Head and neck cancers affect the lips, oral cavity,
oropharynx, nasopharynx, hypopharynx and larynx, and >836,000
new cases and 431,000 related deaths occur each year worldwide
(1). Head and neck squamous cell
carcinoma (HNSCC), an aggressive malignancy characterized by
relatively high rates of local recurrence, low responsiveness to
therapy and lymph node metastasis (2), comprises ≤90% of head and neck cancers
(3). Although notable progress has
been made over the past few decades in surgical, chemotherapeutic
and tumor-targeting therapies for HNSCC, patient prognosis remains
unsatisfactory (4). To date, the
epidermal growth factor receptor-targeting biological factor
cetuximab, is the only Food and Drug Administration-approved
targeted therapy for HNSCC (5).
However, combinatorial cetuximab treatment with radiation or
chemotherapy provides only a modest benefit to survival time (29.3
vs. 49 months and 7.4 vs. 10.1 months, respectively) (6,7).
Furthermore, in clinical trials, cetuximab resistance was
frequently observed, along with c-mesenchymal-epithelial transition
(Met) activation (8–11). Aberrant hepatocyte growth factor
(HGF)/c-Met signaling has been frequently reported in HNSCC
(11–14); HGF-induced Met activation is a known
mechanism of cetuximab resistance (15). Therefore, given its role in HNSCC,
the HGF/Met pathway may serve as a potential target for treatment
of HNSCC.
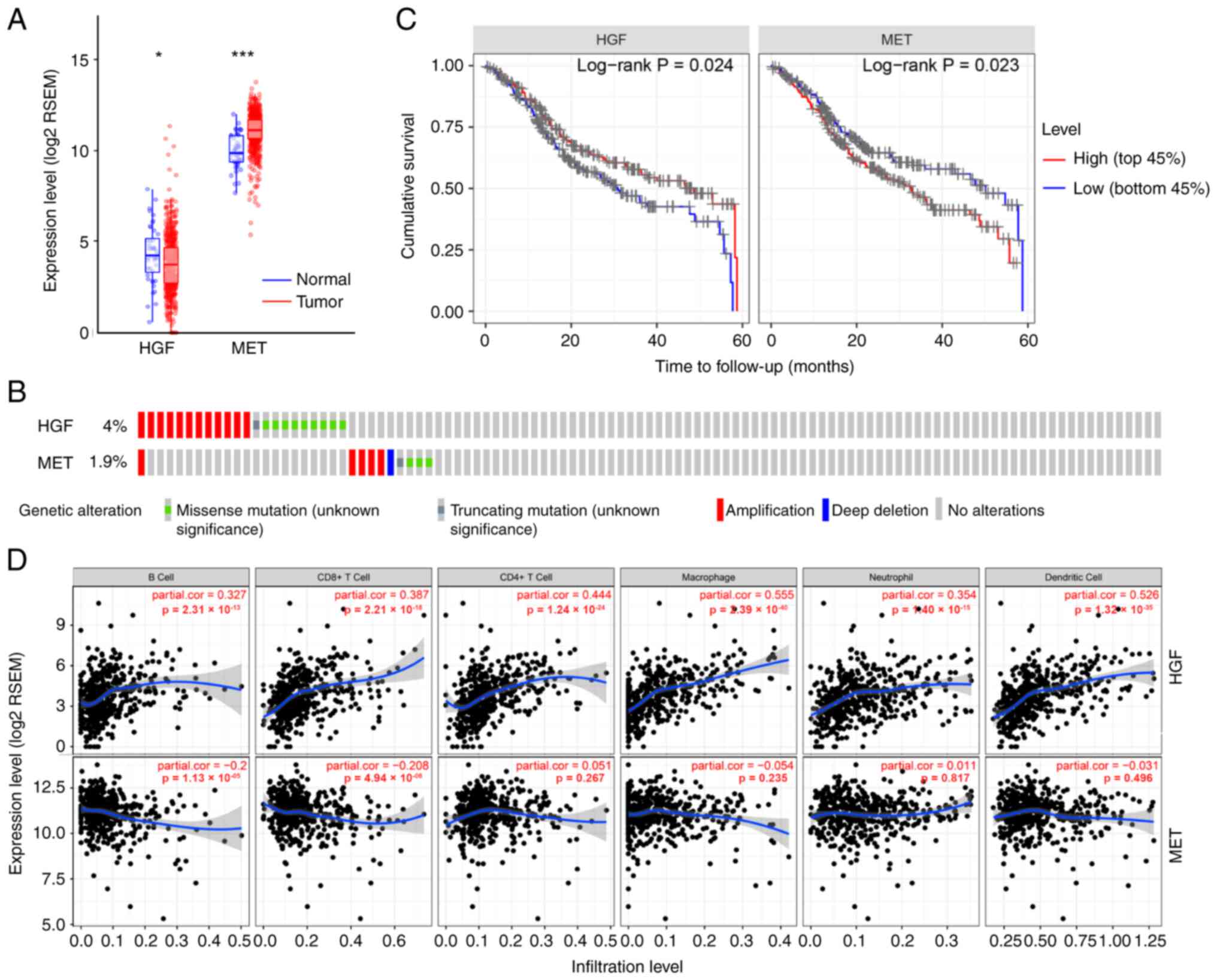
According to The Cancer Genome Atlas (TCGA)
database, Met expression levels are significantly higher in tumors
compared with normal tissues, whereas HGF expression is
downregulated in HNSCC tumor tissues compared with the normal
tissue (Fig. 1A) (34). HGF primarily functions as a
paracrine factor in HNSCC cells. In HNSCC, HGF is primarily
secreted by tumor-associated fibroblasts (TAFs) rather than the
tumor cells themselves. Furthermore, it has been demonstrated that
HGF is overexpressed in HNSCC-TAFs (11,35,36).
Elevated HGF levels have been detected in the supernatant of TAFs
cultured with laryngeal squamous cell carcinoma cells (37). Upregulated expression of Met mRNA
has been frequently reported in HNSCC (12–14).
However, inconsistent with the TCGA data suggesting that the
frequency of alterations in both HGF and MET in HNSCC is very low
(Fig. 1B) (34), the MET mutation frequency may have
been underestimated due to technical difficulties in the detection
of mutations resulting in exon 14 skipping, which are in turn
associated with amplification and overexpression of MET (38,39).
In fact, Met protein expression is often upregulated in HNSCC
tissues (40,41), and phosphorylated Met has been found
to be elevated in 30% of HNSCC samples. Specifically,
phosphorylation of Met at Y1003, Y1230, Y1234 and Y1235 was
observed in 66% of HNSCC samples (42–44).
In addition to exon 14 skipping mutations, other mutations have
been identified in HNSCC tumors, including mutations in the
tyrosine kinase (Y1253D, Y1230C, T1275I, V1333I, V1110I, Y1248C and
R1004G), SEMA (T230M, E168D and N375S) and juxtamembrane (T1010I
and R988C) domains (31,42,45).
Emerging evidence has suggested that the presence of the
constitutively active Y1253D Met mutant protein in primary tumors
is associated with a higher risk of local tumor progression,
recurrence and distant metastasis (46).
It has been shown that HGF/Met signaling promotes
tumor growth, invasion and angiogenesis in several malignancies,
including HNSCC (40,47,48).
HGF promoted the proliferation, migration, invasion and tube
formation of human lymphatic endothelial cells (35), and stimulated the growth and
invasion of HNSCC cells (49,50).
Furthermore, in HNSCC, HGF promoted the expression of the
angiogenic factors interleukin (IL)-8, platelet-derived growth
factor and vascular endothelial growth factor in tumor cells via
both the mitogen-activated protein kinase and PI3K-dependent
pathways (51,52). Additionally, HGF upregulated the
secretion of matrix metalloproteinase-1 (MMP-1) in HNSCC cells
(53). Combined inhibition of Met
and FGFR significantly inhibited TAF-induced HNSCC growth both
in vitro and in vivo (54). Furthermore, the Met/Akt pathway
promoted epithelial-mesenchymal transition, invasion and migration
of tongue squamous cell carcinoma cells (41). In oral cancer and preneoplastic cell
lines, Met was activated, whereas its upregulation in oral
leukoplakia was associated with a high hazard ratio for developing
oral cancer (55).
The association between HGF levels and the
clinicopathological parameters of HNSCC remains contested. Kim
et al (56) demonstrated
that the serum levels of HGF were significantly restored as HNSCC
progressed. In addition, Uchida et al (57) found that the concentration of HGF in
metastatic cancer tissues was significantly higher compared with
that in non-metastatic tissues. However, Hong et al
(58) suggested that in HNSCC there
was no significant association between HGF serum levels and several
clinicopathological parameters. Several studies have reported that
Met expression is associated with various clinicopathological
characteristics of patients with HNSCC, including tumor stage,
tumor size and lymph node metastasis (41,59–62).
TCGA data suggested that MET upregulation was a
predictor of decreased overall survival (OS, P=0.023; Fig. 1C) (34). Furthermore, the expression of HGF
was a positive prognostic factor for HNSCC patient survival
(P=0.024; Fig. 1C) (34), which could be attributed to the
positive correlation between HGF expression and the infiltration of
immune cells, including B cells and CD4+ and
CD8+ T cells. The association between HGF/Met and tumor
immunity is discussed further below. Numerous studies have
supported the notion that high expression of HGF and/or MET is
significantly correlated with a poor outcome (60,63–67);
however, other studies have not identified such a correlation
(58,68–70).
Some studies demonstrated that the expression of HGF was a negative
prognostic factor for the survival of HNSCC patients (63,64),
whereas one study failed to reveal a correlation between HGF
expression and prognosis in patients with HNSCC (58). In addition, another study
demonstrated that upregulated expression of HGF had a significant
effect on OS in human papillomavirus (HPV)-negative HNSCC patients,
whereas no such effects on OS or progression-free survival were
observed in HPV-positive HNSCC patients (71). The majority of studies have shown
that high Met expression is associated with a worse outcome
(60,65–67),
whereas other studies have not confirmed the role of Met as a
prognostic marker in patients with HNSCC (69,70).
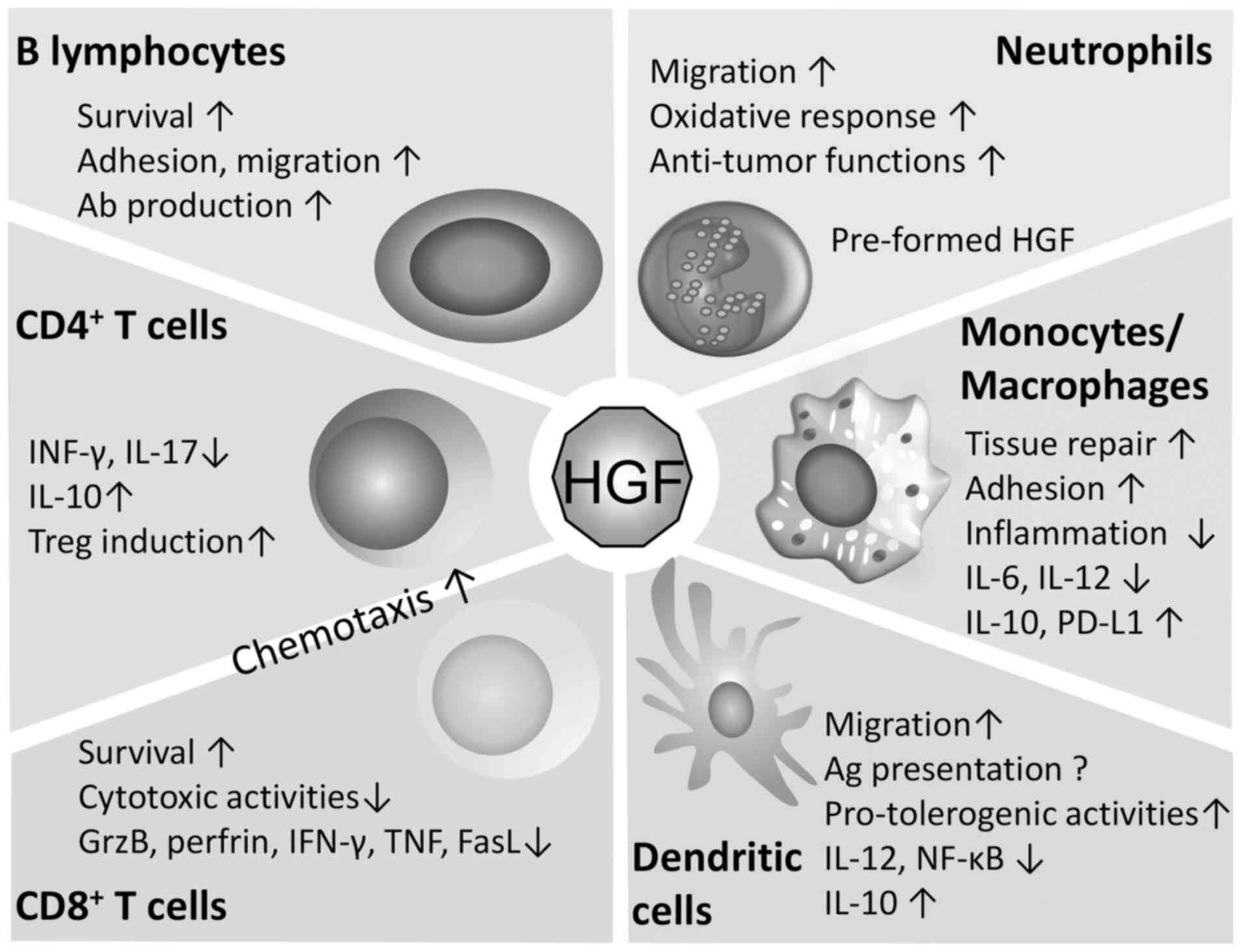
Neutrophils contain pro-HGF in secretory granules
that can be cleaved, activated and released; therefore, de
novo protein synthesis is not required upon cell stimulation
(86). It has been reported that
the treatment of chemotherapy-induced neutropenia using
granulocyte-colony-stimulating factor, results in a significant
increase in plasma HGF levels (87). HGF has been shown to promote the
adhesion and migration of neutrophils (88). The HGF/Met axis has both pro- and
anti-tumor effects in neutrophils. Neutrophil infiltration is
considered as a predictor of poor prognosis in patients with
bronchioloalveolar carcinoma (89).
In addition, tumor neutrophils actively enhanced hepatocellular
carcinoma cell metastasis both in vitro and in vivo
via interactions with HGF/c-Met (90). However, in a murine model,
conditional deletion of Met in neutrophils increased tumor growth
and metastasis, whereas Met was also shown to be required for the
recruitment of anti-tumor neutrophils (91).
In summary, it is hypothesized that the positive
correlation between the expression of HGF and immune cell
infiltration in HNSCC may be associated with the inhibition of
tumorigenesis and may support the notion that HGF overexpression
predicts increased OS. However, additional evidence is required to
support this hypothesis. Therefore, it is hypothesized that
targeting Met and particularly HGF for treatment of HNSCC may
compromise several functions of anti-tumor immune cells. Thus,
clinical trials using HGF or Met inhibitors for HNSCC therapy
should be cautiously evaluated.
Due to its crucial role in cancer cell
proliferation, invasion and metastasis, HGF/Met signaling is an
attractive target for the treatment of HNSCC. The present review
summarizes the current body of knowledge of the role of HGF/Met
signaling in HNSCC, including gene and protein alterations,
biological functions, patient outcomes and tumor-immune system
interactions. Given that patients with tumors exhibiting high-level
MET amplification or MET exon 14-skipping variants are more likely
to benefit from anti-Met therapies (29), the detection of MET alterations is
necessary for assessing whether to use Met inhibitors to treat
HNSCC. Several researchers have hypothesized that HGF is a
promising therapeutic target for HNSCC treatment. However, given
the impact of the HGF/Met signaling in the recruitment of immune
cells, the association between the expression of HGF and HNSCC
outcomes, and the early termination of a phase III study combining
an HGF antibody (rilotumumab) with chemotherapy due to the
increased number of deaths in the rilotumumab arm vs. that in the
placebo arm (106), it is
hypothesized that inhibition of HGF could attenuate several
anti-tumor immune responses. Therefore, intratumoral administration
of HGF inhibitors may not be a suitable approach for the treatment
of HNSCC. Ultimately, a clinical approach that may improve the
efficacy of Met therapy for HNSCC, namely, intratumoral
administration of Met inhibitors in order to reduce the inhibitory
effect on immune cell recruitment may instead improve patient
outcomes. However, further studies are required to provide an
improved understanding of the effects of the HGF/Met pathway on the
tumor microenvironment and to evaluate the therapeutic value of
targeting HGF/Met in HNSCC. In addition, given the important role
of HGF/Met signaling on immune cells, it is necessary to study the
effects of HGF and Met inhibitors on immune cells in the tumor
environment; however, to the best of our knowledge, no studies have
assessed this at present. Therefore, the effects of HGF and Met
inhibitors on immune cells in the tumor environment should be the
focus of future studies and clinical trials using HGF or Met
inhibitors for HNSCC therapy should be cautiously evaluated.
Not applicable.
This study was supported by the National Natural
Science Foundation of China (grant nos. 81902772 and 81800975),
China Postdoctoral Science Foundation (grant nos. 2018M641751,
2018M640269 and 2019T120224) and Liaoning Province Doctoral Science
Foundation (grant no. 20170520058).
Not applicable.
DL collected data and wrote the manuscript. MZ, DZ
and YZ collected data. SL drew figures and edited the manuscript.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hedberg ML, Goh G, Chiosea SI, Bauman JE,
Freilino ML, Zeng Y, Wang L, Diergaarde BB, Gooding WE, Lui VW, et
al: Genetic landscape of metastatic and recurrent head and neck
squamous cell carcinoma. J Clin Invest. 126:16062016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart B and Wild CP: World Cancer
Report. World Cancer Report. 45:12–351. 2014.
|
|
4
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guigay J, Tahara M, Licitra L, Keilholz U,
Friesland S, Witzler P and Mesía R: The evolving role of taxanes in
combination with cetuximab for the treatment of recurrent and/or
metastatic squamous cell carcinoma of the head and neck: Evidence,
advantages, and future directions. Front Oncol. 9:6682019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stabile LP, He G, Lui VW, Thomas S, Henry
C, Gubish CT, Joyce S, Quesnelle KM, Siegfried JM and Grandis JR:
c-Src activation mediates erlotinib resistance in head and neck
cancer by stimulating c-Met. Clin Cancer Res. 19:380–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brand TM, Iida M and Wheeler DL: Molecular
mechanisms of resistance to the EGFR monoclonal antibody cetuximab.
Cancer Biol Ther. 11:777–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leonard B, Brand TM, O'Keefe RA, Lee ED,
Zeng Y, Kemmer JD, Li H, Grandis JR and Bhola NE: BET inhibition
overcomes receptor tyrosine kinase-mediated cetuximab resistance in
HNSCC. Cancer Res. 78:4331–4343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madoz-Gurpide J, Zazo S, Chamizo C, Casado
V, Caramés C, Gavín E, Cristóbal I, García-Foncillas J and Rojo F:
Activation of MET pathway predicts poor outcome to cetuximab in
patients with recurrent or metastatic head and neck cancer. J
Transl Med. 13:2822015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho YA, Kim EK, Heo SJ, Cho BC, Kim HR,
Chung JM and Yoon SO: Alteration status and prognostic value of MET
in head and neck squamous cell carcinoma. J Cancer. 7:2197–2206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morello S, Olivero M, Aimetti M, Bernardi
M, Berrone S, Di Renzo MF and Giordano S: MET receptor is
overexpressed but not mutated in oral squamous cell carcinomas. J
Cell Physiol. 189:285–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sablin MP, Dubot C, Klijanienko J, Vacher
S, Ouafi L, Chemlali W, Caly M, Sastre-Garau X, Lappartient E,
Mariani O, et al: Identification of new candidate therapeutic
target genes in head and neck squamous cell carcinomas. Oncotarget.
7:47418–47430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liska D, Chen CT, Bachleitner-Hofmann T,
Christensen JG and Weiser MR: HGF rescues colorectal cancer cells
from EGFR inhibition via MET activation. Clin Cancer Res.
17:472–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scagliotti G, Moro-Sibilot D, Kollmeier J,
Favaretto A, Cho EK, Grosch H, Kimmich M, Girard N, Tsai CM, Hsia
TC, et al: A randomized-controlled phase 2 Study of the MET
antibody emibetuzumab in combination with erlotinib as first-line
treatment for EGFR-mutation positive NSCLC patients. J Thorac
Oncol. 15:80–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sim WJ, Iyengar PV, Lama D, Lui SKL, Ng
HC, Haviv-Shapira L, Domany E, Kappei D, Tan TZ, Saei A, et al:
c-Met activation leads to the establishment of a TGFβ-receptor
regulatory network in bladder cancer progression. Nat Commun.
10:43492019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giordano S, Di Renzo MF, Narsimhan RP,
Cooper CS, Rosa C and Comoglio PM: Biosynthesis of the protein
encoded by the c-met proto-oncogene. Oncogene. 4:1383–1388.
1989.PubMed/NCBI
|
|
19
|
Ferracini R, Longati P, Naldini L, Vigna E
and Comoglio PM: Identification of the major autophosphorylation
site of the Met/hepatocyte growth factor receptor tyrosine kinase.
J Biol Chem. 266:19558–19564. 1991.PubMed/NCBI
|
|
20
|
Zhen Z, Giordano S, Longati P, Medico E,
Campiglio M and Comoglio PM: Structural and functional domains
critical for constitutive activation of the HGF-receptor (Met).
Oncogene. 9:1691–1697. 1994.PubMed/NCBI
|
|
21
|
Ponzetto C, Bardelli A, Maina F, Longati
P, Panayotou G, Dhand R, Waterfield MD and Comoglio PM: A novel
recognition motif for phosphatidylinositol 3-kinase binding
mediates its association with the hepatocyte growth factor/scatter
factor receptor. Mol Cell Biol. 13:4600–4608. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weidner KM, Di Cesare S, Sachs M,
Brinkmann V, Behrens J and Birchmeier W: Interaction between Gab1
and the c-Met receptor tyrosine kinase is responsible for
epithelial morphogenesis. Nature. 384:173–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu H, Naujokas MA, Fixman ED, Torossian K
and Park M: Tyrosine 1356 in the carboxyl-terminal tail of the
HGF/SF receptor is essential for the transduction of signals for
cell motility and morphogenesis. J Biol Chem. 269:29943–29948.
1994.PubMed/NCBI
|
|
24
|
Hardy-Werbin M, Del Rey-Vergara R,
Galindo-Campos MA, Moliner L and Arriola E: MET inhibitors in small
cell lung cancer: From the bench to the bedside. Cancers (Basel).
11:14042019. View Article : Google Scholar
|
|
25
|
Czyz M: HGF/c-MET signaling in melanocytes
and melanoma. Int J Mol Sci. 19:38442018. View Article : Google Scholar
|
|
26
|
Huang X, Gan G, Wang X, Xu T and Xie W:
The HGF-MET axis coordinates liver cancer metabolism and autophagy
for chemotherapeutic resistance. Autophagy. 15:1258–1279. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan
C, Wu Y, Li X, Li X, Li G, et al: Function of the c-Met receptor
tyrosine kinase in carcinogenesis and associated therapeutic
opportunities. Mol Cancer. 17:452018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Feng Q, Chen WD and Wang YD:
HGF/c-MET: A promising therapeutic target in the digestive system
cancers. Int J Mol Sci. 19:32952018. View Article : Google Scholar
|
|
29
|
Comoglio PM, Trusolino L and Boccaccio C:
Known and novel roles of the MET oncogene in cancer: A coherent
approach to targeted therapy. Nat Rev Cancer. 18:341–358. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hartmann S, Bhola NE and Grandis JR:
HGF/Met signaling in head and neck cancer: Impact on the tumor
microenvironment. Clin Cancer Res. 22:4005–4013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szturz P, Raymond E, Abitbol C, Albert S,
de Gramont A and Faivre S: Understanding c-MET signalling in
squamous cell carcinoma of the head and neck. Crit Rev Oncol
Hematol. 111:39–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nandagopal L, Sonpavde GP and Agarwal N:
Investigational MET inhibitors to treat Renal cell carcinoma.
Expert Opin Investig Drugs. 28:851–860. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng F and Guo D: MET in glioma:
Signaling pathways and targeted therapies. J Exp Clin Cancer Res.
38:2702019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cancer Genome Atlas Network, .
Comprehensive genomic characterization of head and neck squamous
cell carcinomas. Nature. 51:576–582. 2015.
|
|
35
|
Gao P, Li C, Chang Z, Wang X and Xuan M:
Carcinoma associated fibroblasts derived from oral squamous cell
carcinoma promote lymphangiogenesis via c-Met/PI3K/AKT in vitro.
Oncol Lett. 15:331–337. 2018.PubMed/NCBI
|
|
36
|
Knowles LM, Stabile LP, Egloff AM,
Rothstein ME, Thomas SM, Gubish CT, Lerner EC, Seethala RR, Suzuki
S, Quesnelle KM, et al: HGF and c-Met participate in paracrine
tumorigenic pathways in head and neck squamous cell cancer. Clin
Cancer Res. 15:3740–3750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang M, Wu C, Guo Y, Cao X, Zheng W and
Fan GK: The primary growth of laryngeal squamous cell carcinoma
cells in vitro is effectively supported by paired cancer-associated
fibroblasts alone. Tumour Bio. 39:10104283177055122017.
|
|
38
|
Awad MM, Oxnard GR, Jackman DM, Savukoski
DO, Hall D, Shivdasani P, Heng JC, Dahlberg SE, Jänne PA, Verma S,
et al: MET exon 14 mutations in non-small-cell lung cancer are
associated with advanced age and stage-dependent MET genomic
amplification and c-Met overexpression. J Clin Oncol. 34:721–730.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rotow JK, Gui P, Wu W, Raymond VM, Lanman
RB, Kaye FJ, Peled N, Fece de la Cruz F, Nadres B, Corcoran RB, et
al: Co-occurring alterations in the RAS-MAPK pathway limit response
to MET inhibitor treatment in MET exon 14 skipping
mutation-positive lung cancer. Clin Cancer Res. 26:439–449. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vsiansky V, Gumulec J, Raudenska M and
Masarik M: Prognostic role of c-Met in head and neck squamous cell
cancer tissues: A meta-analysis. Sci Rep. 8:103702018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang H, Wen L, Wen M, Liu T, Zhao L, Wu B,
Yun Y, Liu W, Wang H, Wang Y and Wen N: FoxM1 promotes
epithelial-mesenchymal transition, invasion, and migration of
tongue squamous cell carcinoma cells through a c-Met/AKT-dependent
positive feedback loop. Anticancer Drugs. 29:216–226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seiwert TY, Jagadeeswaran R, Faoro L,
Faoro L, Janamanchi V, Nallasura V, El Dinali M, Yala S, Kanteti R,
Cohen EE, et al: The MET receptor tyrosine kinase is a potential
novel therapeutic target for head and neck squamous cell carcinoma.
Cancer Res. 69:3021–3031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen YS, Wang JT, Chang YF, Liu BY, Wang
YP, Sun A and Chiang CP: Expression of hepatocyte growth factor and
c-met protein is significantly associated with the progression of
oral squamous cell carcinoma in Taiwan. J Oral Pathol Med.
33:209–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim CH, Moon SK, Bae JH, Lee JH, Han JH,
Kim K and Choi EC: Expression of hepatocyte growth factor and c-Met
in hypopharyngeal squamous cell carcinoma. Acta Otolaryngol.
126:88–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chu LP, Franck D, Parachoniak CA, Gregg
JP, Moore MG, Farwell DG, Rao S, Heilmann AM, Erlich RL, Ross JS,
et al: MET genomic alterations in head and neck squamous cell
carcinoma (HNSCC): Rapid response to crizotinib in a patient with
HNSCC with a novel MET R1004G mutation. Oncologist. 24:1305–1308.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Renzo MF, Olivero M, Martone T, Maffe
A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G and
Comoglio PM: Somatic mutations of the MET oncogene are selected
during metastatic spread of human HNSC carcinomas. Oncogene.
19:1547–1555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Simiczyjew A, Pietraszek-Gremplewicz K,
Dratkiewicz E, Podgórska M, Matkowski R, Ziętek M and Nowak D:
Combination of selected MET and EGFR inhibitors decreases melanoma
cells' invasive abilities. Front Pharmacol. 10:11162019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu Y, Abudula M, Li C, Chen Z, Zhang Y and
Chen Y: Icotinib-resistant HCC827 cells produce exosomes with mRNA
MET oncogenes and mediate the migration and invasion of NSCLC.
Respi Res. 20:2172019. View Article : Google Scholar
|
|
49
|
Lee BS, Kang S, Kim KA, Song YJ, Cheong
KH, Cha HY and Kim CH: Met degradation by SAIT301, a Met monoclonal
antibody, reduces the invasion and migration of nasopharyngeal
cancer cells via inhibition of EGR-1 expression. Cell Death Dis.
5:e11592014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zeng Q, McCauley LK and Wang CY:
Hepatocyte growth factor inhibits anoikis by induction of activator
protein 1-dependent cyclooxygenase-2. Implication in head and neck
squamous cell carcinoma progression. J Biol Chem. 277:50137–50142.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC
and Van Waes C: Hepatocyte growth factor/scatter factor-induced
activation of MEK and PI3K signal pathways contributes to
expression of proangiogenic cytokines interleukin-8 and vascular
endothelial growth factor in head and neck squamous cell carcinoma.
Cancer Res. 61:5911–5918. 2001.PubMed/NCBI
|
|
52
|
Worden B, Yang XP, Lee TL, Bagain L, Yeh
NT, Cohen JG, Van Waes C and Chen Z: Hepatocyte growth
factor/scatter factor differentially regulates expression of
proangiogenic factors through Egr-1 in head and neck squamous cell
carcinoma. Cancer Res. 65:7071–7080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sundelin K, Roberg K, Grenman R and
Hakansson L: Effects of cytokines on matrix metalloproteinase
expression in oral squamous cell carcinoma in vitro. Acta
Otolaryngol. 125:765–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kumar D, New J, Vishwakarma V, Joshi R,
Enders J, Lin F, Dasari S, Gutierrez WR, Leef G, Ponnurangam S, et
al: Cancer-associated fibroblasts drive glycolysis in a targetable
signaling loop implicated in head and neck squamous cell carcinoma
progression. Cancer Res. 78:3769–3782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saintigny P, William WN Jr, Foy JP,
Papadimitrakopoulou V, Lang W, Zhang L, Fan YH, Feng L, Kim ES,
El-Naggar AK, et al: Met receptor tyrosine kinase and
chemoprevention of oral cancer. J Natl Cancer Inst. 110:250–257.
2018. View Article : Google Scholar
|
|
56
|
Kim CH, Lee JS, Kang SO, Bae JH, Hong SP
and Kahng H: Serum hepatocyte growth factor as a marker of tumor
activity in head and neck squamous cell carcinoma. Oral Oncol.
43:1021–1025. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Uchida D, Kawamata H, Omotehara F,
Nakashiro Ki, Kimura-Yanagawa T, Hino S, Begum NM, Hoque MO,
Yoshida H, Sato M and Fujimori T: Role of HGF/c-met system in
invasion and metastasis of oral squamous cell carcinoma cells in
vitro and its clinical significance. Int J Cancer. 93:489–496.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hong DY, Lee BJ, Lee JC, Choi JS, Wang SG
and Ro JH: Expression of VEGF, HGF, IL-6, IL-8, MMP-9, telomerase
in peripheral blood of patients with head and neck squamous cell
carcinoma. Clin Exp Otorhinolaryngol. 2:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sawatsubashi M, Sasatomi E, Mizokami H,
Tokunaga O and Shin T: Expression of c-Met in laryngeal carcinoma.
Virchows Archiv. 432:331–335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Arnold L, Enders J and Thomas SM:
Activated HGF-c-Met axis in head and neck cancer. Cancers (Basel).
9:1692017. View Article : Google Scholar
|
|
61
|
Yucel OT, Sungur A and Kaya S: c-met
overexpression in supraglottic laryngeal squamous cell carcinoma
and its relation to lymph node metastases. Otolaryngol Head Neck
Surg. 130:698–703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Galeazzi E, Olivero M, Gervasio FC, De
Stefani A, Valente G, Comoglio PM, Di Renzo MF and Cortesina G:
Detection of MET oncogene/hepatocyte growth factor receptor in
lymph node metastases from head and neck squamous cell carcinomas.
Eur Arch Otorhinolaryngol. 254 (Suppl 1):S138–S143. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Montag M, Dyckhoff G, Lohr J, Helmke BM,
Herrmann E, Plinkert PK and Herold-Mende C: Angiogenic growth
factors in tissue homogenates of HNSCC: Expression pattern,
prognostic relevance, and interrelationships. Cancer Sci.
100:1210–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Allen C, Duffy S, Teknos T, Islam M, Chen
Z, Albert PS, Wolf G and Van Waes C: Nuclear factor-kappaB-related
serum factors as longitudinal biomarkers of response and survival
in advanced oropharyngeal carcinoma. Clin Cancer Res. 13:3182–3190.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li W, Zheng H, Xu J, Cao S, Xu X and Xiao
P: Imaging c-Met expression using 18F-labeled binding peptide in
human cancer xenografts. PLoS One. 13:e01990242018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Szturz P, Budikova M, Vermorken JB, Horová
I, Gál B, Raymond E, de Gramont A and Faivre S: Prognostic value of
c-MET in head and neck cancer: A systematic review and
meta-analysis of aggregate data. Oral Oncol. 74:68–76. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fiedler M, Weber F, Hautmann MG, Haubner
F, Reichert TE, Klingelhöffer C, Schreml S, Meier JK, Hartmann A
and Ettl T: Biological predictors of radiosensitivity in head and
neck squamous cell carcinoma. Clin Oral Investig. 22:189–200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
da Costa AA, Costa FD, Araújo DV,
Camandaroba MP, de Jesus VH, Oliveira A, Alves AC, Stecca C,
Machado L, de Oliveira AC, et al: The roles of PTEN, cMET, and p16
in resistance to cetuximab in head and neck squamous cell
carcinoma. Med Oncol. 36:82018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Freudlsperger C, Alexander D, Reinert S
and Hoffmann J: Prognostic value of c-Met expression in oral
squamous cell carcinoma. Exp Ther Med. 1:69–72. 2010.PubMed/NCBI
|
|
70
|
Brusevold IJ, Soland TM, Khuu C,
Christoffersen T and Bryne M: Nuclear and cytoplasmic expression of
Met in oral squamous cell carcinoma and in an organotypic oral
cancer model. Eur J Oral Sci. 118:342–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kwon MJ, Kim DH, Park HR, Shin HS, Kwon
JH, Lee DJ, Kim JH, Cho SJ and Nam ES: Frequent hepatocyte growth
factor overexpression and low frequency of c-Met gene amplification
in human papillomavirus-negative tonsillar squamous cell carcinoma
and their prognostic significances. Hum Pathol. 45:1327–1338. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Galon J and Bruni D: Tumor immunology and
tumor evolution: Intertwined histories. Immunity. 52:55–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Garner H and de Visser KE: Immune
crosstalk in cancer progression and metastatic spread: A complex
conversation. Nat Rev Immunol. 20:483–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Molnarfi N, Benkhoucha M, Funakoshi H,
Nakamura T and Lalive PH: Hepatocyte growth factor: A regulator of
inflammation and autoimmunity. Autoimmun Rev. 14:293–303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ilangumaran S, Villalobos-Hernandez A,
Bobbala D and Ramanathan S: The hepatocyte growth factor (HGF)-MET
receptor tyrosine kinase signaling pathway: Diverse roles in
modulating immune cell functions. Cytokine. 82:125–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Delaney B, Koh WS, Yang KH, Strom SC and
Kaminski NE: Hepatocyte growth factor enhances B-cell activity.
Life Sci. 53:Pl89–P193. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
van der Voort R, Taher TE, Keehnen RM,
Smit L, Groenink M and Pals ST: Paracrine regulation of germinal
center B cell adhesion through the c-met-hepatocyte growth
factor/scatter factor pathway. J Exp Med. 185:2121–2131. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weimar IS, de Jong D, Muller EJ, Nakamura
T, van Gorp JM, de Gast GC and Gerritsen WR: Hepatocyte growth
factor/scatter factor promotes adhesion of lymphoma cells to
extracellular matrix molecules via alpha 4 beta 1 and alpha 5 beta
1 integrins. Blood. 89:990–1000. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu Z, Cai Y, Yang Y, Li A, Bi R, Wang L,
Shen X, Wang W, Jia Y, Yu B, et al: Activation of MET signaling by
HDAC6 offers a rationale for a novel ricolinostat and crizotinib
combinatorial therapeutic strategy in diffuse large B-cell
lymphoma. J Pathol. 246:141–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nagata T, Murata K, Murata R, Sun SL,
Saito Y, Yamaga S, Tanaka N, Tamai K, Moriya K, Kasai N, et al:
Hepatocyte growth factor regulated tyrosine kinase substrate in the
peripheral development and function of B-cells. Biochem Biophys Res
Commun. 443:351–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tjin EP, Bende RJ, Derksen PW, van
Huijstee AP, Kataoka H, Spaargaren M and Pals ST: Follicular
dendritic cells catalyze hepatocyte growth factor (HGF) activation
in the germinal center microenvironment by secreting the serine
protease HGF activator. J Immunol. 175:2807–2813. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hladikova K, Koucky V, Boucek J, Laco J,
Grega M, Hodek M, Zábrodský M, Vošmik M, Rozkošová K, Vošmiková H,
et al: Tumor-infiltrating B cells affect the progression of
oropharyngeal squamous cell carcinoma via cell-to-cell interactions
with CD8+ T cells. J Immunother Cancer. 7:2612019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tamura S, Sugawara T, Tokoro Y, Taniguchi
H, Fukao K, Nakauchi H and Takahama Y: Expression and function of
c-Met, a receptor for hepatocyte growth factor, during T-cell
development. Scand J Immunol. 47:296–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Grenier A, Chollet-Martin S, Crestani B,
Delarche C, El Benna J, Boutten A, Andrieu V, Durand G,
Gougerot-Pocidalo MA, Aubier M and Dehoux M: Presence of a
mobilizable intracellular pool of hepatocyte growth factor in human
polymorphonuclear neutrophils. Blood. 99:2997–3004. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH,
Ho LJ, Sytwu HK and Yen BL: Induction of immunomodulatory monocytes
by human mesenchymal stem cell-derived hepatocyte growth factor
through ERK1/2. J Leukoc Biol. 96:295–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mine S, Tanaka Y, Suematu M, Aso M,
Fujisaki T, Yamada S and Eto S: Hepatocyte growth factor is a
potent trigger of neutrophil adhesion through rapid activation of
lymphocyte function-associated antigen-1. Lab Invest. 78:1395–1404.
1998.PubMed/NCBI
|
|
89
|
Wislez M, Rabbe N, Marchal J, Milleron B,
Crestani B, Mayaud C, Antoine M, Soler P and Cadranel J: Hepatocyte
growth factor production by neutrophils infiltrating
bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor
progression and death. Cancer Res. 63:1405–1412. 2003.PubMed/NCBI
|
|
90
|
He M, Peng A, Huang XZ, Shi DC, Wang JC,
Zhao Q, Lin H, Kuang DM, Ke PF and Lao XM: Peritumoral stromal
neutrophils are essential for c-Met-elicited metastasis in human
hepatocellular carcinoma. Oncoimmunology. 5:e12198282016.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Finisguerra V, Di Conza G, Di Matteo M,
Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H,
Granot Z, et al: MET is required for the recruitment of
anti-tumoural neutrophils. Nature. 522:349–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dan H, Liu S, Liu J, Liu D, Yin F, Wei Z,
Wang J, Zhou Y, Jiang L, Ji N, et al: RACK1 promotes cancer
progression by increasing the M2/M1 macrophage ratio via the NF-κB
pathway in oral squamous cell carcinoma. Mol Oncol. 14:795–807.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Coudriet GM, He J, Trucco M, Mars WM and
Piganelli JD: Hepatocyte growth factor modulates interleukin-6
production in bone marrow derived macrophages: Implications for
inflammatory mediated diseases. PLoS One. 5:e153842010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Flaquer M, Franquesa M, Vidal A, Bolaños
N, Torras J, Lloberas N, Herrero-Fresneda I, Grinyó JM and Cruzado
JM: Hepatocyte growth factor gene therapy enhances infiltration of
macrophages and may induce kidney repair in db/db mice as a model
of diabetes. Diabetologia. 55:2059–2068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang WZ, Hu WH, Wang Y, Chen J, Hu ZQ,
Zhou J, Liu L, Qiu W, Tang FZ, Zhang SC, et al: A mathematical
modelling of initiation of dendritic cells-induced T cell immune
response. Int J Biol Sci. 15:1396–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Garibaldi S, Barisione C, Marengo B, Ameri
P, Brunelli C, Balbi M and Ghigliotti G: Advanced oxidation protein
products-modified albumin induces differentiation of RAW264.7
macrophages into dendritic-like cells which is modulated by cell
surface thiols. Toxins. 9:272017. View Article : Google Scholar
|
|
97
|
Baek JH, Birchmeier C, Zenke M and
Hieronymus T: The HGF receptor/Met tyrosine kinase is a key
regulator of dendritic cell migration in skin immunity. J Immunol.
189:1699–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kurz SM, Diebold SS, Hieronymus T, Gust
TC, Bartunek P, Sachs M, Birchmeier W and Zenke M: The impact of
c-met/scatter factor receptor on dendritic cell migration. Eur J
Immunol. 32:1832–1838. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Benkhoucha M, Santiago-Raber ML, Schneiter
G, Chofflon M, Funakoshi H, Nakamura T and Lalive PH: Hepatocyte
growth factor inhibits CNS autoimmunity by inducing tolerogenic
dendritic cells and CD25+Foxp3+ regulatory T
cells. Proc Natl Acad Sci USA. 107:6424–6429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Okunishi K, Dohi M, Nakagome K, Tanaka R,
Mizuno S, Matsumoto K, Miyazaki J, Nakamura T and Yamamoto K: A
novel role of hepatocyte growth factor as an immune regulator
through suppressing dendritic cell function. J Immunol.
175:4745–4753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Balan M, Mier y Teran E, Waaga-Gasser AM,
Gasser M, Choueiri TK, Freeman G and Pal S: Novel roles of c-Met in
the survival of renal cancer cells through the regulation of HO-1
and PD-L1 expression. J Biol Chem. 290:8110–8120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tong G, Cheng B, Li J, Wu X, Nong Q, He L,
Li X, Li L and Wang S: MACC1 regulates PDL1 expression and tumor
immunity through the c-Met/AKT/mTOR pathway in gastric cancer
cells. Cancer Med. 8:7044–7054. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Demuth C, Andersen MN, Jakobsen KR, Madsen
AT and Sorensen BS: Increased PD-L1 expression in
erlotinib-resistant NSCLC cells with MET gene amplification is
reversed upon MET-TKI treatment. Oncotarget. 8:68221–68229. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li H, Li CW, Li X, Ding Q, Guo L, Liu S,
Liu C, Lai CC, Hsu JM, Dong Q, et al: MET inhibitors promote liver
tumor evasion of the immune response by stabilizing PDL1.
Gastroenterology. 156:1849–1861.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lui VW, Hedberg ML, Li H, Vangara BS,
Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al:
Frequent mutation of the PI3K pathway in head and neck cancer
defines predictive biomarkers. Cancer Discov. 3:761–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Catenacci DV, Tebbutt NC, Davidenko I,
Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E,
Karaszewska B, Bondarenko I, et al: Rilotumumab plus epirubicin,
cisplatin, and capecitabine as first-line therapy in advanced
MET-positive gastric or gastro-oesophageal junction cancer
(RILOMET-1): A randomised, double-blind, placebo-controlled, phase
3 trial. The Lancet. Oncology. 18:1467–1482. 2017.PubMed/NCBI
|