. For example, intracellular Bcl-2
may be bound by galectin-3 to inhibit T-cell apoptosis (10–12).
Furthermore, galectin-3 binds with T-cell receptor (TCR) on the
cell surface to restrict and downregulate TCR expression, thus
resulting in the inhibition of TCR-mediated early activation of T
cells (13,14). When galectin-3 reaches the
extracellular space, it reacts with several binding partners,
mostly extracellular matrix (ECM) or cell surface
polylactosamine-rich molecules, and plays a key role in regulating
tumor progression extracellularly (15,16).
In the inflammatory response, galectin-3 has been associated with
the activation of neutrophils in several infectious diseases, such
as viral lower respiratory tract infection, bacterial sepsis and
candidemia (17–20). The R186S mutation in galectin-3
alters its affinity for various carbohydrates, thus playing an
important role in its function (21). It was previously demonstrated that
the R186S galectin-3 mutant was able to bind lactose, but not
LacNac, and was unable to enter vesicles to activate primed
neutrophils (22). Galectin-3 has
been shown to direct glycoproteins into vesicles, which in turn are
transported though the membrane in a lipid raft-independent manner
(21). However, it has been
suggested that the R186S galectin-3 mutant is not capable of
mediating the intracellular transport of glycoproteins, such as
gp114 (23).
The survival and prognosis of cancer patients are
not only associated with cancer cells, but also with the tumor
microenvironment (TME), which is constituted of cancer cells,
immune cells, stromal cells, the ECM, as well as other components.
Furthermore, the TME contributes to tumor growth, invasion,
metastasis and immunosuppression. For example, it is well known
that tumor-associated macrophages, fibroblasts and tumor cells
secrete suppressive cytokines and chemokines that are involved in
the immune response. Furthermore, the production of inhibitory
metabolites, migration failure due to rigid ECM, poor antigen
expression and decreased TCR signaling all contribute to tumor
progression (24). Tumor-secreted
galectin-3 has been found to inhibit the permeation of interferon-γ
(IFN-γ) in the TME, thus resulting in reduced CXC motif chemokine
ligand 9 content and decreased recruitment of CD8+
T-cells to the tumor. However, treatment with galectin-3 inhibitors
recovered the content of IFN-γ and chemokines in the TME, whereas
the immune cell infiltration was enhanced (25).
Galectin-3 also participates in cell glycolysis and
mitochondrial metabolism in some tumors, thus improving the
metabolic reprogramming of tumors and enabling them to adapt to the
microenvironment stress caused by oxygen and nutrient deprivation
(4,21,26–28).
Under high-fat diet conditions, galectin-3 knockout (KO) mice
exhibited increased levels of fasting blood glucose, insulin and
HbA1c. However, the levels of the glucose transporters were lower
compared with those in the control group. This finding was
hypothesized to be one of the reasons for the increased blood
glucose levels observed in KO mice (29). It was previously demonstrated that
galectin-3 was co-expressed with glucose transporter 1 (GLUT1) in
breast and lung cancer, and their expression was upregulated in
tumor cells surrounding the necrotic region inside the tumor
(26). Galectin-3 may also be
transported to the mitochondrial membrane and interact with Bcl-2,
thereby inhibiting the release of mitochondrial cytochrome c
and reducing cell apoptosis (30).
In addition, inconsistent galectin-3 expression patterns have been
identified in different tumors, possibly due to the variation of
the TME content or the cellular localization of galectin-3 in
different tumor cells (4). For
example, previous studies in various cancers have suggested that
intracellular galectin-3 plays an important role in maintaining
mitochondrial homeostasis, whereas extracellular galectin-3 binds
to the CD29 and CD7 glycoproteins on the surface of T-cell lymphoma
cells and activates mitochondrial apoptotic signaling (31–33). A
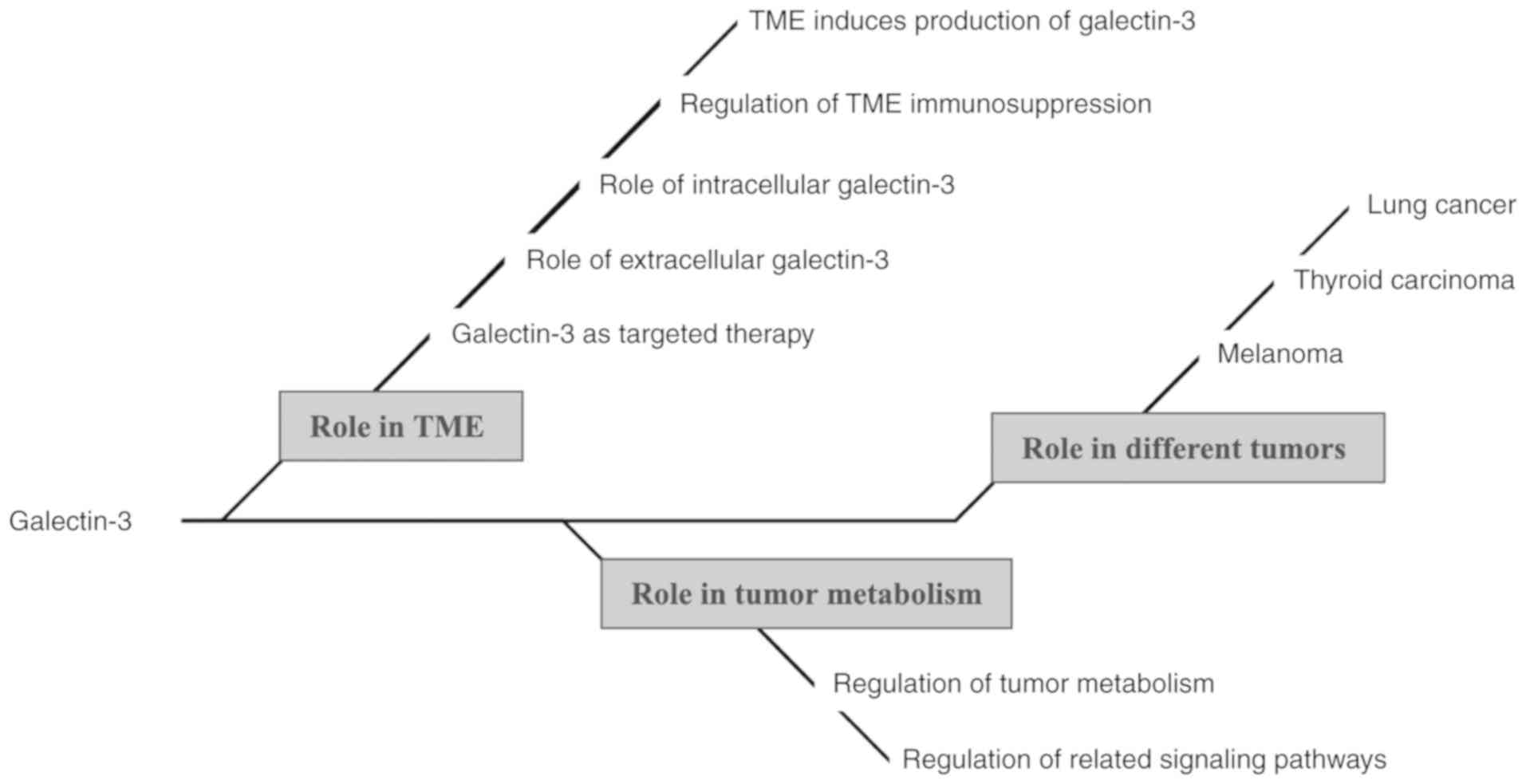
mind map of the present study is presented in Fig. 1.
It has been reported that galectin-3 may be
activated in chronic myeloid leukemia (CML), following interaction
of leukemic cells in the TME with stromal and mesenchymal stem
cells (MSCs) (34–37). A study demonstrated that galectin-3
was upregulated, particularly when leukemic cells were co-cultured
with bone marrow stromal cells (BMSCs) in vitro, while its
expression was predominant in CML cells (35). Additionally, the expression of
galectin-3 was significantly increased when CML cells were
co-cultured with MSCs, and the protein expression was the highest
during the chronic phase (35). In
addition, overexpression of galectin-3 in CML cells promoted CML
cell and BMSC proliferation, thereby accelerating the deposition of
leukemia cells in the bone marrow. In acute myeloid leukemia, high
galectin-3 expression was associated with poor prognosis (38). It has been suggested that galectin-3
supports leukemic cell survival in the TME via the AKT-mediated
inactivation of glycogen synthase kinase (GSK)3, which is involved
in the anti-apoptotic pathway, pro-cell proliferation cascade,
metabolic pathway, and other processes (39–43).
In addition, Krause et al demonstrated that galectin-3 was
induced when t(1;19)-positive acute lymphoblastic leukemia (ALL)
cells were co-cultured with glioma-derived U343 cells. Galectin-3
was considered as ligand of Mer tyrosine kinase and the feedback
mechanism between those two elements may mediate the relapse of ALL
in the central nervous system (CNS) (44).
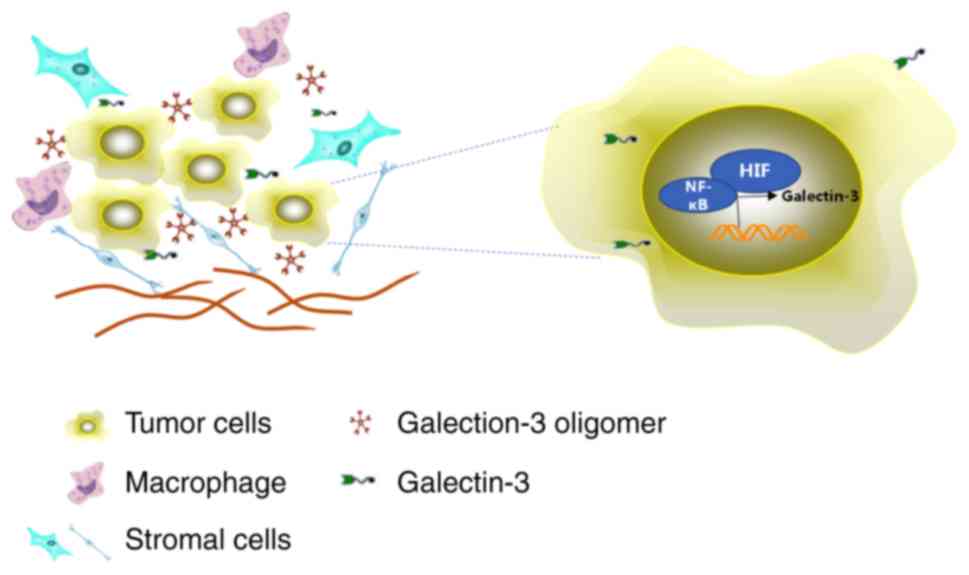
The TME, a hypoxic environment, may be regulated by
the primary regulator hypoxia inducible factor-1α (HIF-1α), which
is known to upregulate the expression of several genes, including
galectin-3, to maintain cellular homeostasis and promote cell
survival in skeletal tissues (45).
Galectin-3 was found to be increased in hypoxic/nutrient-deprived
areas from both glioblastoma and mammary tumor tissues (26,46–48).
In addition, several studies have demonstrated that the
interference of nuclear factor (NF)-κB activation inhibits
galectin-3 expression, resulting in cell apoptosis (49). Taken together, the interactions
among galectin-3, NF-κB, HIF-1α and common stress conditions in the
TME are crucial for tumor progression (Fig. 2).
Galectin-3 plays a key role in promoting
tumor-driven immunosuppression. The specific effects of galectin-3
on the innate and adaptive immunity are summarized in Table I (15). A study revealed that co-culture of T
cells from the peripheral blood with autologous tumor cells
suppressed galectin-3 expression in tumor cells and mediated
galectin-3-induced expansion of tumor-reactive T cells (6). In addition, tumor cell-secreted
galectin-3 may regulate the polarization of macrophages from the M1
(antitumor) to the M2 (tumor-promoting) subtype, trigger
CD8+ T-cell apoptosis, and downregulate the expression
of TCRs (50). Emerging evidence
has suggested that galectin-3 binds to lymphocyte-activation gene-3
(LAG-3) on activated terminally differentiated T cells, and
functional LAG-3 is required for galectin-3-mediated T-cell
suppression (51). In addition,
depletion of galectin-3 was associated with increased activation of
the proinflammatory signaling pathways in CD8+ T cells
(51). Galectin-3 suppressed T-cell
function via inducing T-cell anergy, and this effect was rescued by
depleting surface galectin-3 (52,53).
The mechanism underlying galectin-3-induced immunosuppression is
mainly mediated by triggering apoptosis via its binding to
antitumor T cells (54,55), and shielding the ligands on the
surface of tumor cells from the activated receptors of natural
killer (NK) cells (56,57). Furthermore, galectin-3 is a soluble
ligand of NKp30, which is expressed on the surface of NK cells and
acts as an immunomodulator to mediate immune escape of tumor cells
from NK cells (58).
Galectin-3 regulates T-cell function through several
mechanisms, including the negative regulation of the TCR-mediated
cell response (13). Demotte et
al demonstrated that treating CD8+
tumor-infiltrating T lymphocytes (TILs) with an anti-galectin-3
antibody could restore their ability of IFN-γ secretion (52). Furthermore, the proliferation of
tumor-reactive T cells was improved following treatment with
supernatants isolated from galectin-3-depleted cells, indicating an
important role of the tumor-secreted galectin-3 in the suppression
of T-cell activation (59).
CD146/MCAM has been reported to act as a functional binding ligand
of galectin-3 on the surface of endothelial cells, and is
responsible for galectin-3-induced secretion of
metastasis-promoting cytokines (60). Certain cytokines, such as
interleukin (IL)-1, IL-6, tumor necrosis factor-α and INF-γ, are
associated with metastasis and prognosis in several types of cancer
(61). The interaction of
galectin-3 with endothelial CD146/MCAM in the circulation resulted
in increased secretion of IL-6, granulocyte colony-stimulating
factor and other cytokines; therefore, they may exert an important
effect on the progression and metastasis of cancer (60).
Extracellular and intracellular galectin-3 exert
different effects on lymphocytes; therefore, understanding the
function of galectin-3 is complicated (6). Interestingly, the cellular
localization of galectin-3 determines whether it exerts apoptotic
or anti-apoptotic effects on T cells. It has been reported that
extracellular galectin-3 induces apoptosis, whereas intracellular
galectin-3 inhibits apoptosis by promoting cell proliferation and
stimulating TCR signaling (5).
Therefore, extracellular galectin-3 may induce apoptosis of human
thymocytes and T cells via directly binding to the glycoprotein
receptors CD45 and CD71 (62). On
the contrary, overexpression of galectin-3 in the intracellular
compartment of Jurkat T cells was associated with the inhibition of
apoptosis induced by an anti-Fas antibody and staurosporine
(63). In addition, a study
revealed that depletion of galectin-3 in CD4+ T cells
upregulated TCR expression and IFN-γ secretion compared with
wild-type CD4+ T cells (13).
An increasing number of studies have investigated
the expression levels of galectin-3 in different types of cancer,
and its expression was found to differ among diverse malignancies
(64). Galectin-3 was shown to be
upregulated in thyroid, liver, stomach and CNS cancers, and
downregulated in breast, ovarian, uterine and prostate cancers
(65–67). During tumor progression, galectin-3
is often localized in the cytoplasm, as has been reported for
tongue and prostate cancer (64),
and its expression was decreased in the nucleus during the
transition of tongue tissue from normal to cancerous (64). It was, therefore, hypothesized that
the nuclear translocation of galectin-3 observed during tumor
progression may be a prognostic factor for patients with tongue
cancer (68). A similar research,
including 145 prostate cancer patients, reported that galectin-3
was usually not expressed or decreased in prostate cancer compared
with normal prostate tissues (64).
When galectin-3 was detected in cancer cells, it was always absent
from the nucleus and was only present in the cytoplasm (69). In addition, it has been reported
that the expression of galectin-3 in the cytoplasm is closely
associated with vascular invasion, cell differentiation and tumor
progression (70).
Galectin-3 exerts opposite biological effects,
depending on its cellular localization; therefore, nuclear and
cytoplasmic galectin-3 exert antitumor and tumorigenic effects,
respectively (69,71,72).
It has been suggested that galectin-3 is involved in several
different signal transduction cascades and pro-survival processes,
including the Ras, Bcl-2 and Myc signaling pathways (73–75).
For example, galectin-3 regulated Bcl-2 and other members of the
Bcl-2 family by directly binding to them (76). The expression of galectin-3 between
the cytoplasmic and nucleal regions differs in different types of
skin cancer. For example, the cytoplasmic galectin-3 expression in
cutaneous squamous cell carcinoma was significantly higher compared
with that in circumscribed and infiltrative basal cell carcinoma.
Furthermore, the immunoreactivity of galectin-3 in the cytoplasm
was increased compared with that in the nuclei of non-melanoma skin
cancer cells (77). In addition, it
has been hypothesized that tumor size is associated with the
cytoplasmic expression of galectin-3. The nuclear and cytoplasmic
expression of galectin-3 has been considered as an important factor
in the malignant progression of non-melanoma skin cancer.
Therefore, the expression of galectin-3 was reported to be
decreased in the nucleus and increased in the cytoplasm during the
transition of normal cells to tumor cells (50). Consistently, melanoma patients with
low survival rate exhibited increased cytoplasmic galectin-3
expression compared with its nuclear expression (78).
Notably, several studies have shown that the
behavior of tumor-stromal cells, including endothelial cells,
immune cells, cancer-associated fibroblasts, myofibroblasts and
MSCs, is affected by the extracellular expression of galectin-3,
whereas it has been found that these cells may also secrete
galectin-3 (5,79–82).
Upregulation of the galectin-3 expression increases the ability of
cancer cell migration and invasion in several tumors, including
breast, melanoma, lung, sarcoma, gastric cancer and CML (12,35,82–85).
In addition, galectin-3 interacts with ECM glycoproteins, such as
fibronectin, collagen IV, elastin and laminin, which play pivotal
roles in cell migration (86–89).
Studies have shown that galectin-3 also interacts with epidermal
growth factor receptor (EGFR) to induce its phosphorylation and
re-localization from the membrane to the cytoplasm. In the case of
colon cancer cell migration, extracellular galectin-3 may bind with
EGFR to affect EGFR dynamics (90).
Researchers demonstrated that galectin-3 exhibited an increased
affinity to β-1,6-N-acetylglucosamine branched glycans. This
interaction mediated the binding of galectin-3 to several types of
glycoproteins and glycolipids on the cell membrane, including
carcinoembryonic antigen, mucin-1 and glycosylated transmembrane
tyrosine kinase receptors of EGF (91,92).
The aforementioned findings suggest that galectin-3 may play
multiple roles in regulating cell-matrix and cell-cell interactions
in cancer.
In addition, it has been reported that
tumor-secreted galectin-3 is involved in angiogenesis via binding
through carbohydrate recognition, thus affecting endothelial cell
behavior and regulating capillary formation during tumor
progression (79). The mechanism
underlying galectin-3-mediated angiogenesis has been associated
with the binding of galectin-3 with αvβ3 integrins on endothelial
cells to induce aggregation of integrin clusters and the activation
of several signaling pathways. As a result, galectin-3 may affect
the angiogenic activity of vascular endothelial growth factor and
basic fibroblast growth factor, as well as the promotion of focal
adhesion kinase phosphorylation (93).
Due to the immunosuppressive effects of galectin-3,
its role in promoting tumor invasion, migration and angiogenesis in
the TME has been attracting increasing attention. Therefore,
galectin-3 is considered as a potential target for the clinical
therapy of cancer. Although the pre-clinical study of a clinical
grade galectin antagonist (GM-CT-01) is still at an early stage, a
report demonstrated that this antagonist restored CD8+
T-cell function, suggesting that this compound may be an effective
approach to cancer therapy (94).
Furthermore, the effects of galectin-3 antagonists, combined with
immune checkpoint inhibitors or T-cell agonists, were investigated
to reveal their potential role on enhancing antitumor immunity and
promoting regression of solid tumors (95). Preclinical studies demonstrated that
treatment with a galectin-3 inhibitor, namely GR-MD-02, a
carbohydrate-based drug that binds to galectin-3, promoted
antigen-specific T-cell proliferation in patients with advanced
cancer (95,96). In addition, GR-MD-02 combined with
an irritant (anti-OX40) improved the survival rate of MCA-205
sarcoma, 4T1 breast cancer and transgenic adenocarcinoma of mouse
prostate cell (TRAMP-C1) models (95,97).
In addition, GR-MD-02 attenuated liver pathological changes,
collagen deposition and fibrosis in mice with non-alcoholic
steatohepatitis (96). The
combination of GR-MD-02 with anti-OX40 treatment also reduced lung
metastasis in a 4T1 breast cancer model (98). The successful application of lectin
inhibitors indicates that these inhibitors may represent a
potential promising approach to cancer therapy.
Differentiated or undifferentiated normal cells rely
heavily on the glycolytic pathway to generate energy. Glycolysis
refers to the anaerobic conversion of glucose to pyruvate through a
series of intercellular enzymatic reactions to produce adenosine
triphosphate (ATP), a high-energy phosphate compound (99). The Warburg effect describes a type
of mitochondrial dysfunction, where cancer cells do not allow
pyruvate to enter mitochondria; instead, lactate dehydrogenase
(LDH) enters mitochondria to degrade pyruvate to lactic acid, which
in turn enters the Cori cycle (99,100).
In tumor cells, galectin-3 overexpression was associated with
HIF-1α and p53 activity (21,101),
and enhanced phosphoinositide 3-kinase (PI3K) signaling to promote
GLUT1-mediated aerobic glycolysis in tumor cells (28,32,102).
In addition, galectin-3 promoted RAS and extracellular
signal-regulated kinase (ERK) 1/2 activation to induce GLUT1
expression and the activity of hexokinase, phosphofructokinase and
LDHA (103–106).
It has been reported that galectin-3 is involved in
maintaining mitochondrial homeostasis (107). Therefore, in ovarian cancer,
cisplatin promoted the release of cytochrome c and
mitochondrial reactive oxygen species in cells with
galectin-3-silencing. However, the effect of cisplatin was
attenuated following galectin-3 overexpression (108). Inhibition of galectin-3 expression
in colorectal cancer cells reduced epirubicin-induced ATP-binding
cassette transporter expression and activated the mitochondrial
apoptotic pathway (33). Galectin-3
has also been suggested to be associated with pivotal regulators of
mitochondrial metabolism, such as AMP-activated protein kinase and
peroxisome proliferator-activated receptor, two indicators of fatty
acid oxidation in mitochondria that regulate metabolic balance in
tumor cells (109,110).
Galectin-3 was found to be significantly upregulated
in human glioblastoma T98G cells under conditions of hypoxia and
nutrient deprivation (48).
Consistently, overexpression of galectin-3 enhanced T98G cell
survival and adaptation viability. These findings suggested that
galectin-3 mediated tumor progression via promoting angiogenesis
and maintaining homeostasis in the TME (48,111).
In addition, previous studies in melanoma cells have demonstrated
that extracellular galectin-3 activates the p38 mitogen-activated
protein kinase pathway, thereby inducing the expression of matrix
metalloproteinase (MMP)9, which in turn provides nutritional
support for tumor angiogenesis (32,112).
Therefore, galectin-3 overexpression may be considered as an
adaptive metabolic mechanism of the tumor to maintain cellular
viability and homeostasis under conditions of TME stress induced by
hypoxia and nutrient deficiency.
It has been reported that the AKT-mediated PI3K
signaling pathway is involved in the regulation of GLUT1, glucose
uptake and phosphofructokinase activity, thus affecting cell
survival, cell cycle progression and therapeutic outcome (99,113–117). Furthermore, galectin-3 has a high
affinity for and is cross-linked with β1,6-GlcNAc-branched
N-glycans and glycoproteins to form molecular complexes on the cell
surface and ECM, thus affecting the distribution of glycoproteins
and cell signal transduction (66).
Kariya et al demonstrated that the reactivation of PI3K
mediated by β4-integrin N-glycans was inhibited following treatment
with a neutralizing antibody against galectin-3 (118). Elad-Sfadia et al revealed
that galectin-3 was required for the RAS-induced PI3K/AKT
activation in response to growth factor stimulation (11). Additionally, galectin-3 increased
β-catenin expression and accumulation in the nucleus, thereby
enhancing Wnt signaling in human colon cancer cells via regulating
GSK-3β phosphorylation/activity through the PI3K/AKT signaling
pathway (39).
NF-κB serves an important role in inducing pro-
inflammatory cytokines in several types of cancer (119,120). Emerging evidence has suggested
that HIF-1α, NF-κB, caveolin-1 and TP53-inducible glycolysis and
apoptosis regulator acted as inducers of cancer-stroma metabolic
coupling via modulating oxidative stress and autophagy (121). Upregulation of galectin-3 in a
hypoxic microenvironment relies on transcription factors such as
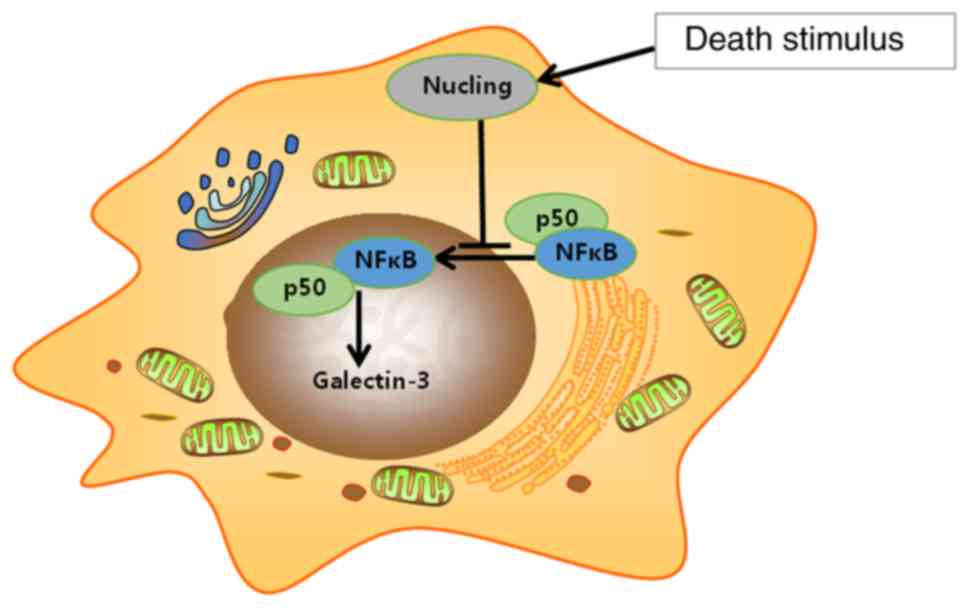
HIF-1α and NF-κB (48,122). Nucling, an apoptosis-associated
molecule, downregulated galectin-3 mRNA and protein expression via
mediating the nuclear translocation of NF-κB/p65 (Fig. 3). Therefore, nucling-deficient cells
were resistant to pro-apoptotic stress, whereas the expression of
galectin-3 and the incidence of inflammatory injury were increased
in mice lacking nucling (49).
Galectin-3 also promoted IL-8 transcription and secretion via NF-κB
signaling in pancreatic stellate cells; however, treatment with a
NF-κB inhibitor and integrin-linked kinase (cdp33) completely
inhibited the galectin-3-mediated transcriptional activities of
NF-κB and IL-8 (123).
Several signaling pathways have been found to be
involved in the metabolic process in tumors. Overexpression of
galectin-3 in the hypoxic TME was considered to regulate tumor cell
migration, invasion and adaptability (32). In addition, the Wnt/β-catenin
pathway was found to be involved in the regulation of cell
migration via MMPs (124). Shimura
et al demonstrated that galectin-3 interacted with the
β-catenin/TCF complex and was co-localized with β-catenin in the
nucleus, thereby regulating the transcriptional activity of
transcription factor 4 (TCF4) in breast cancer cells (125). Consistently, Song et al
reported that galectin-3 mediated β-catenin expression and TCF4
activity by regulating GSK-3β phosphorylation and activation via
the PI3K/AKT pathway in colon cancer cells (39). Additionally, downregulation of
galectin-3 resulted in reduced phosphorylated (p)-AKT and p-GSK-3β
expression, and increased GSK-3β activity, thus mediating the
phosphorylation of β-catenin. This effect was considered as a
critical step for the recognition of β-catenin by the F-box protein
β-Trcp (126). These findings
indicated that galectin-3 participates in multiple signaling
pathways to regulate tumor metabolism.
Lung cancer is one of the most common types of
cancer worldwide, and non-small cell lung cancer (NSCLC) accounts
for ~80% of lung cancer cases (127). The expression of galectin-3 varies
among different types of lung cancer. For example, in small-cell
lung cancer, galectin-3 is not expressed or downregulated compared
with NSCLC, in which galectin-3 is upregulated in the majority of
cases (128). mRNA microarrays
revealed that forkhead box D1 (FOXD1) was associated with poor
prognosis and lung cancer cell proliferation (129). FOXD1 has been shown to promote
lung cancer cell invasiveness via its binding with ERK1/2 and
targeting galectin-3 (95). In
addition, it has been demonstrated that galectin-3 regulates the
expression of FOXD1 via the integrin-β1/ERK/E26 transformation
specific-1 cascade, which in turn mediates the formation of a
positive ring between FOXD1 and galectin-3 and promotes lung cancer
invasiveness (130). A study
suggested that the expression of galectin-3 may be a potential
biomarker for predicting NSCLC recurrence after radical resection
(131). However, the level of
galectin-3 in the serum had no prognostic value in NSCLC, and no
significant correlation was observed between NSCLC and galectin-3
serum levels (131). Knockdown of
galectin-3 in NSCLC cell line-derived spheres decreased the
expression of stemness-associated genes, suggesting that galectin-3
may play a synergistic role by interacting with β-catenin and
increasing the transcriptional activity of downstream
stemness-associated genes. Furthermore, cells lacking galectin-3
were less invasive, more vulnerable to chemotherapy, and
inefficient in initiating tumor formation (132).
In NSCLC, galectin-3 not only mediates the
malignancy of cancer cells, but also attenuates the effect of
immune cells on inducing tumor cell evasion from the immune
response. It has been demonstrated that the intracellular
expression of galectin-3 and galectin-1 in tumor cells may block
apoptosis; however, the extracellular galectins within the TME
induce T-cell apoptosis via binding with CD45 and CD7 on the
surface of T cells, and exacerbating the immune escape of tumor
cells (133). In particular, the
galectin-3 multivalent N-glycan complex impaired TCR clustering on
the T-cell surface and increased the agonist threshold for TCR
signaling (133). In fact,
molecular interactions between T-cell surface glycans and certain
galectins are functionally capable of regulating T-lymphocyte death
and inflammatory responses (25,133–136). Antigen-presenting cells (APCs) and
macrophages also play an important role in establishing immune cell
homeostasis (137). Significant
changes in the glycan chain have been identified during dendritic
cell maturation in order to regulate the binding of specific
galectins to mature or immature APCs (138).
A recent meta-analysis has suggested that galectin-3
may be considered as a potentially useful immune marker for
distinguishing patients with papillary thyroid cancer (PTC) from
those with non-PTC (139). PTC
patients with positive galectin-3 expression are prone to lymph
node metastasis (139). A study
compared the serum galectin-3 levels between patients with thyroid
cancer and healthy individuals. The results revealed that the serum
galectin-3 levels in patients with thyroid cancer were
significantly higher compared with those in patients with benign
thyroid lesions or healthy controls (140,141). PTC and papillary thyroid
micro-carcinoma (PTMC) are the most common types of thyroid
malignancies (142,143). However, distinguishing PTC and
PTMC from thyroid papillary hyperplasia is challenging due to tumor
heterogeneity (144,145). Furthermore, the expression
profiles of galectin-3, cytokeratin 19, CD56, thyroid peroxidase
and BRAF mutations are commonly used for the diagnosis of PTC and
PTMC (145–148).
It has been suggested that the galectin family plays
an important role in Ras membrane anchoring and Ras-mediated cell
transformation (11,149). Ras proteins (H-Ras, K-Ras and
N-Ras) are important members of the GTPase family, which regulate
cell differentiation, proliferation and cell death (150). Ras mutations are known to be
involved in 25–30% of all human cancers (151,152). In addition, galectin-3 interacts
with oncogenic Ras proteins, preferentially with K-Ras, to promote
the activation of important signaling cascades, including
serine/threonine kinase (RAF1), PI3K and Ras signaling pathways,
and to regulate gene expression at the transcriptional level
(11). A study demonstrated that
the combination of galectin-3 inhibitor,
S-trans,trans-farnesylthiosalicylic acid and modified citrus
pectin was able to induce cell cycle arrest and apoptosis, thereby
inhibiting the growth of anaplastic thyroid carcinoma in
vitro and in vivo (153–155).
Melanoma is the most aggressive skin cancer and is
considered as a highly immunogenic tumor (156). Over the past few years, studies
have identified the characteristics of progressive biomarkers and
their underlying mechanisms based on deep research on the invasion
and chemoresistance abilities of melanoma cells, and the
association between galectin-3 expression and melanoma pathogenesis
(59). The results demonstrated
that, compared with benign nevus, the galectin-3 expression levels
were increased in thin primary melanomas. However, this expression
pattern was lost during tumor progression, and galectin-3
expression was decreased in both thick and metastatic melanomas
(70,157). Other studies indicated that the
expression of galectin-3 in melanocytes and melanoma cells treated
with a mutated BRAF inhibitor, vemurafenib, exerted a pivotal
effect on reducing autophagic activity and determining cell fate
(158,159).
It was previously demonstrated that the expression
levels of galectin-3 and its nuclear:cytoplasmic ratio was higher
in metastatic lesions compared with that in primary melanoma
lesions. Additionally, an association between the nuclear
expression of galectin-3 and prognosis was proposed (78). Mourad-Zeidan et al
demonstrated that melanoma cells lacking galectin-3 expression
exhibited reduced tumorigenic potential and decreased expression of
tumor markers (160). However,
other studies reported the opposite result. Therefore, a study
using a xenograft melanoma model constructed with human melanoma
cell lines demonstrated that the expression of galectin-3 was
upregulated in thin primary melanoma lesions compared with benign
pigmented skin lesions or metastases, and was negatively correlated
with cell invasiveness (2). It was
also suggested that, in more advanced melanoma lesions, attenuated
galectin-3 expression may be associated with high risk of tumor
metastasis. Related results indicate that melanoma cells may
separate from the basement membrane and enter the circulation via
attenuating their interaction with the ECM (2).
The unique molecular structure of galectin-3
determines its importance in the TME and tumor metabolism. In the
TME, tumor cells are more prone to inducing the production of
galectin-3 in order to promote their proliferation and survival. In
addition, galectin-3 interacts with immune cells and inhibits the
normal functions of lymphocytes, thereby mediating the immune
escape of tumor cells. In the TME, intracellular and extracellular
galectin-3 serve different functions. Of note, galectin-3 is also
involved in metabolic pathways in tumors, not only affecting
mitochondrial homeostasis, but also contributing to tumor cell
adaptation to a hypoxic metabolic environment and metastasis. The
characteristic functions of galectin-3 in the TME provide a novel
direction for cancer immunotherapy in the clinical setting;
however, further studies are required to elucidate the more
comprehensive mechanisms underlying the multifaceted biological
functions of galectin-3.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81772907), the
Fundamental Research Funds for the Central Universities (grant no.
lzujbky-2020-sp16) and the Gansu Youth Science Foundation Project
(grant no. 17JR2JA227).
Not applicable.
YG was a major contributor to the writing of the
manuscript and was mainly responsible for reviewing the role of
galectin-3 in the TME. RS was mainly responsible for reviewing the
role of galectin-3 in tumor metabolism. YS and DW critically
revised the manuscript for important intellectual content. LY, XZ
and RC were mainly responsible for reviewing the role of galectin-3
in different tumors. All the authors have read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Xue H, Liu L, Zhao Z, Zhang Z, Guan Y,
Cheng H, Zhou Y and Tai G: The N-terminal tail coordinates with
carbohydrate recognition domain to mediate galectin-3 induced
apoptosis in T cells. Oncotarget. 8:49824–49838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vereecken P, Debray C, Petein M, Awada A,
Lalmand MC, Laporte M, Van Den Heule B, Verhest A and Pochet R:
Expression of galectin-3 in primary and metastatic melanoma:
Immunohistochemical studies on human lesions and nude mice
xenograft tumors. Arch Dermatol Res. 296:353–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newlaczyl AU and Yu LG: Galectin-3-a
jack-of-all-trades in cancer. Cancer Lett. 313:123–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li YS, Li XT, Yu LG, Wang L, Shi ZY and
Guo XL: Roles of galectin-3 in metabolic disorders and tumor cell
metabolism. Int J Biol Macromol. 142:463–473. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fortuna-Costa A, Gomes AM, Kozlowski EO,
Stelling MP and Pavão MS: Extracellular galectin-3 in tumor
progression and metastasis. Front Oncol. 4:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farhad M, Rolig AS and Redmond WL: The
role of Galectin-3 in modulating tumor growth and immunosuppression
within the tumor microenvironment. Oncoimmunology. 7:e14344672018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmed H and AlSadek DM: Galectin-3 as a
potential target to prevent cancer metastasis. Clin Med Insights
Oncol. 9:113–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmad N, Gabius HJ, André S, Kaltner H,
Sabesan S, Roy R, Liu B, Macaluso F and Brewer CF: Galectin-3
precipitates as a pentamer with synthetic multivalent carbohydrates
and forms heterogeneous cross-linked complexes. J Biol Chem.
279:10841–10847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nangia-Makker P, Hogan V and Raz A:
Galectin-3 and cancer stemness. Glycobiology. 28:172–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehul B and Hughes RC: Plasma membrane
targetting, vesicular budding and release of galectin 3 from the
cytoplasm of mammalian cells during secretion. J Cell Sci.
110:1169–1178. 1997.PubMed/NCBI
|
|
11
|
Elad-Sfadia G, Haklai R, Balan E and Kloog
Y: Galectin-3 augments K-Ras activation and triggers a Ras signal
that attenuates ERK but not phosphoinositide 3-kinase activity. J
Biol Chem. 279:34922–34930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honjo Y, Nangia-Makker P, Inohara H and
Raz A: Down-regulation of galectin-3 suppresses tumorigenicity of
human breast carcinoma cells. Clin Cancer Res. 7:661–668.
2001.PubMed/NCBI
|
|
13
|
Chen HY, Fermin A, Vardhana S, Weng IC, Lo
KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML and Liu FT:
Galectin-3 negatively regulates TCR-mediated CD4+ T-cell
activation at the immunological synapse. Proc Natl Acad Sci USA.
106:14496–14501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grigorian A and Demetriou M: Manipulating
cell surface glycoproteins by targeting n-glycan-galectin
interactions. Methods Enzymol. 480:245–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Lella S, Sundblad V, Cerliani JP,
Guardia CM, Estrin DA, Vasta GR and Rabinovich GA: When galectins
recognize glycans: From biochemistry to physiology and back again.
Biochemistry. 50:7842–7857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato S and Hughes RC: Binding specificity
of a baby hamster kidney lectin for H type I and II chains,
polylactosamine glycans, and appropriately glycosylated forms of
laminin and fibronectin. J Biol Chem. 267:6983–6990.
1992.PubMed/NCBI
|
|
17
|
Yamaoka A, Kuwabara I, Frigeri LG and Liu
FT: A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates
superoxide production by neutrophils. J Immunol. 154:3479–3487.
1995.PubMed/NCBI
|
|
18
|
Kuwabara I and Liu FT: Galectin-3 promotes
adhesion of human neutrophils to laminin. J Immunol. 156:3939–3944.
1996.PubMed/NCBI
|
|
19
|
Karlsson A, Follin P, Leffler H and
Dahlgren C: Galectin-3 activates the NADPH-oxidase in exudated but
not peripheral blood neutrophils. Blood. 91:3430–3438. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ten Oever J, Giamarellos-Bourboulis EJ,
van de Veerdonk FL, Stelma FF, Simon A, Janssen M, Johnson M,
Pachot A, Kullberg BJ, Joosten LA and Netea MG: Circulating
galectin-3 in infections and non-infectious inflammatory diseases.
Eur J Clin Microbiol Infect Dis. 32:1605–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruvolo PP: Galectin 3 as a guardian of the
tumor microenvironment. Biochim Biophys Acta. 1863:427–437. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salomonsson E, Carlsson MC, Osla V,
Hendus-Altenburger R, Kahl-Knutson B, Oberg CT, Sundin A, Nilsson
R, Nordberg-Karlsson E, Nilsson UJ, et al: Mutational tuning of
galectin-3 specificity and biological function. J Biol Chem.
285:35079–35091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delacour D, Greb C, Koch A, Salomonsson E,
Leffler H, Le Bivic A and Jacob R: Apical sorting by
galectin-3-dependent glycoprotein clustering. Traffic. 8:379–388.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson KG, Stromnes IM and Greenberg PD:
Obstacles posed by the tumor microenvironment to T cell activity: A
case for synergistic therapies. Cancer Cell. 31:311–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon-Alonso M, Hirsch T, Wildmann C and
van der Bruggen P: Galectin-3 captures interferon-gamma in the
tumor matrix reducing chemokine gradient production and T-cell
tumor infiltration. Nat Commun. 8:7932017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Oliveira JT, Ribeiro C, Barros R, Gomes
C, de Matos AJ, Reis CA, Rutteman GR and Gärtner F: Hypoxia
up-regulates galectin-3 in mammary tumor progression and
metastasis. PLoS One. 10:e01344582015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun W, Li L, Li LJ, Yang QQ, Zhang ZR and
Huang Y: Two birds, one stone: Dual targeting of the cancer cell
surface and subcellular mitochondria by the galectin-3-binding
peptide G3-C12. Acta Pharmacol Sin. 38:806–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bacchi PS, Bloise AC, Bustos SO,
Zimmermann L, Chammas R and Rabbani SR: Metabolism under hypoxia in
Tm1 murine melanoma cells is affected by the presence of
galectin-3, a metabolomics approach. Springerplus. 3:4702014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pejnovic NN, Pantic JM, Jovanovic IP,
Radosavljevic GD, Milovanovic MZ, Nikolic IG, Zdravkovic NS, Djukic
AL, Arsenijevic NN and Lukic ML: Galectin-3 deficiency accelerates
high-fat diet-induced obesity and amplifies inflammation in adipose
tissue and pancreatic islets. Diabetes. 62:1932–1944. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu F, Finley RL Jr, Raz A and Kim HR:
Galectin-3 translocates to the perinuclear membranes and inhibits
cytochrome c release from the mitochondria. A role for synexin in
galectin-3 translocation. J Biol Chem. 277:15819–15827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sciacchitano S, Lavra L, Morgante A,
Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB and
Ricci A: Galectin-3: One molecule for an alphabet of diseases, from
A to Z. Int J Mol Sci. 19:3792018. View Article : Google Scholar
|
|
32
|
Cardoso AC, Andrade LN, Bustos SO and
Chammas R: Galectin-3 determines tumor cell adaptive strategies in
stressed tumor microenvironments. Front Oncol. 6:1272016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YK, Lin TH, Chang CF and Lo YL:
Galectin-3 silencing inhibits epirubicin-induced ATP binding
cassette transporters and activates the mitochondrial apoptosis
pathway via β-catenin/GSK-3 β modulation in colorectal carcinoma.
PLoS One. 8:e824782013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fei F, Joo EJ, Tarighat SS, Schiffer I,
Paz H, Fabbri M, Abdel-Azim H, Groffen J and Heisterkamp N: B-cell
precursor acute lymphoblastic leukemia and stromal cells
communicate through galectin-3. Oncotarget. 6:11378–11394. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto-Sugitani M, Kuroda J, Ashihara E,
Nagoshi H, Kobayashi T, Matsumoto Y, Sasaki N, Shimura Y, Kiyota M,
Nakayama R, et al: Galectin-3 (Gal-3) induced by leukemia
microenvironment promotes drug resistance and bone marrow lodgment
in chronic myelogenous leukemia. Proc Natl Acad Sci USA.
108:17468–17473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silverman AM, Nakata R, Shimada H, Sposto
R and DeClerck YA: A galectin-3-dependent pathway upregulates
interleukin-6 in the microenvironment of human neuroblastoma.
Cancer Res. 72:2228–2238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakayama R, Kuroda J, Taniyama N,
Yamamoto-Sugitani M, Wada S, Kiyota M, Mizutani S, Chinen Y,
Matsumoto Y, Nagoshi H, et al: Suppression of SERPINA1-albumin
complex formation by galectin-3 overexpression leads to paracrine
growth promotion of chronic myelogenous leukemia cells. Leuk Res.
38:103–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruvolo PP, Ruvolo VR, Burks JK, Qiu Y,
Wang RY, Shpall EJ, Mirandola L, Hail N Jr, Zeng Z, McQueen T, et
al: Role of MSC-derived galectin 3 in the AML microenvironment.
Biochim Biophys Acta Mol Cell Res. 1865:959–969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song S, Mazurek N, Liu C, Sun Y, Ding QQ,
Liu K, Hung MC and Bresalier RS: Galectin-3 mediates nuclear
beta-catenin accumulation and Wnt signaling in human colon cancer
cells by regulation of glycogen synthase kinase-3beta activity.
Cancer Res. 69:1343–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCubrey JA, Davis NM, Abrams SL, Montalto
G, Cervello M, Basecke J, Libra M, Nicoletti F, Cocco L, Martelli
AM and Steelman LS: Diverse roles of GSK-3: Tumor promoter-tumor
suppressor, target in cancer therapy. Adv Biol Regul. 54:176–196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hermida MA, Dinesh Kumar J and Leslie NR:
GSK3 and its interactions with the PI3K/AKT/mTOR signalling
network. Adv Biol Regul. 65:5–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ricciardi MR, Mirabilii S, Licchetta R,
Piedimonte M and Tafuri A: Targeting the Akt, GSK-3, Bcl-2 axis in
acute myeloid leukemia. Adv Biol Regul. 65:36–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruvolo PP: GSK-3 as a novel prognostic
indicator in leukemia. Adv Biol Regul. 65:26–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krause S, Pfeiffer C, Strube S, Alsadeq A,
Fedders H, Vokuhl C, Loges S, Waizenegger J, Ben-Batalla I, Cario
G, et al: Mer tyrosine kinase promotes the survival of
t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central
nervous system (CNS). Blood. 125:820–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zeng Y, Danielson KG, Albert TJ, Shapiro
IM and Risbud MV: HIF-1 alpha is a regulator of galectin-3
expression in the intervertebral disc. J Bone Miner Res.
22:1851–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Neder L, Marie SK, Carlotti CG Jr, Gabbai
AA, Rosemberg S, Malheiros SM, Siqueira RP, Oba-Shinjo SM, Uno M,
Aguiar PH, et al: Galectin-3 as an immunohistochemical tool to
distinguish pilocytic astrocytomas from diffuse astrocytomas, and
glioblastomas from anaplastic oligodendrogliomas. Brain Pathol.
14:399–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rêgo MJ, Vieira de Mello GS, da Silva
Santos CA, Chammas R and Beltrão EI: Implications on
glycobiological aspects of tumor hypoxia in breast ductal carcinoma
in situ. Med Mol Morphol. 46:92–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ikemori RY, Machado CM, Furuzawa KM,
Nonogaki S, Osinaga E, Umezawa K, de Carvalho MA, Verinaud L and
Chammas R: Galectin-3 up-regulation in hypoxic and nutrient
deprived microenvironments promotes cell survival. PLoS One.
9:e1115922014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu L, Sakai T, Sano N and Fukui K:
Nucling mediates apoptosis by inhibiting expression of galectin-3
through interference with nuclear factor kappaB signalling. Biochem
J. 380:31–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Radosavljevic G, Jovanovic I, Majstorovic
I, Mitrovic M, Lisnic VJ, Arsenijevic N, Jonjic S and Lukic ML:
Deletion of galectin-3 in the host attenuates metastasis of murine
melanoma by modulating tumor adhesion and NK cell activity. Clin
Exp Metastasis. 28:451–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kouo T, Huang L, Pucsek AB, Cao M, Solt S,
Armstrong T and Jaffee E: Galectin-3 shapes antitumor immune
responses by suppressing CD8+ T cells via LAG-3 and
inhibiting expansion of plasmacytoid dendritic cells. Cancer
Immunol Res. 3:412–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Demotte N, Wieërs G, Van Der Smissen P,
Moser M, Schmidt C, Thielemans K, Squifflet JL, Weynand B, Carrasco
J, Lurquin C, et al: A galectin-3 ligand corrects the impaired
function of human CD4 and CD8 tumor-infiltrating lymphocytes and
favors tumor rejection in mice. Cancer Res. 70:7476–7488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gordon-Alonso M, Demotte N and van der
Bruggen P: Sugars boost exhausted tumor-infiltrating lymphocytes by
counteracting immunosuppressive activities of galectins.
Oncoimmunology. 3:e287832014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng W, Wang HY, Miyahara Y, Peng G and
Wang RF: Tumor-associated galectin-3 modulates the function of
tumor-reactive T cells. Cancer Res. 68:7228–7236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zubieta MR, Furman D, Barrio M, Bravo AI,
Domenichini E and Mordoh J: Galectin-3 expression correlates with
apoptosis of tumor-associated lymphocytes in human melanoma
biopsies. Am J Pathol. 168:1666–1675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka
N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T,
et al: A novel strategy for evasion of NK cell immunity by tumours
expressing core2 O-glycans. EMBO J. 30:3173–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suzuki Y, Sutoh M, Hatakeyama S, Mori K,
Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Habuchi T, et al:
MUC1 carrying core 2 O-glycans functions as a molecular
shield against NK cell attack, promoting bladder tumor metastasis.
Int J Oncol. 40:1831–1838. 2012.PubMed/NCBI
|
|
58
|
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun
R and Tian Z: Tumor-released galectin-3, a soluble inhibitory
ligand of human NKp30, plays an important role in tumor escape from
NK cell attack. J Biol Chem. 289:33311–33319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Melief SM, Visser M, van der Burg SH and
Verdegaal EME: IDO and galectin-3 hamper the ex vivo generation of
clinical grade tumor-specific T cells for adoptive cell therapy in
metastatic melanoma. Cancer Immunol Immunother. 66:913–926. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Colomb F, Wang W, Simpson D, Zafar M,
Beynon R, Rhodes JM and Yu LG: Galectin-3 interacts with the
cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion
of metastasis-promoting cytokines from vascular endothelial cells.
J Biol Chem. 292:8381–8389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pop VV, Seicean A, Lupan I, Samasca G and
Burz CC: IL-6 roles-molecular pathway and clinical implication in
pancreatic cancer-A systemic review. Immunol Lett. 181:45–50. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Stillman BN, Hsu DK, Pang M, Brewer CF,
Johnson P, Liu FT and Baum LG: Galectin-3 and galectin-1 bind
distinct cell surface glycoprotein receptors to induce T cell
death. J Immunol. 176:778–789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang RY, Hsu DK and Liu FT: Expression of
galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad
Sci USA. 93:6737–6742. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Haudek KC, Spronk KJ, Voss PG, Patterson
RJ, Wang JL and Arnoys EJ: Dynamics of galectin-3 in the nucleus
and cytoplasm. Biochim Biophys Acta. 1800:181–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
van den Brûle F, Califice S and Castronovo
V: Expression of galectins in cancer: A critical review. Glycoconj
J. 19:537–542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nakahara S, Oka N and Raz A: On the role
of galectin-3 in cancer apoptosis. Apoptosis. 10:267–275. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Honjo Y, Inohara H, Akahani S, Yoshii T,
Takenaka Y, Yoshida J, Hattori K, Tomiyama Y, Raz A and Kubo T:
Expression of cytoplasmic galectin-3 as a prognostic marker in
tongue carcinoma. Clin Cancer Res. 6:4635–4640. 2000.PubMed/NCBI
|
|
69
|
van den Brûle FA, Waltregny D, Liu FT and
Castronovo V: Alteration of the cytoplasmic/nuclear expression
pattern of galectin-3 correlates with prostate carcinoma
progression. Int J Cancer. 89:361–367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brown ER, Doig T, Anderson N, Brenn T,
Doherty V, Xu Y, Bartlett JM, Smyth JF and Melton DW: Association
of galectin-3 expression with melanoma progression and prognosis.
Eur J Cancer. 48:865–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Califice S, Castronovo V, Bracke M and van
den Brûle F: Dual activities of galectin-3 in human prostate
cancer: Tumor suppression of nuclear galectin-3 vs tumor promotion
of cytoplasmic galectin-3. Oncogene. 23:7527–7536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dumic J, Dabelic S and Flögel M:
Galectin-3: An open-ended story. Biochim Biophys Acta.
1760:616–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Levy R, Biran A, Poirier F, Raz A and
Kloog Y: Galectin-3 mediates cross-talk between K-Ras and Let-7c
tumor suppressor microRNA. PLoS One. 6:e274902011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Song S, Ji B, Ramachandran V, Wang H,
Hafley M, Logsdon C and Bresalier RS: Overexpressed galectin-3 in
pancreatic cancer induces cell proliferation and invasion by
binding Ras and activating Ras signaling. PLoS One. 7:e426992012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Streetly MJ, Maharaj L, Joel S, Schey SA,
Gribben JG and Cotter FE: GCS-100, a novel galectin-3 antagonist,
modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death.
Blood. 115:3939–3948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Harazono Y, Nakajima K and Raz A: Why
anti-Bcl-2 clinical trials fail: A solution. Cancer Metastasis Rev.
33:285–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Song L, Tang JW, Owusu L, Sun MZ, Wu J and
Zhang J: Galectin-3 in cancer. Clin Chim Acta. 431:185–191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Prieto VG, Mourad-Zeidan AA, Melnikova V,
Johnson MM, Lopez A, Diwan AH, Lazar AJ, Shen SS, Zhang PS, Reed
JA, et al: Galectin-3 expression is associated with tumor
progression and pattern of sun exposure in melanoma. Clin Cancer
Res. 12:6709–6715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nangia-Makker P, Honjo Y, Sarvis R,
Akahani S, Hogan V, Pienta KJ and Raz A: Galectin-3 induces
endothelial cell morphogenesis and angiogenesis. Am J Pathol.
156:899–909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Henderson NC, Mackinnon AC, Farnworth SL,
Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ and Sethi T:
Galectin-3 regulates myofibroblast activation and hepatic fibrosis.
Proc Natl Acad Sci USA. 103:5060–5065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sioud M, Mobergslien A, Boudabous A and
Fløisand Y: Evidence for the involvement of galectin-3 in
mesenchymal stem cell suppression of allogeneic T-cell
proliferation. Scand J Immunol. 71:267–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Henderson NC, Mackinnon AC, Farnworth SL,
Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J and Sethi T:
Galectin-3 expression and secretion links macrophages to the
promotion of renal fibrosis. Am J Pathol. 172:288–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
O'Driscoll L, Linehan R, Liang YH, Joyce
H, Oglesby I and Clynes M: Galectin-3 expression alters adhesion,
motility and invasion in a lung cell line (DLKP), in vitro.
Anticancer Res. 22:3117–3125. 2002.PubMed/NCBI
|
|
84
|
Melo FH, Butera D, Junqueira Mde S, Hsu
DK, da Silva AM, Liu FT, Santos MF and Chammas R: The promigratory
activity of the matricellular protein galectin-3 depends on the
activation of PI-3 kinase. PLoS One. 6:e293132011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kim SJ, Shin JY, Lee KD, Bae YK, Choi IJ,
Park SH and Chun KH: Galectin-3 facilitates cell motility in
gastric cancer by up-regulating protease-activated receptor-1
(PAR-1) and matrix metalloproteinase-1 (MMP-1). PLoS One.
6:e251032011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hughes RC: Galectins as modulators of cell
adhesion. Biochimie. 83:667–676. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ochieng J, Leite-Browning ML and Warfield
P: Regulation of cellular adhesion to extracellular matrix proteins
by galectin-3. Biochem Biophys Res Commun. 246:788–791. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ochieng J, Warfield P, Green-Jarvis B and
Fentie I: Galectin-3 regulates the adhesive interaction between
breast carcinoma cells and elastin. J Cell Biochem. 75:505–514.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nangia-Makker P, Balan V and Raz A:
Regulation of tumor progression by extracellular galectin-3. Cancer
Microenviron. 1:43–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu KL, Kuo CM, Huang EY, Pan HM, Huang CC,
Chen YF, Hsiao CC and Yang KD: Extracellular galectin-3 facilitates
colon cancer cell migration and is related to the epidermal growth
factor receptor. Am J Transl Res. 10:24022018.PubMed/NCBI
|
|
91
|
Partridge EA, Le Roy C, Di Guglielmo GM,
Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL and Dennis JW:
Regulation of cytokine receptors by Golgi N-glycan processing and
endocytosis. Science. 306:120–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Saeland E, Belo AI, Mongera S, van Die I,
Meijer GA and van Kooyk Y: Differential glycosylation of MUC1 and
CEACAM5 between normal mucosa and tumour tissue of colon cancer
patients. Int J Cancer. 131:117–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Markowska AI, Liu FT and Panjwani N:
Galectin-3 is an important mediator of VEGF- and bFGF-mediated
angiogenic response. J Exp Med. 207:1981–1993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Demotte N, Bigirimana R, Wieërs G,
Stroobant V, Squifflet JL, Carrasco J, Thielemans K, Baurain JF,
Van Der Smissen P, Courtoy PJ and van der Bruggen P: A short
treatment with galactomannan GM-CT-01 corrects the functions of
freshly isolated human tumor-infiltrating lymphocytes. Clin Cancer
Res. 20:1823–1833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dong R, Zhang M, Hu Q, Zheng S, Soh A,
Zheng Y and Yuan H: Galectin-3 as a novel biomarker for disease
diagnosis and a target for therapy (Review). Int J Mol Med.
41:599–614. 2018.PubMed/NCBI
|
|
96
|
Traber PG and Zomer E: Therapy of
experimental NASH and fibrosis with galectin inhibitors. PLoS One.
8:e834812013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bayes-Genis A, de Antonio M, Vila J,
Peñafiel J, Galán A, Barallat J, Zamora E, Urrutia A and Lupón J:
Head-to-head comparison of 2 myocardial fibrosis biomarkers for
long-term heart failure risk stratification: ST2 versus galectin-3.
J Am Coll Cardiol. 63:158–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Linch S, Kasiewicz MJ, McNamara M, Hilgart
I, Farhad M and Redmond W: Galectin-3 inhibition using novel
inhibitor GR-MD-02 improves survival and immune function while
reducing tumor vasculature. J Immunother Cancer. 3 (Suppl
2):P3062015. View Article : Google Scholar
|
|
99
|
Courtnay R, Ngo DC, Malik N, Ververis K,
Tortorella SM and Karagiannis TC: Cancer metabolism and the Warburg
effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng J, Lu W, Wang C, Xing Y, Chen X and
Ai Z: Galectin-3 induced by hypoxia promotes cell migration in
thyroid cancer cells. Oncotarget. 8:101475–101488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rinaldi G, Rossi M and Fendt SM: Metabolic
interactions in cancer: Cellular metabolism at the interface
between the microenvironment, the cancer cell phenotype and the
epigenetic landscape. Wiley Interdiscip Rev Syst Biol Med. 10:2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gao X, Balan V, Tai G and Raz A:
Galectin-3 induces cell migration via a calcium-sensitive
MAPK/ERK1/2 pathway. Oncotarget. 5:2077–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Nakahara S and Raz A: Regulation of
cancer-related gene expression by galectin-3 and the molecular
mechanism of its nuclear import pathway. Cancer Metastasis Rev.
26:605–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yu F, Finley RL Jr, Raz A and Kim HR:
Galectin-3 translocates to the perinuclear membranes and inhibits
cytochrome c release from the mitochondria. A role for synexin in
galectin-3 translocation. J Biol Chem. 277:15819–15827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang D, You D and Li L: Galectin-3
regulates chemotherapy sensitivity in epithelial ovarian carcinoma
via regulating mitochondrial function. J Toxicol Sci. 44:47–56.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dupont J, Reverchon M, Cloix L, Froment P
and Ramé C: Involvement of adipokines, AMPK, PI3K and the PPAR
signaling pathways in ovarian follicle development and cancer. Int
J Dev Biol. 56:959–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wu Y, Sarkissyan M, Mcghee E, Lee S and
Vadgama JV: Combined inhibition of glycolysis and AMPK induces
synergistic breast cancer cell killing. Breast Cancer Res Treat.
151:529–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dos Santos SN, Sheldon H, Pereira JX,
Paluch C, Bridges EM, El-Cheikh MC, Harris AL and Bernardes ES:
Galectin-3 acts as an angiogenic switch to induce tumor
angiogenesis via Jagged-1/Notch activation. Oncotarget.
8:49484–49501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dange MC, Agarwal AK and Kalraiya RD:
Extracellular galectin-3 induces MMP9 expression by activating p38
MAPK pathway via lysosome-associated membrane protein-1 (LAMP1).
Mol Cell Biochem. 404:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Simons AL, Orcutt KP, Madsen JM,
Scarbrough PM and Spitz DR: The role of Akt pathway signaling in
glucose metabolism and metabolic oxidative stress. Oxidative stress
in cancer biology and therapy. Oxidative Stress in Applied Basic
Research and Clinical Practice. Spitz D, Dornfeld K, Krishnan K and
Gius D: Humana Press; Totowa, NJ: pp. 21–46. 2012, http://doi-org-443.webvpn.fjmu.edu.cn/10.1007/978-1-61779-397-4_2
View Article : Google Scholar
|
|
114
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L,
Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional
regulation of vascular endothelial cell responses to hypoxia by
HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Minet E, Michel G, Remacle J and Michiels
C: Role of HIF-1 as a transcription factor involved in embryonic
development, cancer progression and apoptosis (Review). Int J Mol
Med. 5:253–262. 2000.PubMed/NCBI
|
|
117
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kariya Y, Oyama M, Hashimoto Y, Gu J and
Kariya Y: β4-Integrin/PI3K signaling promotes tumor progression
through the galectin-3-N-glycan complex. Mol Cancer Res.
16:1024–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lippert E, Falk W, Bataille F, Kähne T,
Naumann M, Goeke M, Herfarth H, Schoelmerich J and Rogler G:
Soluble galectin-3 is a strong, colonic epithelial-cell-derived,
lamina propria fibroblast-stimulating factor. Gut. 56:43–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia
X, He S, Qiang F, Li A, Shu Y, et al: CHIP functions as a novel
suppressor of tumour angiogenesis with prognostic significance in
human gastric cancer. Gut. 62:496–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wilde L, Roche M, Domingo-Vidal M, Tanson
K, Philp N, Curry J and Martinez-Outschoorn U: Metabolic coupling
and the reverse Warburg effect in cancer: Implications for novel
biomarker and anticancer agent development. Semin Oncol.
44:198–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Greijer AE, van der Groep P, Kemming D,
Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van
Diest PJ and van der Wall E: Up-regulation of gene expression by
hypoxia is mediated predominantly by hypoxia-inducible factor 1
(HIF-1). J Pathol. 206:291–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhao W, Ajani JA, Sushovan G, Ochi N,
Hwang R, Hafley M, Johnson RL, Bresalier RS, Logsdon CD, Zhang Z
and Song S: Galectin-3 mediates tumor cell-stroma interactions by
activating pancreatic stellate cells to produce cytokines via
integrin signaling. Gastroenterology. 154:1524–1537.e6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
McCubrey JA, Rakus D, Gizak A, Steelman
LS, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Yang LV, Montalto
G, Cervello M, et al: Effects of mutations in Wnt/β-catenin,
hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects
on cell growth, metabolism and cancer. Biochim Biophys Acta.
1863:2942–2976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shimura T, Takenaka Y, Tsutsumi S, Hogan
V, Kikuchi A and Raz A: Galectin-3, a novel binding partner of
beta-catenin. Cancer Res. 64:6363–6367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sun S, Schiller JH, Spinola M and Minna
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yoshimura A, Gemma A, Hosoya Y, Komaki E,
Hosomi Y, Okano T, Takenaka K, Matuda K, Seike M, Uematsu K, et al:
Increased expression of the LGALS3 (galectin 3) gene in human
non-small-cell lung cancer. Genes Chromosomes Cancer. 37:159–164.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nakayama S, Soejima K, Yasuda H, Yoda S,
Satomi R, Ikemura S, Terai H, Sato T, Yamaguchi N, Hamamoto J, et
al: FOXD1 expression is associated with poor prognosis in non-small
cell lung cancer. Anticancer Res. 35:261–268. 2015.PubMed/NCBI
|
|
130
|
Li CH, Chang YC, Hsiao M and Liang SM:
FOXD1 and Gal-3 form a positive regulatory loop to regulate lung
cancer aggressiveness. Cancers (Basel). 11:18972019. View Article : Google Scholar
|
|
131
|
Kataoka Y, Igarashi T, Ohshio Y, Fujita T
and Hanaoka J: Predictive importance of galectin-3 for recurrence
of non-small cell lung cancer. Gen Thorac Cardiovasc Surg.
67:704–711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY
and Sun KH: Galectin-3 augments tumor initiating property and
tumorigenicity of lung cancer through interaction with β-catenin.
Oncotarget. 6:4936–4952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rabinovich GA and Toscano MA: Turning
‘sweet’ on immunity: Galectin-glycan interactions in immune
tolerance and inflammation. Nat Rev Immunol. 9:338–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Toscano MA, Bianco GA, Ilarregui JM, Croci
DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum
LG and Rabinovich GA: Differential glycosylation of TH1, TH2 and
TH-17 effector cells selectively regulates susceptibility to cell
death. Nat Immunol. 8:825–834. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
135
|
Demetriou M, Granovsky M, Quaggin S and
Dennis JW: Negative regulation of T-cell activation and
autoimmunity by Mgat5 N-glycosylation. Nature. 409:733–739. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Novak R, Dabelic S and Dumic J: Galectin-1
and galectin-3 expression profiles in classically and alternatively
activated human macrophages. Biochim Biophys Acta. 1820:1383–1390.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Capalbo C, Scafetta G, Filetti M,
Marchetti P and Bartolazzi A: Predictive biomarkers for checkpoint
inhibitor-based immunotherapy: The Galectin-3 signature in NSCLCs.
Int J Mol Sci. 20:16072019. View Article : Google Scholar
|
|
138
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tang W, Huang C, Tang C, Xu J and Wang H:
Galectin-3 may serve as a potential marker for diagnosis and
prognosis in papillary thyroid carcinoma: A meta-analysis. Onco
Targets Ther. 9:455–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xue G, Liu J, Huang J, Zhang J, Zhang W,
Wu J and Shang X: Detection of galectin-3 in both serum and tissue
for early diagnosis of thyroid carcinoma. Nan Fang Yi Ke Da Xue Xue
Bao. 33:1027–1030. 2013.(In Chinese). PubMed/NCBI
|
|
141
|
Yılmaz E, Karşıdağ T, Tatar C and Tüzün S:
Serum galectin-3: Diagnostic value for papillary thyroid carcinoma.
Ulus Cerrahi Derg. 31:192–196. 2015.PubMed/NCBI
|
|
142
|
Shi RL, Qu N, Liao T, Wang YL, Wang Y, Sun
GH and Ji QH: Expression, clinical significance and mechanism of
Slit2 in papillary thyroid cancer. Int J Oncol. 48:2055–2062. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Shi RL, Qu N, Liao T, Wei WJ, Lu ZW, Ma B,
Wang YL and Ji QH: Relationship of body mass index with BRAF
(V600E) mutation in papillary thyroid cancer. Tumour Biol.
37:8383–8390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Park YJ, Kim YA, Lee YJ, Kim SH, Park SY,
Kim KW, Chung JK, Youn YK, Kim KH, Park DJ and Cho BY: Papillary
microcarcinoma in comparison with larger papillary thyroid
carcinoma in BRAF(V600E) mutation, clinicopathological features,
and immunohistochemical findings. Head Neck. 32:38–45.
2010.PubMed/NCBI
|
|
145
|
Batistatou A, Charalabopoulos K, Nakanishi
Y, Vagianos C, Hirohashi S, Agnantis NJ and Scopa CD: Differential
expression of dysadherin in papillary thyroid carcinoma and
microcarcinoma: Correlation with E-cadherin. Endocr Pathol.
19:197–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang L, Wang X, Huang X, Gui H, Li Y,
Chen Q, Liu D and Liu L: Diagnostic significance of CK19,
galectin-3, CD56, TPO and Ki67 expression and BRAF mutation in
papillary thyroid carcinoma. Oncol Lett. 15:4269–4277.
2018.PubMed/NCBI
|
|
147
|
Lu ZZ, Zhang Y, Wei SF, Li DS, Zhu QH, Sun
SJ, Li M and Li LI: Outcome of papillary thyroid microcarcinoma:
Study of 1,990 cases. Mol Clin Oncol. 3:672–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Nasr MR, Mukhopadhyay S, Zhang S and
Katzenstein AL: Absence of the BRAF mutation in HBME1+ and
CK19+ atypical cell clusters in Hashimoto thyroiditis: Supportive
evidence against preneoplastic change. Am J Clin Pathol.
132:906–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Paz A, Haklai R, Elad-Sfadia G, Ballan E
and Kloog Y: Galectin-1 binds oncogenic H-Ras to mediate Ras
membrane anchorage and cell transformation. Oncogene. 20:7486–7493.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Trent JC II, McConkey DJ, Loughlin SM,
Harbison MT, Fernandez A and Ananthaswamy HN: Ras signaling in
tumor necrosis factor-induced apoptosis. EMBO J. 15:4497–4505.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Crul M, de Klerk GJ, Beijnen JH and
Schellens JH: Ras biochemistry and farnesyl transferase inhibitors:
A literature survey. Anticancer Drugs. 12:163–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Adjei AA: Blocking oncogenic Ras signaling
for cancer therapy. J Natl Cancer Inst. 93:1062–1074. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Levy R, Grafi-Cohen M, Kraiem Z and Kloog
Y: Galectin-3 promotes chronic activation of K-Ras and
differentiation block in malignant thyroid carcinomas. Mol Cancer
Ther. 9:2208–2219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nangia-Makker P, Conklin J, Hogan V and
Raz A: Carbohydrate-binding proteins in cancer, and their ligands
as therapeutic agents. Trends Mol Med. 8:187–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Menachem A, Bodner O, Pastor J, Raz A and
Kloog Y: Inhibition of malignant thyroid carcinoma cell
proliferation by Ras and galectin-3 inhibitors. Cell Death Discov.
1:150472015. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Rosenberg SA, Yannelli JR, Yang JC,
Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA,
Einhorn JH and White DE: Treatment of patients with metastatic
melanoma with autologous tumor-infiltrating lymphocytes and
interleukin 2. J Natl Cancer Inst. 86:1159–1166. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Li ZW, Wang Y, Xue WC, Si L, Cui CL, Cao
DF, Zhou LX, Guo J and Lu AP: Expression and prognostic
significance of galectin-1 and galectin-3 in benign nevi and
melanomas. Zhonghua Bing Li Xue Za Zhi. 42:801–805. 2013.(In
Chinese). PubMed/NCBI
|
|
158
|
Ma XH, Piao SF, Dey S, Mcafee Q,
Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, et al:
Targeting ER stress-induced autophagy overcomes BRAF inhibitor
resistance in melanoma. J Clin Invest. 124:1406–1417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Villanueva J, Vultur A, Lee JT,
Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu
X, Gimotty PA, Kee D, et al: Acquired resistance to BRAF inhibitors
mediated by a RAF kinase switch in melanoma can be overcome by
cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 18:683–695. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Mourad-Zeidan AA, Melnikova VO, Wang H,
Raz A and Bar-Eli M: Expression profiling of Galectin-3-depleted
melanoma cells reveals its major role in melanoma cell plasticity
and vasculogenic mimicry. Am J Pathol. 173:1839–1852. 2008.
View Article : Google Scholar : PubMed/NCBI
|