Introduction
Osteosarcoma (OS) is the most common primary
malignant tumor, with high incidence rates in children and
adolescents for nearly 20 years, in the world (1). Cisplatin, doxorubicin (Dox) and
methotrexate (MTX) are commonly used anticancer drugs for OS in a
clinical setting (2), and patients
who do not respond to these drugs have a poor prognosis (3). Lung metastasis is present in 20% of
patients with OS at time of diagnosis, and current active
treatments, including chemo- and radio-therapy, do not guarantee
long-term survival, for example >5 years (4,5),
suggesting that chemoresistance and metastasis are still the
primary challenges in the treatment of OS. However, the mechanisms
leading to chemoresistance and metastasis development in OS remain
unclear.
Cancer stem cells (CSCs) are a small subset of cells
located within a tumor, and studies show that CSCs are implicated
in chemotherapy resistance and metastasis in cancer such as
non-small cell lung cancer and breast cancer (6–8). CSCs
present high resistance to chemotherapy drugs that are commonly
used in the treatment of OS, including cisplatin and doxorubicin
(5,7). In addition, studies have revealed that
CSCs are able to regenerate all of the cell types (HuO9, HuO9-M132,
Saos-2, Saos-2-LM5 and MCF7, SKBR3, MDA-MB-231, MDA-MB-435) in the
tumor due to their stem cell-like behavior, resulting in metastatic
relapse (5,8). Therefore, CSCs are important
therapeutic targets in cancer. However, the mechanism of CSC
regulation in OS remains unknown.
MicroRNAs (miRNA/miR) are a class of non-coding RNAs
that are 18–22 nucleotides in length and negatively regulate gene
expression through the interaction with the 3′ untranslated region
(UTR) of the target gene mRNA (9).
Dysregulated expression of miRNAs (miR-125b, miR-199a, let-7 g,
miR-433, miR-214 and miR-100) has been detected in most types of
cancer, and dysregulation of even a single miRNA can lead to
tumorigenesis and stimulate cancer progression (10,11).
In addition, dysregulated expression of miRNAs (let-7, miR-135 and
miR-200) was demonstrated in CSCs, and such aberrantly regulated
miRNAs are involved in the development of CSCs and the maintenance
of stemness (6,12). Decreased expression levels of
miR-382, miR-369, miR-134, miR-127-3p, located on chromosome 14q32,
have been identified in OS (13),
and previous studies have revealed that the expression level of
these miRNAs is associated with the progression of OS, including
rapid growth of the tumor, recrudescence and metastasis (13,14).
miR-487b-3p is one of the aforementioned miRNAs and is associated
with progression of OS; however, its function and mechanism in OS
remains unknown. In the present study the functional role of
miR-487b-3p was determined and the miRNA was found to be a tumor
inhibitor, regulating invasion, chemoresistance and the development
of CSCs by targeting ALDH1A3 in OS. ALDH1A3 plays an important role
in cancer stemness maintenance and has been associated with the
development, progression and prognosis of different cancer types
(15–17). In addition, ALDH1A3 is involved with
a diverse range of biological characteristics within cancer stem
cells and can act as a marker for these cells (17). ALDH1A3 could therefore provide novel
targets for the treatment of osteosarcoma.
Materials and methods
Cell culture and human specimens
All osteosarcoma cells (U2OS, Saos2, MG63, HOS) and
the 293T cell line were purchased from the American Type Culture
Collection and cultured in RPMI 1640 media (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare). Human specimens were collected during diagnostic
biopsies (paraffin embedded tissue) at First Affiliated Hospital of
Nanchang University (Jiangxi, China) between May 2017 and September
2018. A total of 40 diagnostic patient specimens and 24 healthy
bone specimens were used in the present study (Table I), which were also obtained from
First Affiliated Hospital of Nanchang University (Jiangxi, China).
All the OS cases were diagnosed by pathological evaluation using
the National Comprehensive Cancer Network Guidelines version 2.2017
(18) and had complete clinical
information. The TNM stage was graded according to the eighth
edition of American Joint Committee on Cancer TNM staging (19). Written informed consent was provided
by all the participants. Ethics approval was granted by the
Research Ethics Board of the First Affiliated Hospital of Nanchang
University (approval no. 2019042).
 | Table I.Characteristics of healthy control
and patients with OS. |
Table I.
Characteristics of healthy control
and patients with OS.
| Variable | Patients | Healthy
controls | P-value |
|---|
| Sex, n |
|
|
|
|
Male | 26 | 13 | 0.365 |
|
Female | 14 | 11 |
|
| Mean age ± SD,
years (range) | 40.2±4.5
(22–67) | 42.3±3.8
(21–62) |
|
| Age, years, n |
|
|
|
|
≤35 | 22 | 10 | 0.314 |
|
>35 | 18 | 14 |
|
| TNM stages, n |
|
|
|
|
I–II | 24 | – |
|
|
III–IV | 16 | – |
|
| Histology,
na |
|
|
|
|
Poor | 9 | – |
|
|
Moderate | 17 | – |
|
|
Well | 14 | – |
|
| Total | 40 | 24 |
|
Transfection
Cell transfection was performed using
Lipofectamine® 3000 transfection reagent according to
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.) and the protein or total RNA was extracted after 72 h.
miR-487b-3p mimics (5′-AAUCGUACAGGGUCAUCCACUU-3′), inhibitors
(5′-AAGUGGAUGACCCUGUACGAUU-3′) and negative control (NC)
oligonucleotides (5′-UUCUCCGACGUGUCACGUTT-3′), the aldehyde
dehydrogenase 1 family member A3 (ALDH1A3) expression vector
(pcDNA3.1 plasmid), and the ALDH1A3 short inhibiting (si)RNA
(5′-GCAGAGAACUAGGUGAAUAdTdT-3′) and control siRNA
(5′-GTATCGAAGAAGTGATAAA-3′) were purchased from Guangzhou RiboBio
Co., Ltd.. The concentration of miRNA and siRNA used was 50
pM/96-well, and for the plasmid was 5 µg/6-well.
For transfection of Saos2 cells, they were seeded
into 60 mm dishes and incubated overnight at 37°C in humidified
incubator with 5% CO2. Following which, 10 µl lentivirus
with a titer of 1×108 TU was added and the cells were
incubated at 37°C in a humidified incubator with 5% CO2
for 72 h. Lastly, puromycin, at a final concentration of 2 µg/ml
was added and the cells were cultured for 48 h to select the
transfected cells.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells or tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc).
miR-487b-3p and RNU6 expression was detected using RT-qPCR using
SYBR-Green and the following primers purchased from Guangzhou
RiboBio Co., Ltd: miR-487b-3p forward, 5′-AATCGTACAGGGTCATC-3 and
reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The sequences of the primers for
ALDH1A3 and GAPDH are as follows: ALDH1A3 forward,
5′-TGAGTGATTTAGCAGGCTGCA-3′, and reverse
5′-TGGCCACATACACCAATAGGTTC-3′; and GAPDH forward
5′-GCAGGGGGGAGCCAAAAGGGT-3′ and reverse
5′-TGGGTGGCAGTGATGGCATGG-3′. miRNA was reverse transcribed using
the polyA tailing method (All-in-One™ miRNA RT-qPCR detection kit),
while mRNA was reverse transcribed using the All-in-One™
first-strand cDNA synthesis kit, (both from GeneCopoeia, Inc.). D
The following temperature protocol was used: Incubation at 37°C for
60 min, and 72°C for 5 min, then the samples were store at 4°C. The
following thermocycling conditions were used: Initial denaturation
at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec. 58°C
for 20 sec and 72°C for15 sec. The relative expression levels of
miR487b-3pa and ALDH1A3 were normalized against RNU6 and GAPDH,
respectively. The 2−ΔΔCq method was used to analyze the
relative fold-changes (20).
Apoptosis analysis
Saos-2 cells were seeded in 6-well plates, at a
density of 2×105, after 6 h of transfection. Cells were
incubated with or without Dox (0.2 uM). The cells were harvested
and stained with annexin V and 7-aminoactinomycin D (7-AAD) at room
temperature for 30 min, according to the manufacturer's protocol in
(Biotium Inc.) 24 h later. The cells were subsequently analyzed
using flow cytometry (CytoFLEX) and analyzed using CytoFLEX v2.0
software (both Beckman Coulter, Inc).
MTT assay
The MTT assay was performed according to the
manufacturer's protocol using MTT assay kit (Promega Corporation).
SAOS2 cells were transfected with miR-487b-3p mimics, inhibitors
and NC oligonucleotides using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's instructions. After 6 h of transfection, cells were
reseeded in a 96-well plate at a density 10,000/well. After 12 h of
incubation, cells were treated with 0.2 µM doxorubicin for 24 h,
after which the medium was removed and 100 µl DMSO was added. The
absorbance was measured at 570 nm.
Transwell and osteosphere assays
The Transwell and osteosphere assays were performed
as described previously by Xu et al (5). Briefly, 1×105 transfected
cells in serum-free growth DMEM were seeded in the upper wells of
the chamber (12-well plate). The lower wells contained the same
medium with 10% serum. After 24 h, the cells that had migrated to
the lower side of the chamber were fixed with 2.5% glutaraldehyde
for 15 min, stained with 0.1% crystal violet for 30 min at room
temperature and counted using ImageJ software (v1.8.0; National
Institutes of Health), from 3 fields of view. For the osteosphere
assay, 1,000 cells were plated in 24-well ultra-low attachment
plates in N2B27-defined serum free medium and cultured for 9 days
at 37°C in a humidified incubator with 5% CO2. Spheres
were counted in each plate using a Leica MZ12 inverted light
microscope, at ×4 magnification, and using 3 fields of view.
Luciferase reporter assay
To identify target genes of miR-487b-3p, the online
database miRDB (www.mirdb.org) was used. A total of
3′UTRs from ALDH1A3 were predicted to interact with miR-487b-3p and
these were amplified using PCR from human genomic DNA (purchased
from Shanghai GenChem Co., Ltd.) and ligated into the Luciferase
reporter vector (pGL4; Hanheng Biotechnology Co., Ltd) using the
HindIII and SacI restriction sites. The primer
sequences for amplifying the 3′UTR of ALDH1A3 are as follows:
forward,
5′-AAAGATCCTTTATTAAGCTTTAATAAAATGAGGGCCCGTAACAGAACCAGTG-3′; and
reverse, 5′-GCGCACTAGTGAGGGAGCTCTTGTGGGATGCGATCTGCAGCTAGGA-3′.
For the luciferase reporter experiments, the 293T
cells were seeded into 24-well cell culture plates and transfected
with the aforementioned luciferase reporter plasmids and either the
miR-487b-3p or NC oligonucleotides, using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
subjected to a luciferase reporter assay, 48 h following
incubation. Luciferase activity was measured using the
dual-luciferase assay system (Promega Corporation) according to the
manufacturer's protocol. The luminous intensity of firefly
fluorescein was normalized with the luminous intensity of
Renilla fluorescein as a reference, then the data was
analyzed. Renilla luciferase was used as an internal
reference. The relative value was used as the control.
Western blot analysis
The cells were lysed using RIPA (Sigma-Aldrich;
Merck KGaA), and subsequently quantified using bicinchoninic acid
(Beijing Dingguo Changsheng Biotechnology Co. Ltd). A total of 25
µg of protein was separated using 10% SDS-PAGE, and the proteins
were transferred to PVDF membrane, which were subsequently blocked
with 5% skimmed milk for 1 h at room temperature. The primary
antibodies E-cadherin (cat. no. 17952-1-AP; 1:1,000, ProteinTech
Group, Inc.), vimentin (cat. no. 5471; 1:2,000; Cell Signaling
Technology, Inc.), cleaved-PARP (cat. no. 5625; 1:1,500; Cell
Signaling Technology, Inc.), PARP (cat. no. ab74290; 1:3,000;
Abcam), ALDH1A3 (cat. no. ab129815; 1:800; Abcam), SOX-2 (cat. no.
11064-1-AP; 1:2,000; ProteinTech Group, Inc.), OCT-4 (cat. no.
11263-1-AP; 1:2,000; ProteinTech Group, Inc.), polycomb complex
protein BMI-1 (BMI-1; cat. no. ab38295; 1:2,000 Abcam), β-actin
(cat. no. 3700; 1:5,000; Cell Signaling Technology, Inc.), were
used and incubated overnight at 4°C. Following which, the membranes
were incubated with secondary antibodies (goat anti-rabbit; cat.
no, A16104; 1:10,000; goat anti-mouse; cat. no, 31430; 1:10,000)
(both Thermo Fisher Scientific, Inc.) conjugated to horseradish
peroxidase for 2 h at room temperature. Then the proteins were
visualized using an ECL kit (Bio-Rad Laboratories, Inc.) and
quantified using Quantity One software (v4.6.2; Bio-Rad
Laboratories, Inc.)
CD133 detection
The transplanted tumor tissue was washed with Hanks'
buffer then cut into 3 mm3 sections. A total of 3 ml
serum-free RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) was
subsequently added followed by 3 ml collagenase (0.1%) and the
tissue sections were incubated for 3 h at 37°C for digestion.
Following which, the tissue fragments were passed through a 70-µm
cell sieve to produce a cell suspension, for cell testing. The
cells were resuspended with RPMI 1640 medium and centrifuged for 10
min at 200 × g at 4°C and washed once with PBS. The four groups of
cells obtained were adjusted to a concentration of 1×106
per 100 µl and transferred to Eppendorf® tubes 5 ml,
following which, 1 µl fragment crystallizable block antibody (BD
Biosciences) was added and the tubes were incubated for 30 min at
4°C then washed once with PBS. A total of 5 µg CD133 antibody (cat.
no. ab252126; 1:2,000; Abcam) was added to each tube and incubated
for 30 min in the dark. Subsequently, 2 ml cell staining buffer was
added, and the samples were centrifuged at 350 × g for 5 min at
4°C, twice. The cells were resuspended in 0.4 ml cell staining
buffer, following which 2 µl nuclear dye 7-AAD (BD Biosciences) was
added, and the cells were incubated on ice for 3–5 min, then
analyzed using flow cytometry.
Animal experiments
ALDH1high cells were sorted from Saos-2
cells using FACS and the ALDEFLUOR kit (Stemcell Technologies,
Inc.) as aforementioned. All animal experiments were performed
using 6-week-old female athymic (nu/nu) mice (17±1.5 g) and housed
under specific pathogen-free conditions, with 12 h light/dark
cycles, 24°C, 70% humidity and ad libitum access to food and
water, and the veterinarian monitored the health and behavior of
the animals every morning. For tumor formation experiments,
1×104 ALDH1high Saos-2 cells that were
previously transfected with miR-487b-3p mimics or NC in 100 µl
serum-free DMEM were subcutaneously (s.c.) injected into the back
of the mouse, on the right side. After 2 months the mice were
sacrificed using cervical dislocation. For chemotherapy
experiments, 1×104 ALDH1high Saos-2 cells in
100 µl serum-free medium were s.c. injected into the back of the
mouse, on the right side. When the tumors reached ~50
mm3 (using Vernier caliper and measured every other day;
volume=long diameter × short diameter2), mice were
intraperitoneally injected with Dox (6 mg/kg body weight), 10 µM
miR-487b-3p mimic or both, for three cycles (days 29, 32 and 36)
and each group included nine mice (total 36 mice). Euthanasia was
performed in mice when the tumor size reached 1,500 mm3.
All animal experiments were approved by the Laboratory Animal
Welfare and Ethics Committee of the Nanchang University (approval
no. AMUWEC2018410).
Statistical analysis
Kaplan-Meier survival analysis was used to analyze
the overall survival rate in patients with OS. Significant
differences between treatment groups were analyzed using Student's
t-test or ANOVA, followed by Tukey's post hoc test. All data were
analyzed using the SAS statistical software package version 6.12
(SAS Institute, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
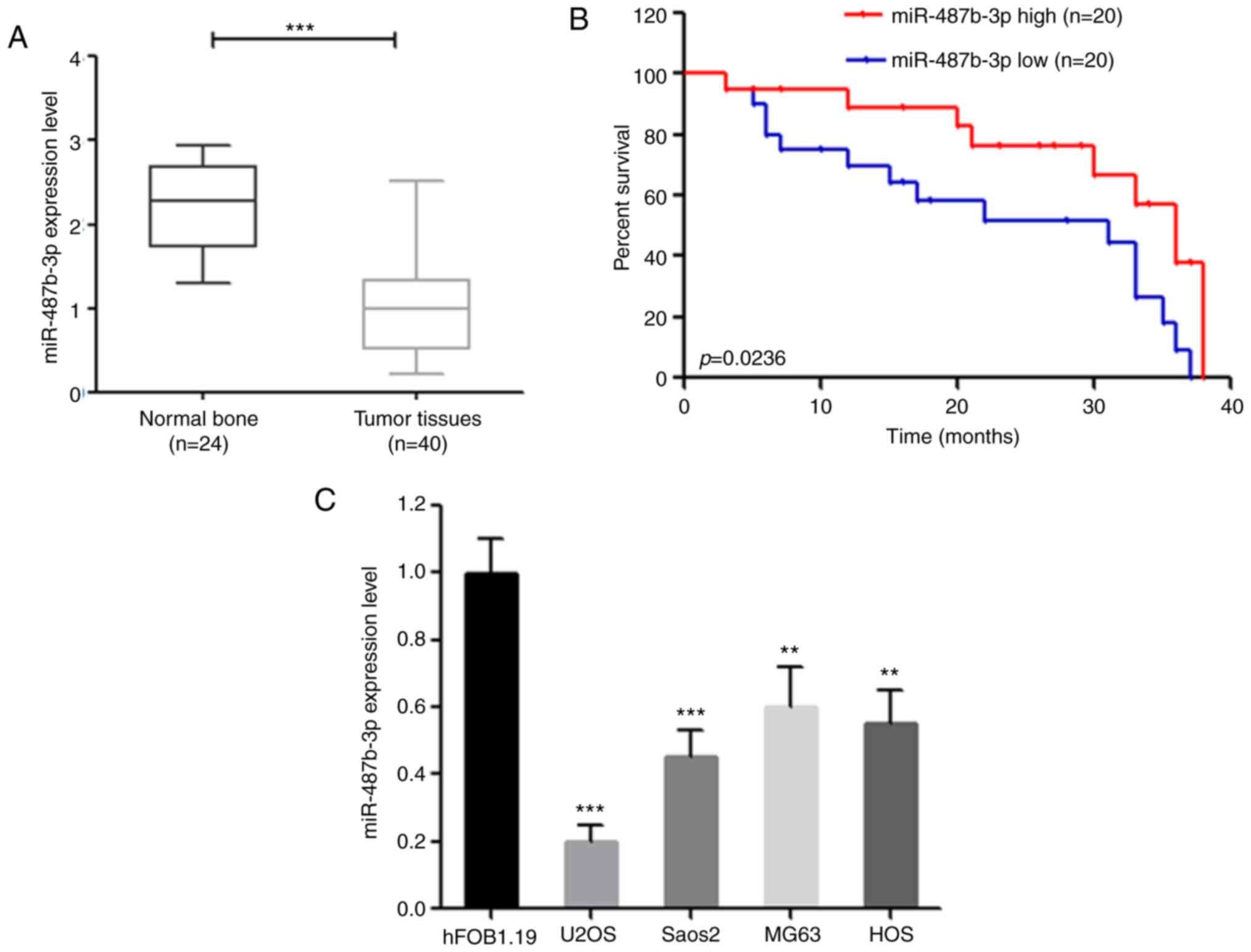
Decreased expression of miR-487b-3p is
associated with poor clinical outcome
Previous studies have shown that ~9 miRNAs at the
14q32 locus are inversely correlated with OS progression
(metastasis and relapse) (13,14),
which suggests that miR-487b-3p may also be associated with
progression of OS. As expected, miR-487b-3p expression was
significantly decreased in OS specimens compared with that in
normal bone tissues (Fig. 1A). In
addition, the clinical data also found that decreased expression of
miR-487b-3p was significantly associated with poor overall survival
in patients with OS (P=0.0236) (Fig.
1B). Consistent with these results, miR-487b-3p expression was
significantly decreased in OS cell lines compared with that in the
human fetal osteoblastic hFOB1.19 cell line (Fig. 1C). Taken together, these data
suggest that miR-487b-3p may play a role in OS, as a tumor
suppressor.
miR-487b-3p inhibits invasion and
chemoresistance in OS
The occurrence of metastasis and chemoresistance
indicates a poor clinical outcome in patients with OS (3,5). Thus,
the effects of miR-487b-3p on OS metastasis and chemoresistance
using OS cell lines transfected with miR-487b-3p mimic or inhibitor
was investigated (Fig. S1 and
Fig. 2). Transwell experiments
demonstrated that the overexpression of miR-487b-3p significantly
suppressed cell migration while the inhibition of miR-487b-3p
stimulated cell invasion in both Saos-2 (Fig. 2B) and MG63 cells (Fig. S1B). In addition, the effects of
miR-487b-3p on OS cell epithelial-mesenchymal transition (EMT) was
also investigated, as EMT plays an important role in cancer
metastasis. miR-487b-3p mimic was found to significantly increase
E-cadherin expression in Saos-2 and MG-63 cells but significantly
decreased vimentin expression (Fig.
2C and Fig. S1C,
respectively), suggesting that miR-487-3p inhibits EMT in OS cells.
Subsequently, the effects of miR-487b-3p on the chemotherapy
sensitivity of OS cells was investigated. The results revealed that
the increased level of miR-487b-3p significantly enhanced the
sensitivity of Saos-2 and MG63 cells to Dox treatment compared with
that the control cells, while the inhibition of miR-487b-3p
inhibited Dox-induced cell viability inhibition (Fig. 2D and Fig. S1D). In addition, the results from
the apoptosis analysis revealed that increased expression level of
miR-487b-3p increased the rate of apoptosis in OS cells and the
combination of miR-487b-3p and Dox significantly increased the rate
of apoptosis compared with that in a single treatment (Fig. 2E and Fig. S1E). In contrast, the inhibition of
miR-487b-3p significantly attenuated Dox-induced apoptosis in OS
cells (Fig. 2E and Fig. S1E). Consistent with apoptosis
analysis, combined treatment of miR-487b-3p and Dox significantly
increased the protein expression level of the pro-apoptotic cleaved
PARP protein compared with that in the Dox and mimic single
treatment groups, while the miR-487b-3p inhibitor and Dox treatment
groups significantly decreased the protein level of cleaved PARP
compared with that in the Dox only treatment group in both Saos-2
and MG63 cells (Fig. 2F and
Fig. S1F). Taken together, the
results from the present study suggest that miR-487b-3p inhibits OS
progression by inhibiting OS cell migration and inducing
chemosensitivity.
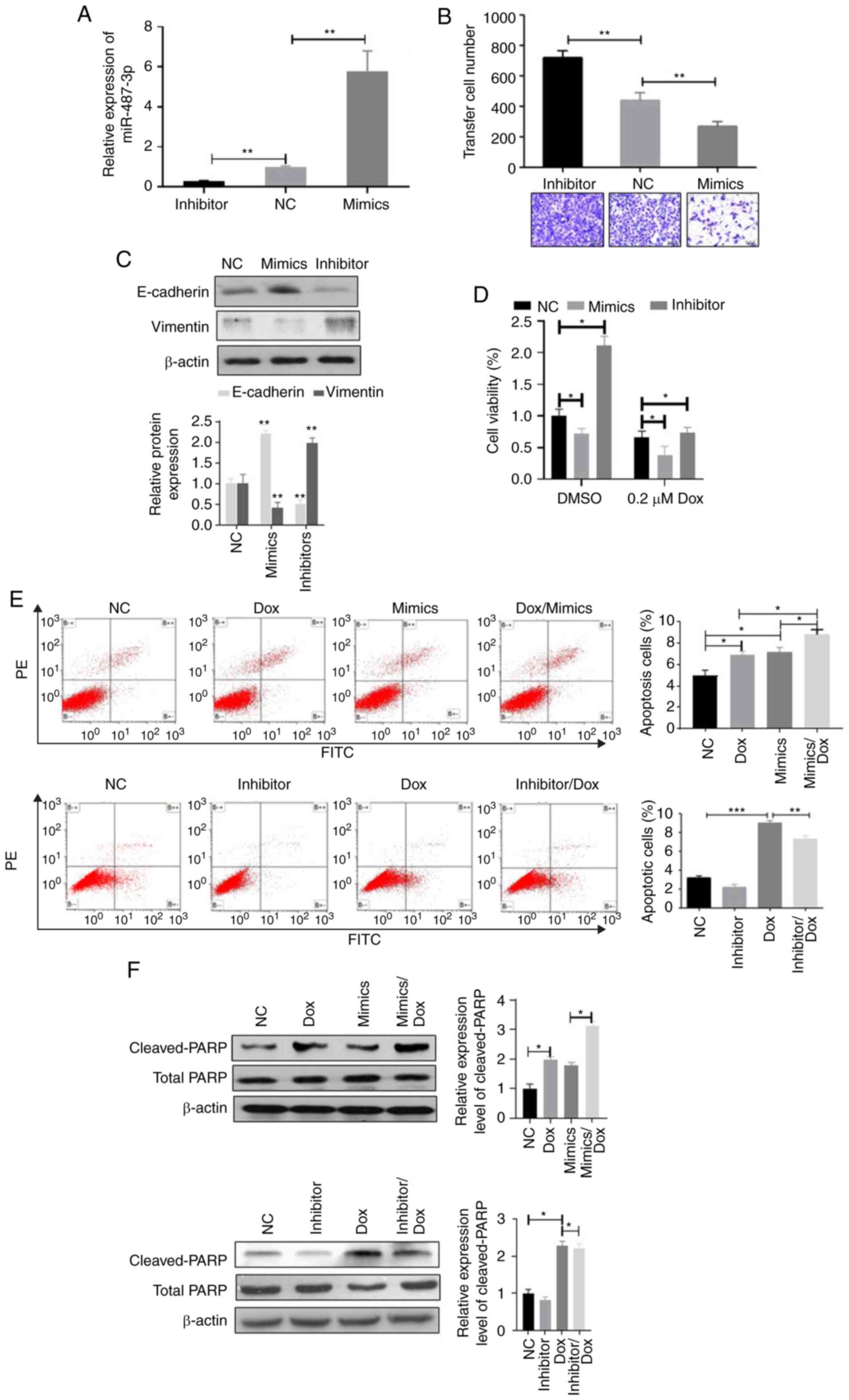 | Figure 2.miR-487b-3p negatively regulates
osteosarcoma cell migration and chemoresistance. (A) Saos-2 cells
were transfected with NC oligonucleotides, miR-487b-3p mimics or
miR-487b-3p inhibitor and extracted mRNA were analyzed using
reverse transcription-quantitative-PCR, 72 h following
transfection. miR-487b-3p mimics and inhibitors significantly
increased and decreased the mRNA expression level of miR-487b-3p in
Saos-2 cells, respectively. miR-487b-3p negatively regulates Saos-2
(B) cell migration and (C) inhibits epithelial-mesenchymal
transition following transfection with NC, miR-487-3p mimics or
inhibitor. (D) miR-487b-3p enhanced the sensitivity of Saos-2 cells
to Dox treatment, following transfection with the aforementioned
oligonucleotides, treated with or without 0.2 µM Dox for 48 h. Cell
viability was measured using MTT assay. (E) The overexpression of
miR-487b-3p increased Dox-induced apoptosis in Saos-2 cells, while
the inhibition of miR-487b-3p inhibited Dox-induced apoptosis in
Saos-2 cells, following transfection with the aforementioned
oligonucleotides and subsequently treated with DMSO or 0.2 µM Dox
for 24 h. (F) miR-487b-3p stimulates Dox-induced expression of
cleaved- and total-PARP in Saos-2 cells, following transfection
with the aforementioned oligonucleotides, and treated with 0.2 µM
Dox for 48 h. *P<0.05; **P<0.01; ***P<0.001. NC, negative
control; miR, microRNA; mimics, miR-487b-3p mimics; inhibitor,
miR-487b-3p inhibitor; Dox, doxorubicin. |
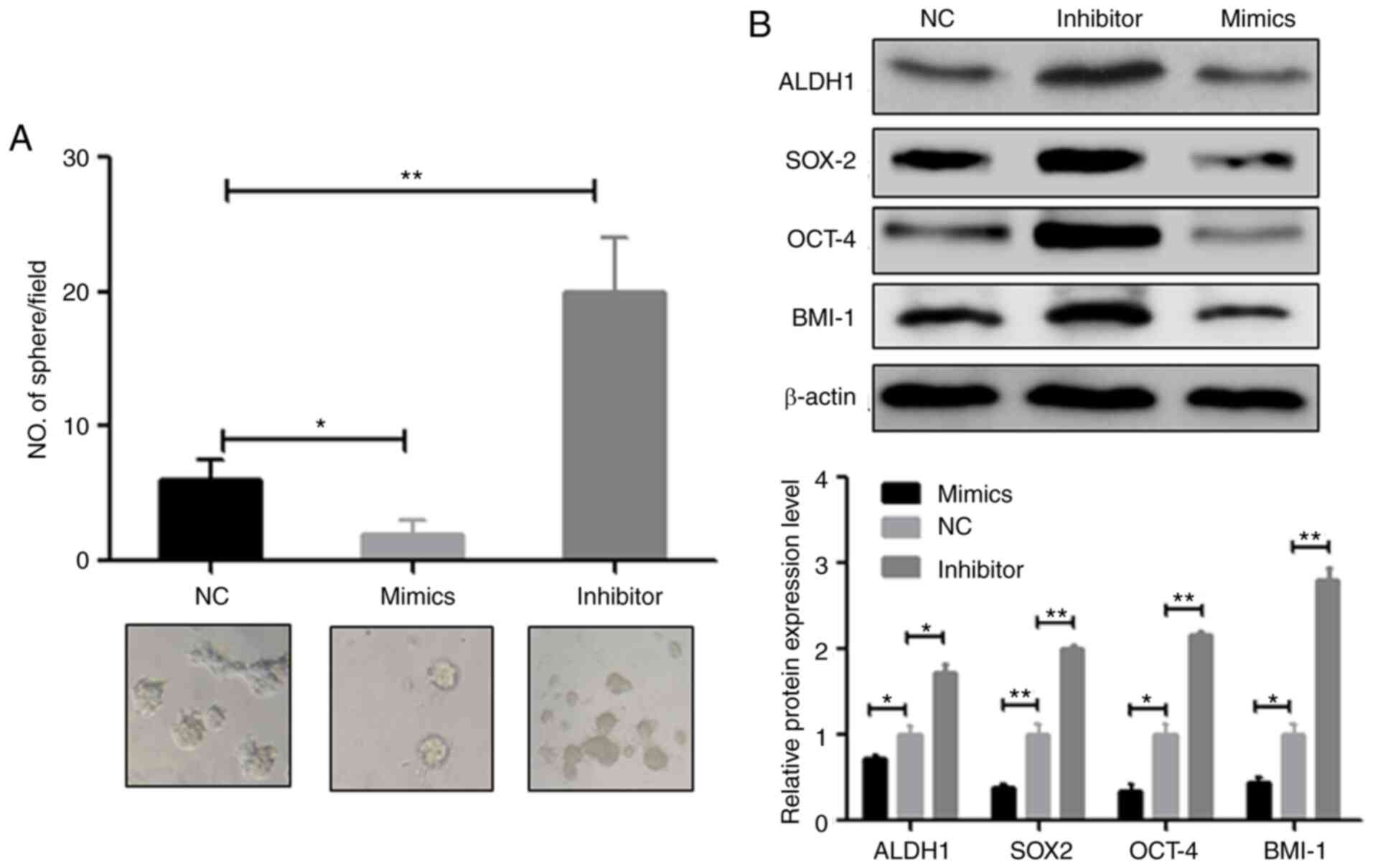
miR-487b-3p inhibits CSCs in OS
Previous studies have suggested that CSCs lead to
the development of metastasis and chemoresistance in multiple
cancers, such as breast cancer and NSCLC (7,10),
thus, it was investigated whether miR-487b-3p is involved in CSC
regulation of OS. As expected, the osteosphere assay revealed that
the inhibition of miR-487b-3p significantly increased, while the
overexpression of miR-487b-3p reduced osteosphere numbers in Saos-2
(Fig. 3A) and MG63 cells (Fig. S2A). Consistent with sphere
formation, the western blot results also showed that the
overexpression of miR-487b-3p significantly inhibited the
expression level of the CSC marker proteins, including ALDH1, SOX2,
OCT4 and BMI-1 (Figs. 3B and
S2B). In contrast, the inhibition
of miR-487b-3p significantly increased protein expression levels of
the aforementioned CSCs marker (Figs.
3B and S2B). These data
suggested that miR-487b-3p exerts its anticancer effects partially
due to the inhibition of CSCs in OS.
miR-487b-3p can target ALDH1A3
To investigate how miR-487b-3p regulates CSCs in OS,
target gene candidates of miR-487b-3p were investigated and
multiple targets were identified, including ALDH1A3 (Fig. 4A). Thus, ALDH1A3 was chosen for
further investigation. To investigate whether miR-487b-3p is
involved in the regulation of ALDH1A3, ALDH1A3 protein and mRNA
expression levels were determined after OS cells were transfected
with miR-487b-3p mimic or inhibitor. The results showed that
ALDH1A3 expression was significantly upregulated by the inhibition
and downregulated by the overexpression of miR-487b-3p, in OS cells
at both the mRNA and protein levels (Fig. 4B and C). Consistent with the in
vitro results, the clinical sample analysis results also showed
an inverse association between ALDH1A3 and miR-478b-3p expression
levels in OS specimens (R2=0.4471; P=0.0129; Fig. 4D). Furthermore, it was verified that
miR-487b-3p can directly target the 3′UTR of ALDH1A3 using the
luciferase reporter assay, as the expression of ALDH1A3 was
decreased in the WT group compared with that in the control group,
indicating that miR-487b-3p can directly bind to the 3′UTR of
ALDH1A3 to regulate its expression (Fig. 4E). These findings indicate that
miR-487b-3p inhibits the mRNA and protein expression levels of
ALDH1A3 by binding to its 3′UTR.
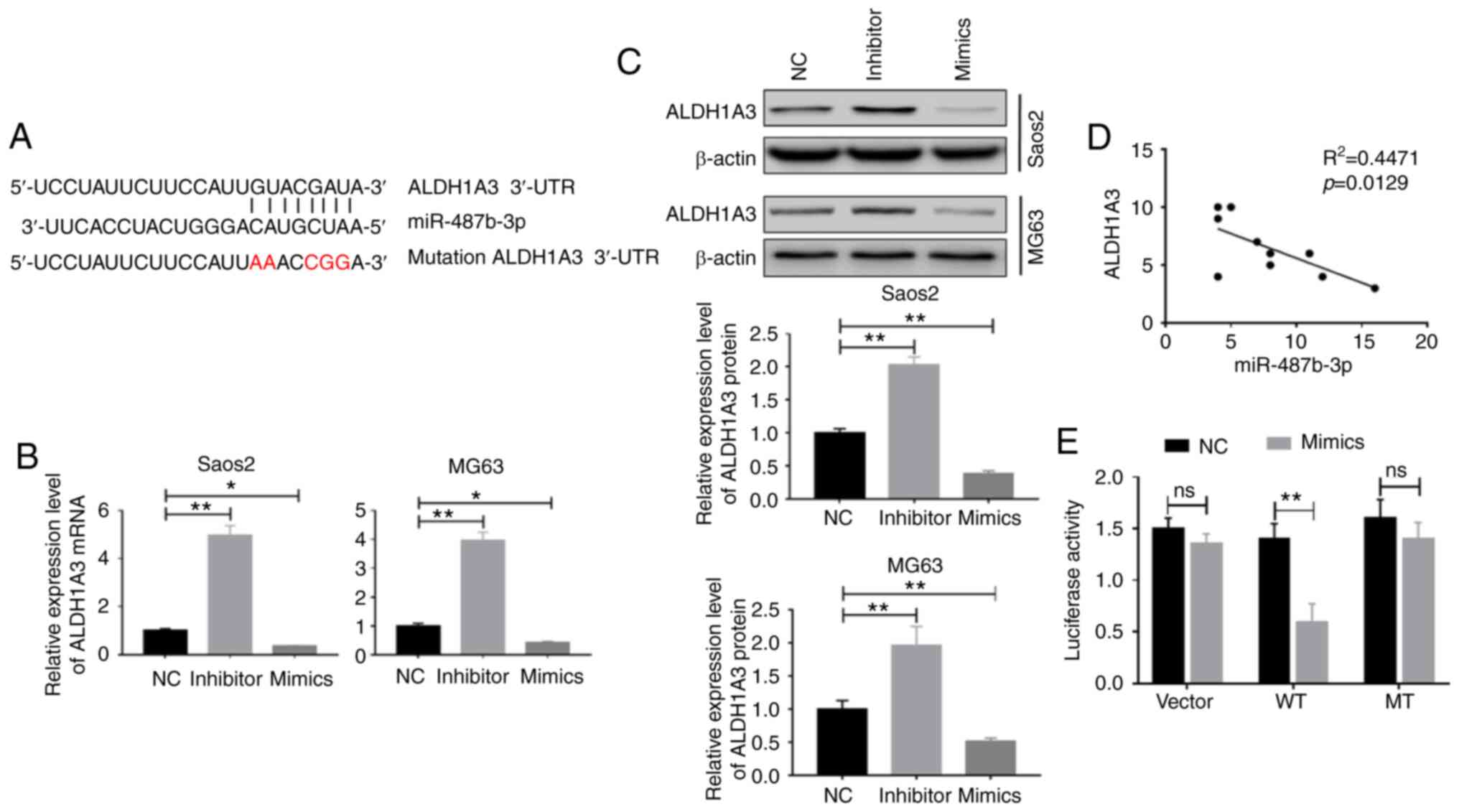 | Figure 4.miR-487b-3p targets ALDH1A3. (A) The
miR-487b-3p seed sequence is complementary to the 3′UTR of ALDH1A3.
Saos-2 and MG63 cells were transfected with either NC, mimic or
inhibitor nucleotides and miR-487b-3p inhibited (B) mRNA and (C)
protein expression levels of ALDH1A3, 72 h following transfection
and were measured using reverse transcription-quantitative PCR and
western blot analysis, respectively. (D) The expression levels of
ALDH1A3 and miR-487-3p revealed a negative correlation in patients
with OS. Tumor samples were obtained from 10 patients with OS. (E)
Activity of the luciferase gene linked to the 3′UTR of ALDH1A3. The
luciferase reporter plasmids of WT or MT 3′UTR sequences of ALDH1A3
were transfected into 293T cells with or without the mimic.
*P<0.05. **P<0.01. NC, negative control oligonucleotides;
mimic, miR-487b-3p mimic; inhibitor, miR-487b-3p inhibitor; ns, no
significance; WT, wild-type; MT, mutated; miR, microRNA; ALDH1A3,
aldehyde dehydrogenase 1 family member A3. |
ALDH1A3 is a functional target of
miR-487b-3p which modulates OS stemness
It was subsequently investigated whether ALDH1A3 is
involved in the miR-487b-3p-mediated regulation of CSCs in OS. The
expression of ALDH1A3 in OS cells was disrupted by transfection of
ALDH1A3 expression vector or siRNA against ALDH1A3 (Fig. S3A). The overexpression or silencing
of ALDH1A3 did not affect the miR-487b-3p expression (Fig. S3B and S3C). The overexpression of ALDH1A3
restored the downregulated expression of CSC marker proteins
induced by miR-487b-3p, including ALDH1, SOX-2, OCT-4 and BMI-1
(Fig. 5A). In contrast, silencing
of ALDH1A3 suppressed the protein expression levels of CSC markers,
which were increased following inhibition of miR-487-3p (Fig. 5B). Consistent with these results,
the overexpression of ALDH1A3 attenuated the miR-487b-3p-induced
antisphere formation effect, while silencing of ALDH1A3 attenuated
miR-487b-3p inhibition-induced stimulation of osteosphere formation
(Fig. 5C). Together, the results
have shown that miR-487b-3p regulates CSCs due to regulation of
ALDH1A3 in OS.
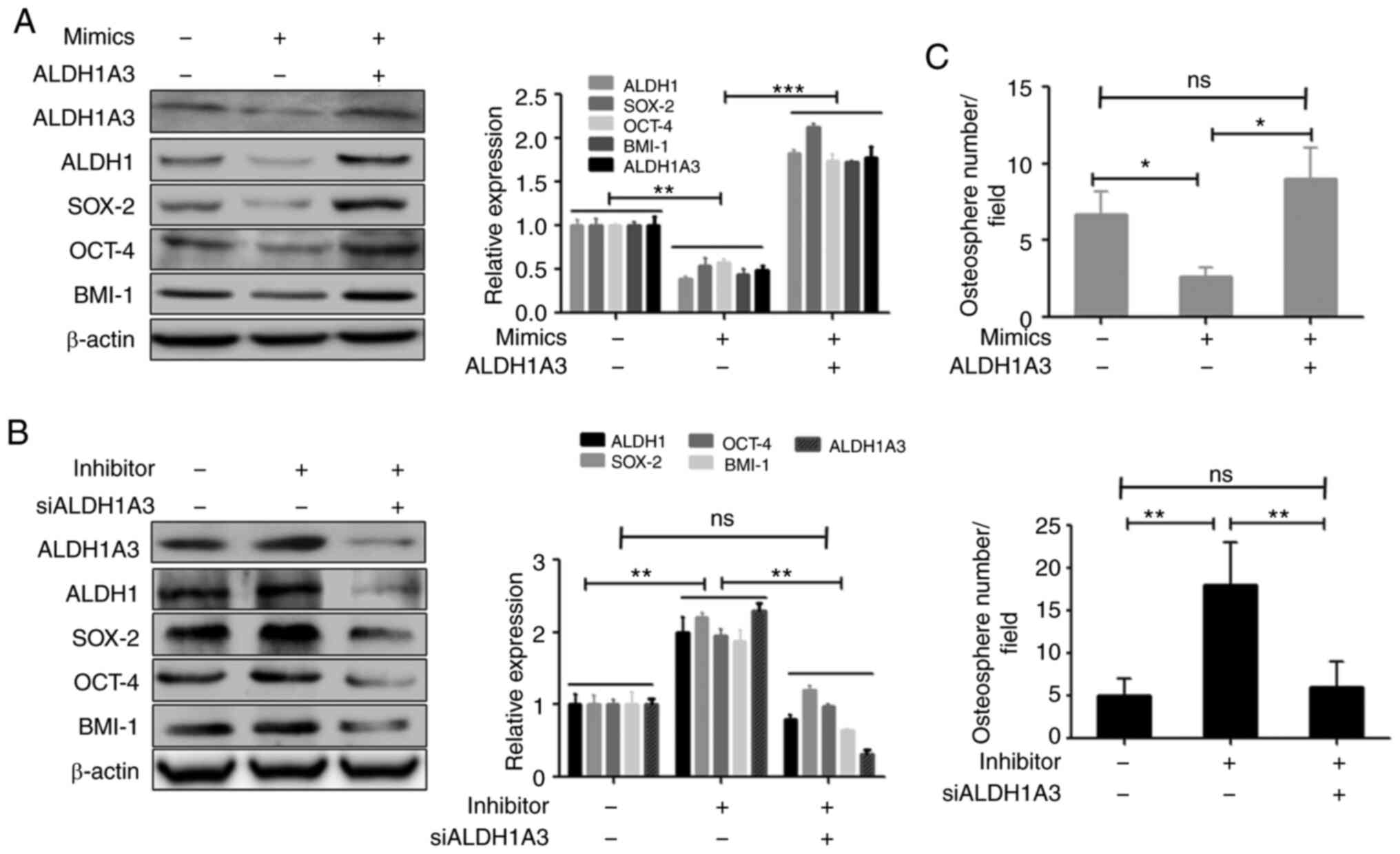 | Figure 5.ALDH1A3 causes miR-487b-3p-induced
effects on OS stemness. (A) The overexpression of ALDH1A3 increased
miR-487b-3p-induced inhibition and (B) The silencing of ALDH1A3
blocked the miR-487b-3p inhibition-induced upregulation of the
protein expression levels of CSC markers. Saos-2 cells were
transfected with mimic and/or ALDH1A3 expression plasmids or with
miR-487b-3p inhibitor and/or ALDH1A3 siRNA, respectively and
analyzed using western blot analysis (C) miR-487b-3p regulated OS
sphere formation due to ALDH1A3. Saos-2 cells were transfected with
the aforementioned nucleotides and plasmids and then subjected to
sphere formation assays. *P<0.05; **P<0.01; ***P<0.001.
NC, negative control; mimic, miR-487b-3p mimic; inhibitor,
miR-487b-3p inhibitor; si, short inhibiting; OS, osteosarcoma;
ALDH1A3, aldehyde dehydrogenase 1 family member A3; ns,
non-significant; +, positive, -, negative; CSC, cancer stem
cell. |
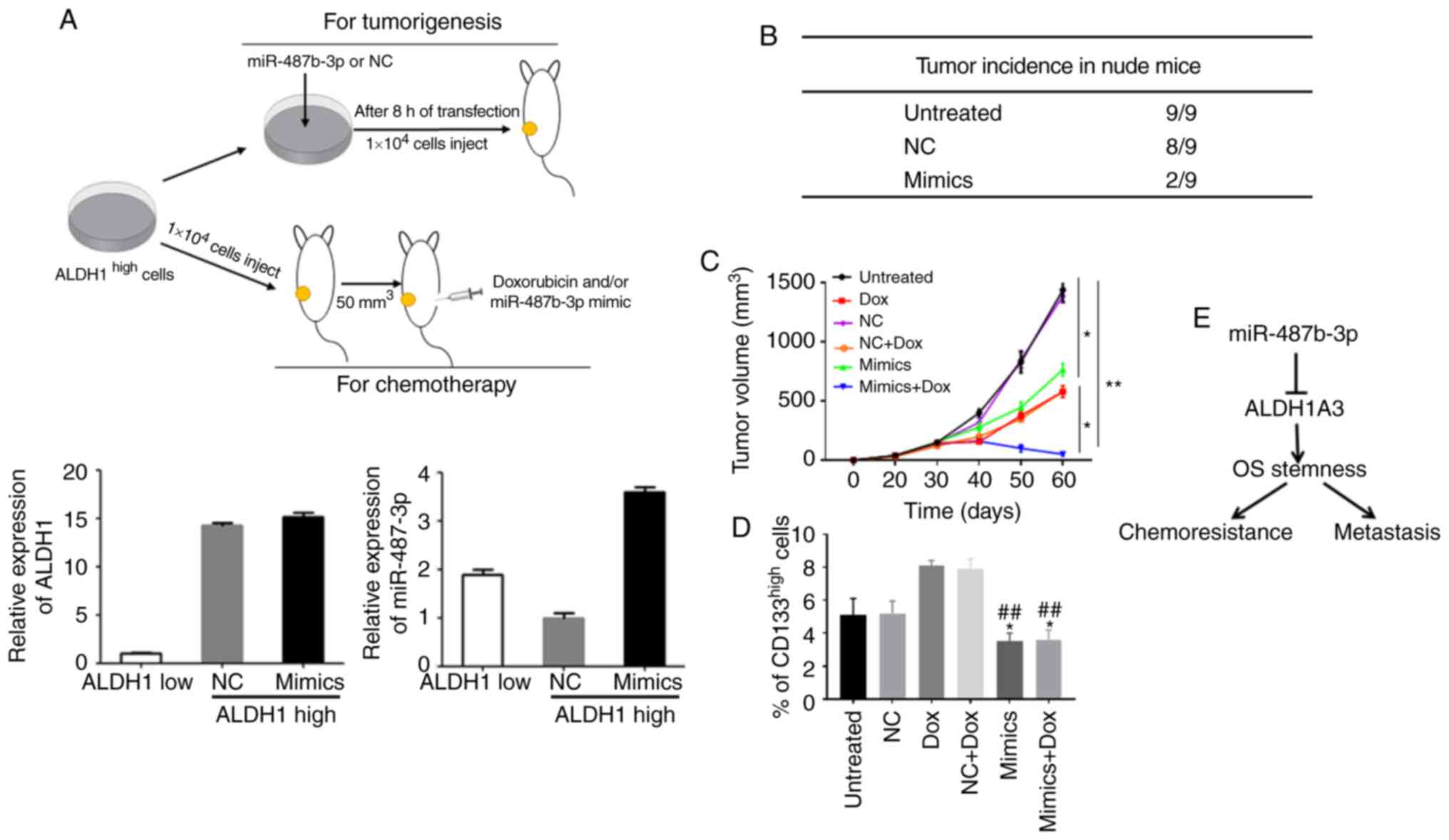
miR-487b-3p significantly inhibits
CSCs-induced tumorigenesis and chemoresistance in vivo
The in vitro experimental results indicated
that upregulation of miR-487b-3p significantly inhibits OS
stemness. Thus, the effects of miR-487b-3p on CSC-induced
tumorigenesis and chemoresistance in vivo were investigated.
ALDH1high-expression cells were separated from OS cell
lines using flow cytometry and transfected with miR-487b-3p mimics
and subsequently s.c. injected into nude mice to investigate the
effect of miR487b-3p on tumorigenesis (Fig. 6A). As expected, treatment with
miR-487b-3p blocked CSC-induced tumor formation in nude mice
(Fig. 6B). Subsequently, to
investigate the effects of miR-487b-3p on chemosensitivity, a
xenograft model was generated using ALDH1high Saos-2
cells. These mice received three cycles (days 29, 32 and 36) of
intraperitoneal injection of Dox, miR-487b-3p or both. The results
revealed that Dox treatment inhibited tumor growth, but regrowth of
the disease occurred after 40 days. However, no tumor regrowth
occurred in the combination treatment group of Dox and miR-487b-3p.
(Fig. 6C). In addition, the CSCs in
the tumor tissues were investigated, and data revealed that the
groups treated with miR-487b-3p had a significantly reduced CSC
population compared with that in the groups that were not treated
with miR-487b-3p (Fig. 6D).
Discussion
The occurrence of chemoresistance and metastasis
indicates poor prognosis in patients with OS (3,4).
Previous studies have revealed that the expression level of these
miRNAs (miR-495, miR-329, miR-487b, miR-410, and miR-656) are
associated with the progression of OS (13,14).
Previous studies have reported that miR-487b-3p suppressed
osteoblastic differentiation and the tumorigenicity of colon cancer
cells in vivo (21,22). In the present study, an insight into
the biological effects of miR-487b-3p in OS metastasis and
chemoresistance has been provided. It was found that decreased
expression of miR-487b-3p was significantly associated with poor
clinical outcomes in patients with OS. In addition, the in
vitro results revealed that the inhibition of miR-487b-3p
promoted OS cell migration and chemoresistance. The findings from
the present study suggests that miR-487b-3p functions as a cancer
suppressor and that may be a novel strategy for the treatment of OS
metastasis and chemoresistance by the restoration of
miR-487b-3p.
In the present study, the anti-OS mechanism of
miR-487b-3p was clarified. Accumulated evidence has shown that
increased cancer stemness can stimulate cancer metastasis and
induce chemoresistance (23–25).
The results from the present study showed that the inhibition of
miR-487b-3p stimulated OS stemness. Notably, the in vitro
and in vivo experiments revealed that the overexpression of
miR-487b-3p significantly suppressed OS stemness and number of CSCs
in tumor tissues, suggesting that miR-487b-3p plays an anticancer
role partially due to the inhibition of CSCs in OS. In addition,
the CSC regulation mechanism of miR-487b-3p in OS was investigated,
in which a series of in vitro experiments were used to
confirm that ALDH1A3 is a target gene of miR-487b-3p in OS.
Previous studies have shown that ALDH1A3 is one of the important
members of the aldehyde dehydrogenase family, which is a CSC marker
and plays an important role in CSC regulation, which can affect the
biological characteristics of CSCs through the regulation of other
stem cell genes, such as CD133 and OCT4 (26,27).
The data in the present study revealed that the ALDH1A3 expression
level was increased or decreased in OS cells by the inhibition or
increased expression of miR-487b-3p, respectively. In addition, a
luciferase reporter assay revealed that miR-487b-3p directly
targets the 3′UTR of ALDH1A3. Furthermore, clinical data revealed
that the expression levels of miR-487b-3p and ALDH1A3 were
inversely correlated in specimens from patient with OS. In
additional, the restoration of ALDH1A3 blocked the miR-487b-3p
overexpression-induced inhibition of CSCs. Taken together, these
data suggest that miR-487b-3p inhibits OS metastasis and
chemoresistance through the inhibition of CSCs by targeting
ALDH1A3.
In summary, a role for miR-487b-3p in OS
chemoresistance and metastasis has been established using a
combination of clinical and experimental studies. The
overexpression of miR-487b-3p enhances the sensitivity of OS cells
to chemotherapy and inhibits OS metastasis through suppressing OS
stemness by targeting ALDH1A3 (Fig.
6E). The findings from the present study may also aid in the
development of potential therapeutics for the treatment of OS
metastasis and chemoresistance.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81760393 and 81401790).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
MC and MD made substantial contributions to
conception and designed the study. ZZG and PGD analyzed and
interpreted the data. MC and MD drafted the manuscript and revised
it critically for important intellectual content; All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was granted by the Research Ethics
Board of the First Affiliated Hospital of Nanchang University
(approval no. 2019042). Written informed consent was provided by
all the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu M, Jin H, Xu CX, Sun B, Song ZG, Bi WZ
and Wang Y: miR-382 inhibits osteosarcoma metastasis and relapse by
targeting Y box-binding protein 1. Mol Ther. 23:89–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C and Tang DG: MicroRNA regulation of
cancer stem cells. Cancer Res. 71:5950–5954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacDonagh L, Gray SG, Breen E, Cuffe S,
Finn SP, O'Byrne KJ and Barr MP: BBI608 inhibits cancer stemness
and reverses cisplatin resistance in NSCLC. Cancer Lett.
428:117–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iliopoulos D, Lindahl-Allen M, Polytarchou
C, Hirsch HA, Tsichlis PN and Struhl K: Loss of miR-200 inhibition
of Suz12 leads to polycomb-mediated repression required for the
formation and maintenance of cancer stem cells. Mol Cell.
39:761–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song SJ, Poliseno L, Song MS, Ala U,
Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, et al:
MicroRNA-antagonism regulates breast cancer stemness and metastasis
via TET-familydependent chromatin remodeling. Cell. 154:311–324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin H, Luo S, Wang Y, Liu C, Piao Z, Xu M,
Guan W, Li Q, Zou H, Tan QY, et al: miR-135b stimulates
osteosarcoma recurrence and lung metastasis via notch and
Wnt/β-catenin signaling. Mol Ther Nucleic Acids. 8:111–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thayanithy V, Sarver AL, Kartha RV, Li L,
Angstadt AY, Breen M, Steer CJ, Modiano JF and Subramanian S:
Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma.
Bone. 50:171–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill KE, Kelly AD, Kuijjer ML, Barry W,
Rattani A, Garbutt CC, Kissick H, Janeway K, Perez-Atayde A,
Goldsmith J, et al: An imprinted non-coding genomic cluster at
14q32 defines clinically relevant molecular subtypes in
osteosarcoma across multiple independent datasets. J Hematol Oncol.
10:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marcato P, Dean CA, Pan D, Araslanova R,
Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, et al:
Aldehyde dehydrogenase activity of breast cancer stem cells is
primarily due to isoform ALDH1A3 and its expression is predictive
of metastasis. Stem Cells. 29:32–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sullivan KE, Rojas K, Cerione RA, Nakano I
and Wilson KF: The stem cell/cancer stem cell marker ALDH1A3
regulates the expression of the survival factor tissue
transglutaminase, in mesenchymal glioma stem cells. Oncotarget.
8:22325–22343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan JJ, Cai J, Guo YF, Bian XW and Yu SC:
ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int J
Cancer. 139:965–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steffner RJ and Jang ES: Staging of bone
and soft-tissue sarcomas. J Am Acad Orthop Surg. 26:e269–e278.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi H, Geng L, Black A, Talmon G, Berim L
and Wang J: The miR-487b-3p/GRM3/TGFβ signaling axis is an
important regulator of colon cancer tumorigenesis. Oncogene.
36:3477–3489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
John AA, Prakash R and Singh D:
miR-487b-3p impairs osteoblastogenesis by targeting Notch-regulated
ankyrin-repeat protein (Nrarp). J Endocrinol. 241:249–263. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: Migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sullivan KE, Cerione RA and Wilson KF:
ALDH1A3 in CSCs. Aging (Albany NY). 9:1351–1352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu X, Chai S, Wang P, Zhang C, Yang Y,
Yang Y and Wang K: Aldehyde dehydrogenases and cancer stem cells.
Cancer Lett. 369:50–57. 2015. View Article : Google Scholar : PubMed/NCBI
|