Introduction
Lung cancer remains the most common malignancy with
the highest morbidity and mortality in the world (1). According to a 2015 research study of
China, the incidence rate of lung cancer ranks first among men and
second among women (2). Lung cancer
can be divided into two main types: Small-cell lung cancer (SCLC)
and non-small cell lung cancer (NSCLC) according to the
pathological classification of lung cancer in clinical practice.
NSCLC accounts for ~85% of all lung cancer patients, with a
five-year survival as low as 15% (3). Currently, with the continuous
development of medicine, great progress has been made in the
diagnosis and treatment of lung cancer, however, some methods
exhibit toxicity-related side effects and present limitations in
their application, which seriously threaten the safety of human
life (4). Therefore, it is urgent
to identify new diagnosis and treatment methods for lung
cancer.
Long non-coding RNAs (lncRNAs) are a class of RNAs
that do not encode proteins and have transcripts longer than 200
nucleotides. Current studies have confirmed that lncRNAs are
closely related to the occurrence and the development of various
human malignant tumors (5). In the
process of tumor formation, lncRNAs play an important role in the
function of oncogenes and tumor suppressor genes (6). Some lncRNAs are differentially
expressed in tumor and normal tissues, which may be involved in the
occurrence and development of tumors (7–10).
Fer-1-like protein 4 (FER1L4), as an important member of lncRNAs,
is closely related to the occurrence and development of tumors.
Studies have reported that lncRNA FER1L4 knockout in liver cancer
cells promoted cell proliferation and invasion (11,12).
Yue et al (13), revealed
that lncRNA FER1L4 inhibited the formation of colon cancer and
predicted the prognosis of patients. Liu et al (14), confirmed that the expression level
of FER1L4 could be an important indicator for early diagnosis of
gastric cancer. However, the role of lncRNA FER1L4 in NSCLC remains
unclear.
From literature review, it was revealed that lncRNA
FER1L4 inhibited the process of osteosarcoma cells by targeting
microRNA-18a-5p (miR-18a-5p) to release the gene phosphatase and
tension homolog deleted on chromosome ten (PTEN) (15). In addition, FER1L4 was revealed to
inhibit proliferation, invasion and migration of hepatocellular
carcinoma (11). Futhermore,
research has revealed that upregulating lncRNA FER1L4 suppressed
the proliferation and migration of the hepatocellular carcinoma via
regulation of the PI3K/AKT signaling pathway (12). However, the expression of FER1L4 in
the plasma and tissues of patients with NSCLC and the role of
PTEN/AKT/p53 signaling proteins in the proliferation, invasion and
migration of NSCLC are still unknown. In the present study, the
expression of lncRNA FER1L4 was examined in the plasma and tissues
of patients with NSCLC, and it was investigated whether lncRNA
FER1L4 inhibited the proliferation of lung cancer cells and
promoted apoptosis through the PTEN/AKT/p53 signaling pathway.
Materials and methods
Tumor tissues and blood samples
A total of 30 tumor tissues and blood samples were
obtained from NSCLC patients (15 females and 15 males; age range,
18–45 years) who received surgical treatment from January to April
in 2019 at Northern Jiangsu People's Hospital. Blood and tissues of
31 healthy volunteers were used as the control group. None of the
patients had received neoadjuvant therapy or endocrine therapy
before the surgery. The present study was approved by the Ethics
Committee of Northern Jiangsu People's Hospital, and all patients
provided written informed consent. All of the procedures were in
compliance with The Declaration of Helsinki and relevant policies
in China.
Cell culture
All cell lines used in this study, including BEAS-2B
(cat. no. SCSP-5067), H1975 (cat. no. SCSP-597), H23 (cat. no.
SCSP-581), A549 (cat. no. TCHu150) and H1299 (cat. no. SCSP-589),
were all obtained from the Type Culture Collection of the Chinese
Academy of Sciences, and were cultured in DMEM supplemented with
10% FBS (both from Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. The HBE cell line is a human bronchial
epithelial cell line, and H1975, H23, A549 and H1299 are all lung
cancer cell lines.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tumor tissues, blood samples and
cultured cells were extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. The RNA concentration and quantification
were assessed using a Nanodrop spectrophotometer (Thermo Fisher
Scientific, Inc.). Reverse transcription of RNA into complementary
DNA (cDNA) was performed using a PrimeScript™ reverse transcription
reagent kit (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. Subsequently, cDNA was analyzed using a
TaqMan® Universal PCR Master Mix kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Amplification conditions were as follows: 95°C for 10 min, followed
by 40 cycles at 95°C for 10 sec and 60°C for 60 sec. Primer
sequences used in PCR were obtained from GenScript. The primer
sequences were as follows: FER1L4 forward,
5′-AATGTGGGCTTCCAGGAAC-3′ and reverse, 5′-CACCAGAAAGTTCCACGTC-3′;
GAPDH forward, 5′-GGTCGGAGTCAACGGATTTG-3′ and reverse,
5′-GGAAGATGGTGATGGGATTTC-3′. The relative gene expression level
quantification was analyzed using the 2−ΔΔCq method, and
normalized to GAPDH expression (16).
Cell transfection
One day prior to transfection, A549 cells
(1×105 cells/well) were seeded into 6-well plates and
cultured at 37°C in an atmosphere containing 5% CO2.
Subsequently, the cells were transfected with FER1L4 or negative
control (NC) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Cells of the blank control group (Control) did not
receive any treatment. When cells were incubated for 48 h, they
were used for the following experiments. Transfection efficiency
was detected by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
Cell Counting Kit-8 (CCK-8) assay
The proliferation of cells was assessed using CCK-8
kit (Dojindo Molecular Technologies, Inc.) according to the
manufacturer's instructions. In brief, cells were seeded into
96-well plates, and trypsinized to prepare a cell suspension with a
density of 2×103 cells/ml. After 4 h of incubation, 10
µl of CCK-8 solution was added to each well and further incubated
for 2 h at 37°C. The absorbance at 450 nm was measured using a
microplate reader (BioTek Instruments, Inc.).
Wound healing assay
Cell migration was determined by wound-healing
assays. Briefly, transfected cells were plated in 12-well plates at
a density of 1×105 cells/well. Once cells reached 80%
confluence, the medium was replaced by serum-free DMEM and cells
were incubated at 37°C overnight before initiating the experiment.
A wound was created on the surface of the cell monolayer using a
200-µl pipette tip. The cells were then rinsed twice with
serum-free medium in order to remove free-floating cells and
debris. An inverted microscope (magnification ×20; BX51; Olympus
Corporation) was used to monitor cells along the edge of the
scratch. Cell mobility=(0-h scratch width −24-h scratch width)/0-h
scratch width.
Cell invasion assay
To assess the invasion of transfected cells, 24-well
Transwell plates (Corning, Inc.) with 8-µm pore inserts were coated
with Matrigel (BD Biosciences). A total of 200 µl serum-free medium
cell suspension containing 5×104 cells/ml was added to
the upper chamber and 600 µl DMEM containing 10% FBS was added to
the lower compartment. After 24 h of incubation at 37°C with 5%
CO2, the Matrigel and the cells remaining on the upper
chamber were removed by a cotton-tipped swab. The filters were
fixed in 4% formaldehyde for 10 min at room temperature and stained
with 0.1% crystal violet solution for 30 min at room temperature.
The cells in five random fields (magnification ×20) were observed
under an inverted microscope (Olympus Corporation).
Western blotting
The protein levels of Ki67, proliferating cell
nuclear antigen (PCNA), matrix metalloproteinase-2 (MMP2), MMP9,
phosphatase and tension homolog deleted on chromosome ten (PTEN),
p-AKT, AKT, Bcl-2, BAX, p53, cleaved caspase-3 and cleaved
caspase-9 were assessed by western blotting. In brief, the total
cell proteins were extracted from cells using SDS lysis buffer
(Cell Signaling Technology, Inc.). Subsequently, a BCA assay
(Thermo Fisher Scientific, Inc.) was used to detect the protein
concentrations. Subsequently, equal amounts of protein samples (25
µg/lane) were separated by 10% SDS-PAGE and then transferred onto
polyvinylidene difluoride membranes. Following blocking with 5%
non-fat milk at room temperature for 2 h, the membranes were
incubated with the primary antibodies against Ki67 (1:1,000;
product no. 9449T), PCNA (1:1,000; product no. 13110T), MMP2
(1:1,000; product no. 40994T), MMP9 (1:1,000; product no. 13667T),
PTEN (1:1,000; product no. 9188T), p-AKT (1:1,000; product no.
13038T), AKT (1:1,000; product no. 4685T), Bcl-2 (1:1,000; product
no. 15071T), BAX (1:1,000; product no. 5023T), p53 (1:1,000;
product no. 2527T), cleaved caspase-3 (1:1,000; product no. 9664T)
and cleaved caspase-9 (1:1,000; product no. 9509T) obtained from
Cell Signaling Technology, Inc. at 4°C overnight. Then, the
membranes were incubated with secondary antibodies: Anti-mouse IgG,
HRP-linked antibody (1:5,000; product no. 7076S; Cell Signaling
Technology, Inc.) at room temperature for 2 h. GAPDH (1:5,000;
product no. 5174T; Cell Signaling Technology, Inc.) was used as the
internal control. At the end of the experiment, the protein bands
were visualized with an enhanced chemiluminescence detection system
(Super Signal West Dura Extended Duration Substrate; Pierce; Thermo
Fisher Scientific, Inc.) and ImageJ software (v1.46; National
Institutes of Health) was used to analyze the fold-changes of the
protein levels.
Cell apoptosis assay
Transfected cells were plated in 6-well plates at a
density of 1×105 cells/well. Seventy-two hours after
adding 2 µm of PTEN inhibitor SF1670 to transfected cells, cells
were collected for Annexin V/PI double staining (Annexin V-FITC;
Vazyme Biotech Co., Ltd.). The staining procedure was conducted
according to the manufacturer's instructions. The activity of
Annexin V/PI was then detected by flow cytometry (FACSCalibur; BD
Biosciences) and quantified by FlowJo software (v7.6.1; FlowJo
LLC).
Hoechst staining
Transfected cells were plated in 6-well plates at a
density of 1×105 cells/well. Seventy-two hours after
adding 2 µm of PTEN inhibitor SF1670 to transfected cells, cells
were incubated with 1 µg/µl Hoechst 33342 (Beyotime Institute of
Biotechnology) for 15 min, washed twice with ddH2O, and
observed under a fluorescence microscope (magnification, ×20).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS v17.0 statistical software (SPSS, Inc.) was used for all
statistical analyses. Comparisons between groups were analyzed by
Student's t-test or one-way analysis of variance (ANOVA) followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA FER1L4 expression is
downregulated in plasma of patients with lung cancer and lung
cancer cell lines
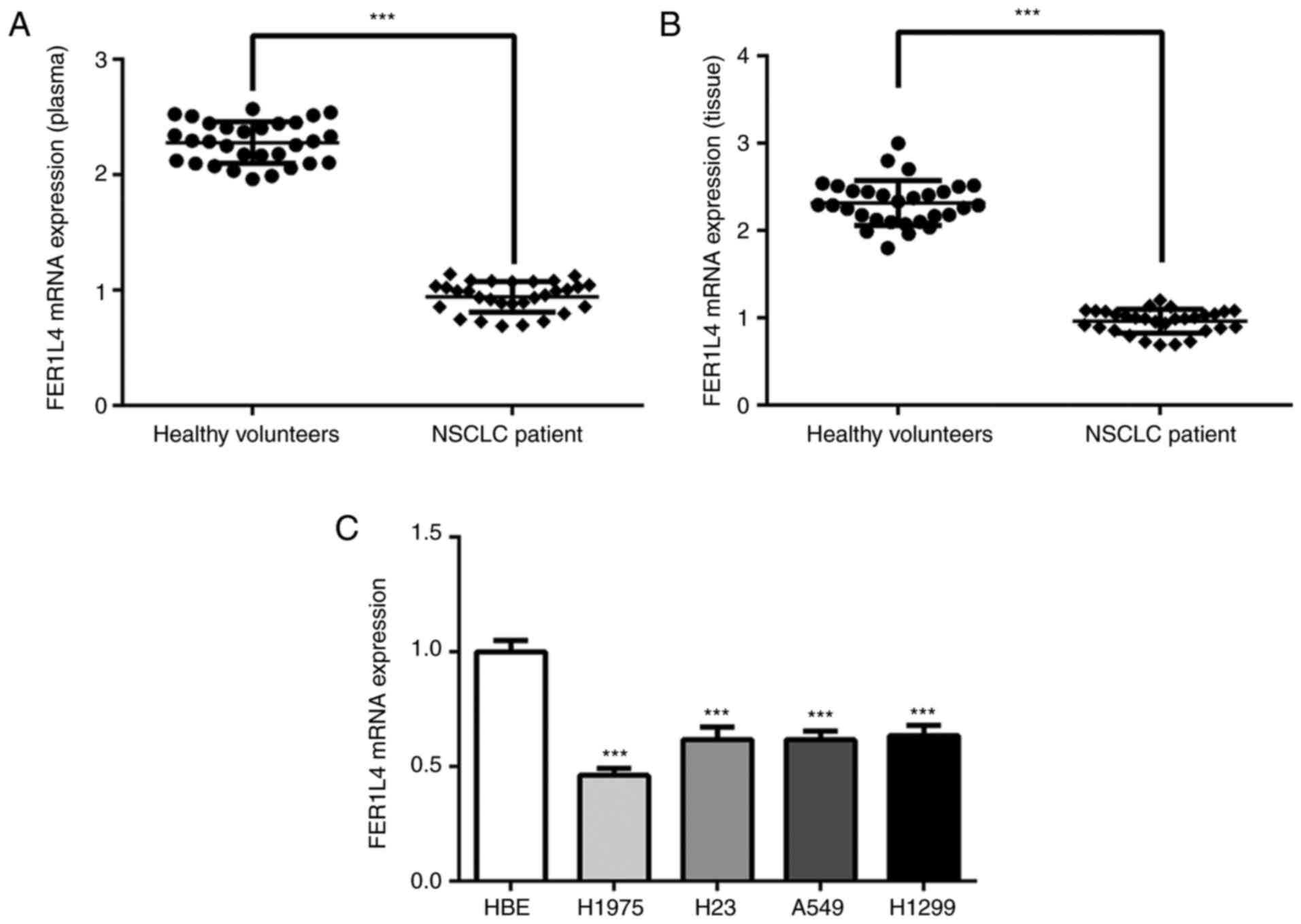
The blood and tissues of lung cancer patients and
normal volunteers were collected, and the expression of FER1L4 in
plasma and tissues was detected by RT-qPCR. A significant decrease
of the expression level of FER1L4 was observed in plasma and
tissues of lung cancer patients (Fig.
1A and B). Then, in order to conduct the functional studies on
FER1L4, its expression in the HBE cell line which was considered as
a control and four lung cancer cell lines (H1975, H23, A549 and
H1299) was also detected. Similarly, FER1L4 expression was
significantly decreased in H1975, H23, A549 and H1299 cells
compared to HBE cells (Fig. 1C).
After comprehensive consideration, since A549 cells were
significantly decreased and are easily cultured this cell line was
selected for the following experiments.
Overexpression of lncRNA FER1L4
inhibits proliferation of A549 cells
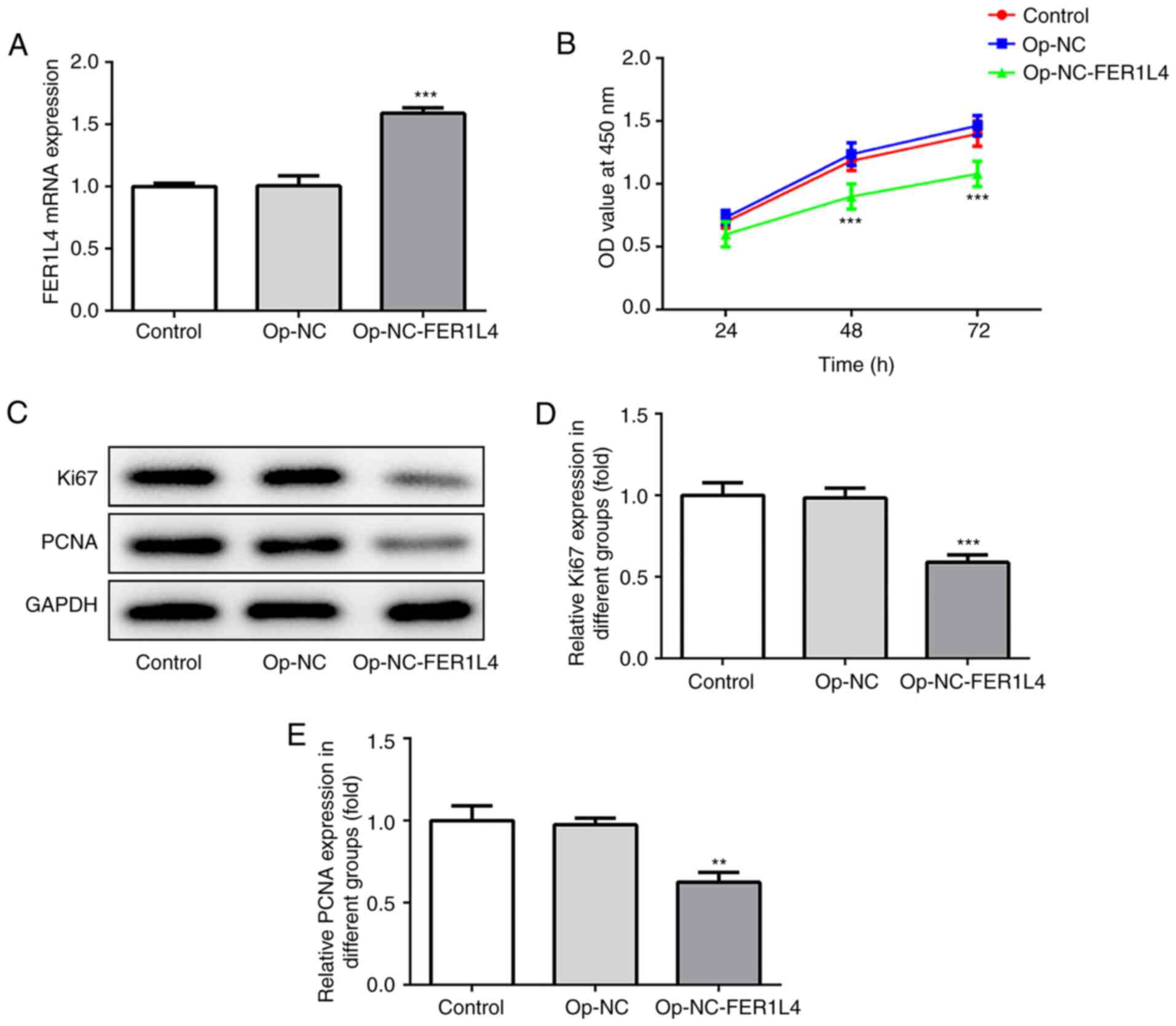
The transfection efficiency of the cells was
detected, and as revealed in Fig
2A, compared to the overexpression-NC group, the expression of
FER1L4 in the overexpression-NC-FER1L4 group was significantly
increased. The proliferation rate of cells was detected by CCK-8
assay (Fig. 2B) and the expression
levels of proliferation-related proteins Ki67 and PCNA were
detected by western blotting (Fig.
2C). The data revealed that compared to the overexpression-NC
group, the proliferation rate and the expression levels of Ki67 and
PCNA proteins were significantly decreased in lung cells, in which
lncRNA FER1L4 was overexpressed (Fig.
2B-E). The results indicated that overexpression of lncRNA
FER1L4 inhibited the proliferation of A549 cells.
Overexpression of lncRNA FER1L4
inhibits the invasion and migration abilities of A549 cells
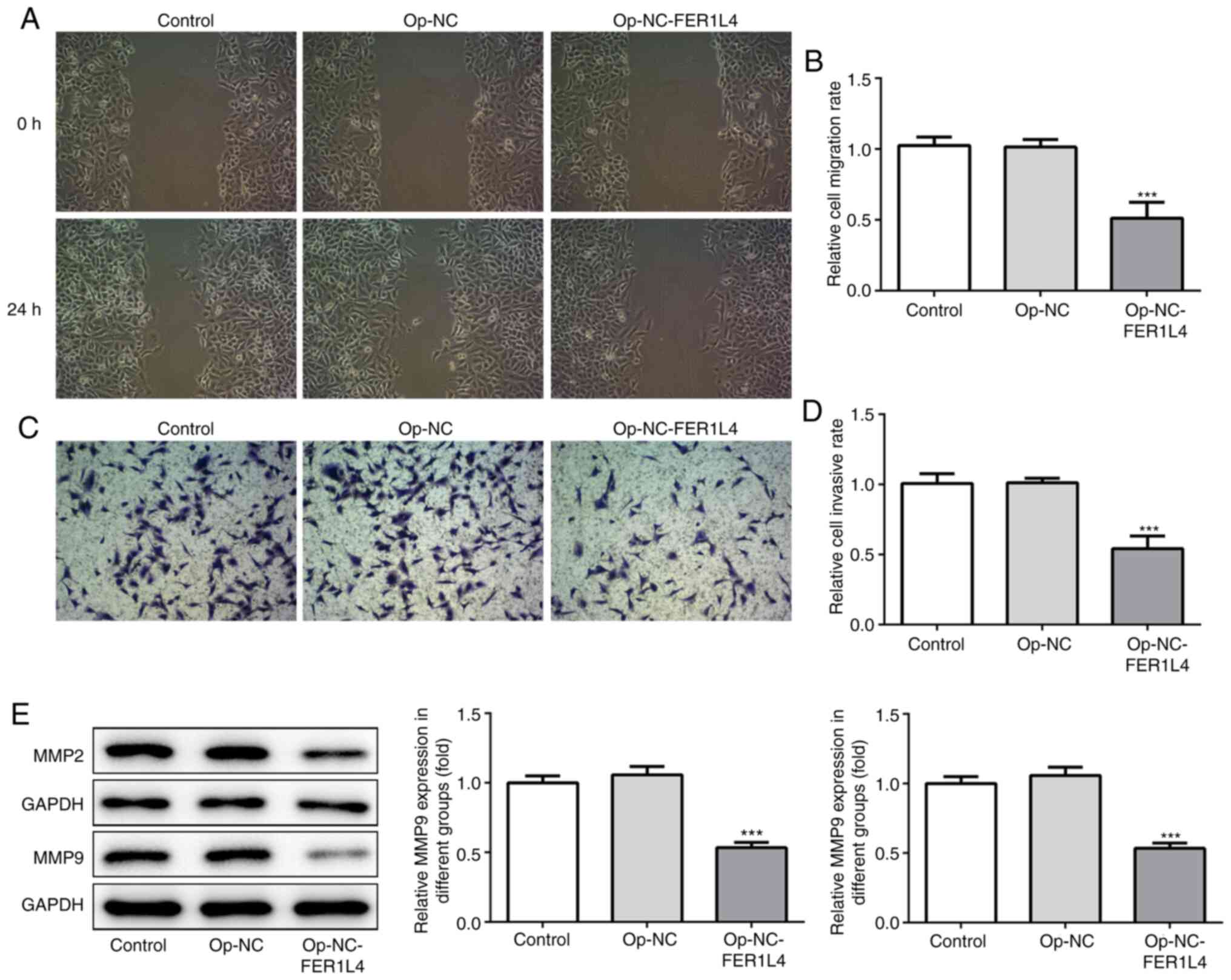
To detect the effect of lncRNA FER1L4 on the
invasion and migration of A549 cells, wound healing (Fig. 3A and B) and Transwell (Fig. 3C and D) assays were employed.
Western blotting was used to detect the expression of invasion- and
migration-related proteins MMP2 and MMP9 (Fig. 3E). The results revealed that
overexpression of lncRNA FER1L4 significantly reduced the invasion
and migration abilities of cells, as well as the expression levels
of MMP2 and MMP9.
Overexpression of lncRNA FER1L4
activates PTEN and inhibits the phosphorylation of AKT
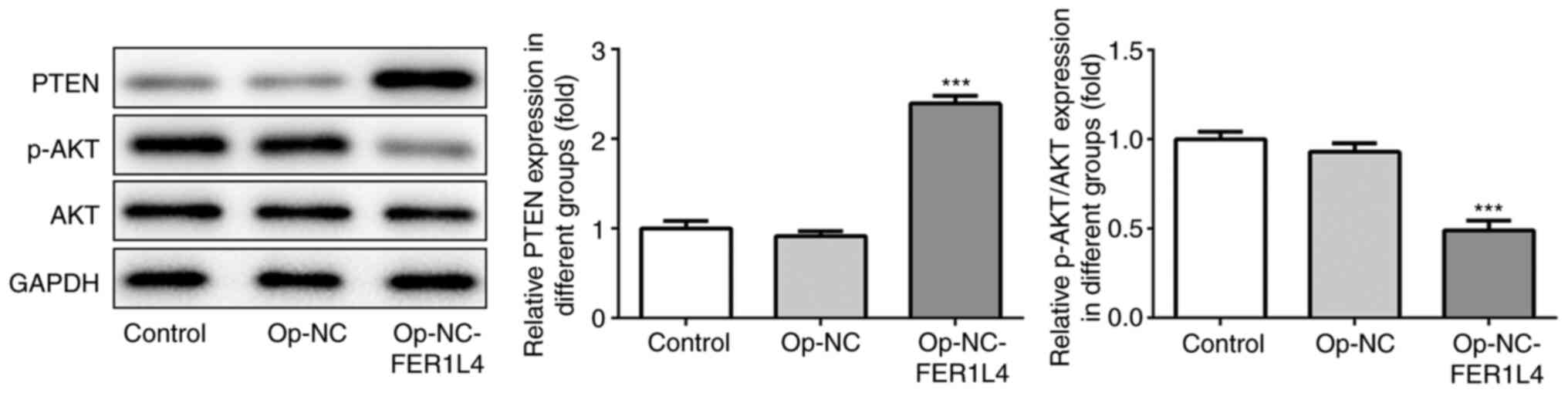
The expression of PTEN protein was detected by
western blotting, and it was revealed that in cells that
overexpressed lncRNA FER1L4, PTEN was significantly increased
compared with the overexpression-NC group. Additionally, the
protein levels of total and phosphorylated AKT (p-AKT) were further
analyzed to explore FER1L4 regulation on AKT signaling. The results
revealed that FER1L4 could also inhibit AKT phosphorylation, since
a decreased level of p-AKT was observed in FER1L4-overexpressed
cells (Fig. 4). These results
revealed that FER1L4 regulated the expression of PTEN protein and
thus inhibited the expression of AKT phosphorylation.
Overexpression of LncRNA FER1L4
promotes apoptosis of lung cancer cells by activating PTEN
AKT signaling pathway is closely related to
apoptosis (17,18). We previously revealed that
overexpressed FER1L4 regulated the PTEN protein and inhibited the
phosphorylation of the AKT protein, which may also be related to
apoptosis. Therefore, PTEN inhibitor SF1670 was added into the
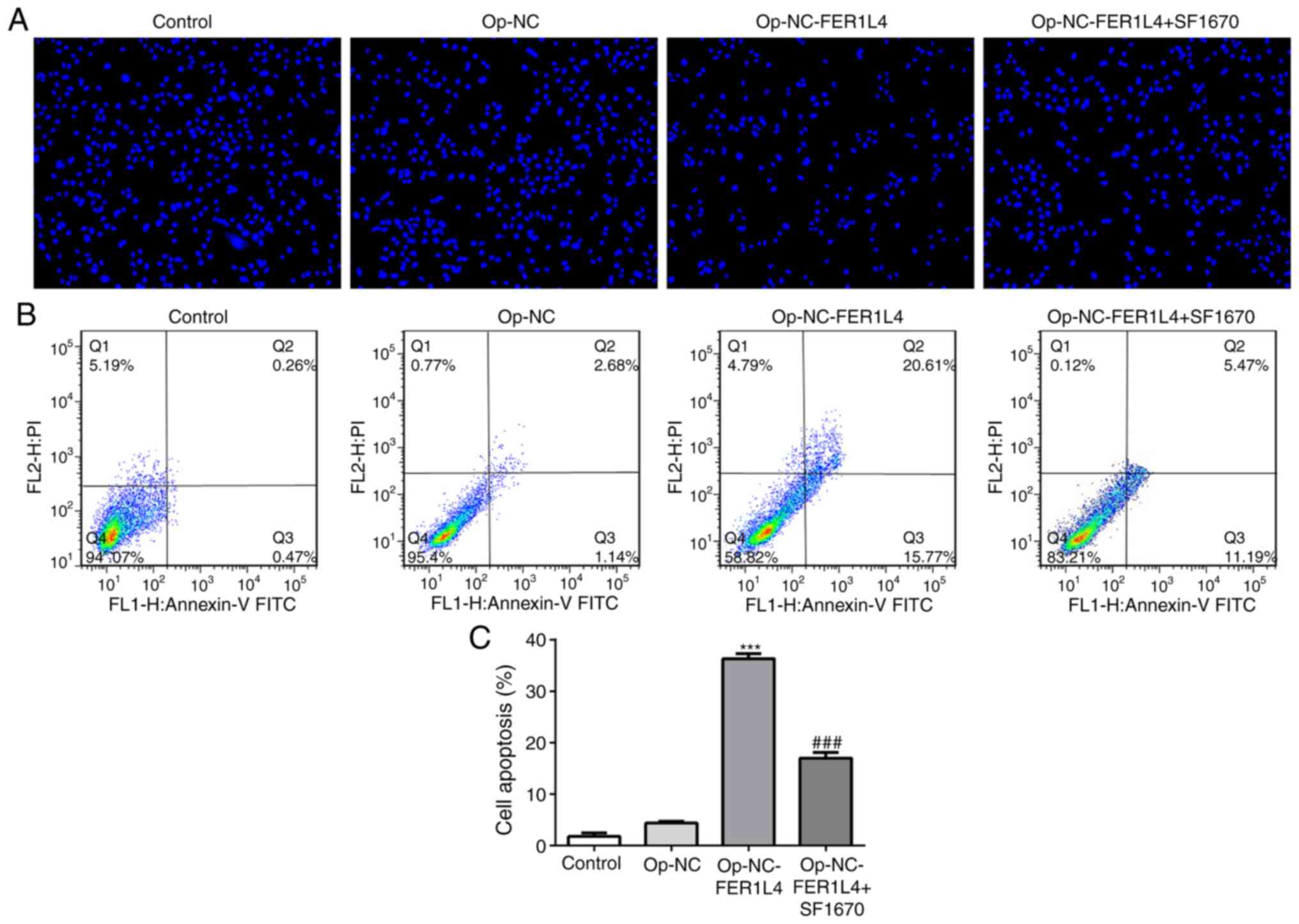
cells, and apoptosis was detected by Hoechst staining (Fig. 5A) and flow cytometry (Fig. 5B and C). Compared with the Control,
there was no difference in the apoptosis of Op-NC. Compared with
the Op-NC group, the apoptosis rate in Op-NC-FER1L4 was
significantly increased after overexpression of FER1L4, but
compared with the Op-NC-FER1L4 group, the cell apoptosis was
decreased after further SF1670 administration. In addition, the
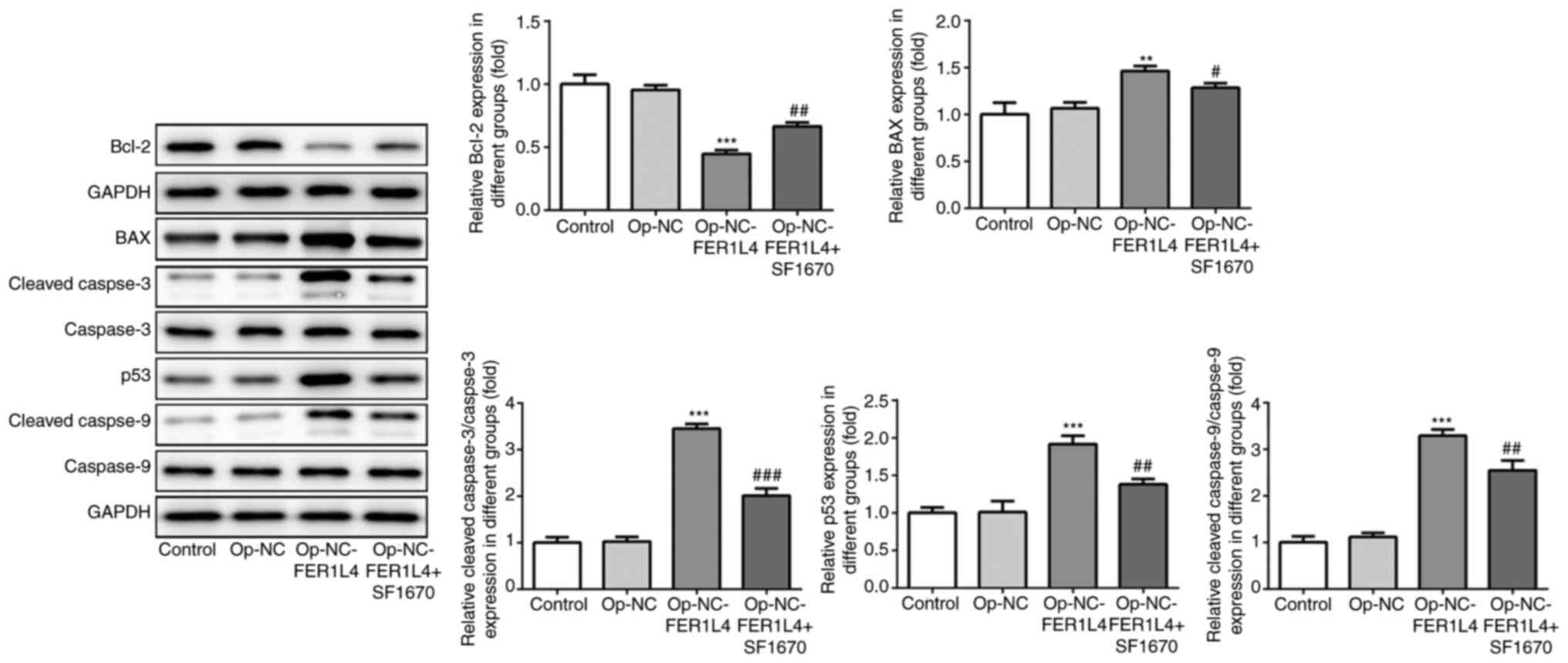
expression levels of apoptosis-related proteins were detected by
western blotting (Fig. 6). The
results revealed that compared with the OP-NC group, FER1L4
increased cell death with a reduction in apoptosis-related protein
Bcl-2 and an increase in BAX, p53, cleaved caspase-3 and cleaved
caspase-9. However, compared with the Op-NC-FER1L4 group, after the
addition of SF1670, cell death was decreased, with an increase in
apoptosis-related protein Bcl-2, and a decrease in BAX, p53,
cleaved caspase-3 and cleaved caspase-9 (Fig. 6). These results indicated that
lncRNA FER1L4 promoted lung cancer cell apoptosis via the
PTEN/AKT/p53 signaling pathway.
Discussion
Lung cancer is currently the most common cancer type
in the world, accounting for 11.6% of all cancer cases. It is one
of the most malignant tumors with the fastest increase in morbidity
and mortality and the greatest threat to human health and life
(3). The World Health Organization
classifies lung cancer into two major groups, namely non-small cell
lung cancer and small-cell lung cancer. NSCLC is more common,
including squamous cell carcinoma, adenocarcinoma and large cell
lung cancer as well as several others (19). Various chemotherapy and targeted
therapies for lung cancer have been studied in the past decade, but
most NSCLC cases metastasize and relapse.
lncRNAs are important regulators of gene expression
at both transcriptional and post-transcriptional levels. In
addition, lncRNAs have been revealed to be involved in regulating
the formation, proliferation, invasion and metastasis of tumors,
which is closely related to the occurrence and development of
tumors (20,21).
Current studies have revealed that the occurrence
and development of NSCLC are related to the aberrant expression of
lncRNAs. There were 953 aberrant lncRNAs expressed in lung
adenocarcinoma and 1,014 aberrant lncRNAs expressed in lung
squamous cell carcinoma (22). Pan
et al (23), revealed that
the expression of FAL1 was significantly upregulated in 78% of
NSCLC cells. Lu et al (24),
confirmed that the upregulated expression of SNHG1 promoted the
proliferation and migration of NSCLC cells through the further
action of miR-145-5p on the expression of MDTH.
lncRNA FER1L4 is an important member of the lncRNA
family, and literature has demonstrated that FER1L4 can serve as a
cancer suppressor gene to inhibit the occurrence and development of
cancer (6). In addition, it can
also inhibit the occurrence of gastrointestinal cancer (14). The expression of FER1L4 in colon
cancer was revealed to be closely related to the depth of tumor
invasion, vascular invasion and lymph node metastasis, and played
an important role in suppressing oncogenesis (13). However, there are few studies on the
role of lncRNA FER1L4 in NSCLC, thus, the present study
innovatively examined the expression of FER1L4 in tissues and
plasma of patients with NSCLC and it was revealed that the
expression of FER1L4 was significantly reduced. In addition, it was
revealed that the expression of FER1L4 in lung cancer cell lines
H1975, H23, A549 and H1299 was also significantly decreased.
Targeted researches on tumors are usually conducted
by examining the effects of drugs or targeted genes on the
proliferation, invasion and migration of tumor cells. lncRNA FER1L4
can inhibit the proliferation, invasion and migration of various
tumor cells, such as liver cancer cells, glioma cells, gastric
cancer cells and endometrial cancer cells (12,25,26).
However, the effect of FER1L4 on NSCLC cells is unknown. Therefore,
A549 cells were selected in the present study to further detect the
effects of FER1L4 on cell proliferation, invasion and migration.
The results revealed that overexpression of lncRNA FER1L4 resulted
in decreased proliferation, invasion and migration. This suggests
that FER1L4 inhibits the proliferation, invasion and migration of
NSCLC cells.
Qiao et al demonstrated that promotion of the
expression of PTEN and inhibition of the phosphorylation of AKT
signaling pathways inhibited proliferation and migration of
endometrial cells (26). FER1L4 was
also revealed to regulate the PI3K/AKT signaling pathway to
suppress the occurrence and development of liver cancer (8,27,28).
In addition, lncRNA FER1L4 inhibited the progression of
osteosarcoma cells by targeting miR-18a-5p to release the
expression of PTEN (15). Our
previous experiments confirmed that lncRNA FER1L4 could inhibit
cell proliferation, invasion and migration, but whether it
functions through the PTEN/AKT signaling pathway remains to be
further studied. Therefore, the expression levels of proteins PTEN,
p-AKT and AKT were detected in NSCLC cells and it was revealed that
the overexpression of lncRNA FER1L4 significantly increased the
expression of PTEN protein and decreased the expression of p-AKT.
It was thus concluded that overexpression of lncRNA FER1L4
activated PTEN and inhibited the phosphorylation of AKT, thereby
inhibiting cell proliferation, invasion and migration.
Notably, the AKT signaling pathway plays an
important role in cell proliferation and apoptosis (29). Furthermore, PTEN was revealed to
regulate and promote apoptosis by mediating the AKT pathway
(30). Therefore, the effect of
FER1L4 overexpression on cell apoptosis was also investigated in
the present study. The results revealed that overexpression of
lncRNA FER1L4 significantly decreased the cell apoptosis rate and
Bcl-2 protein expression, while BAX, cleaved caspase-3, cleaved
caspase-9 protein expression was increased. After the addition of
PTEN inhibitor SF1670, the opposite results were obtained. It is
well known that p53 is a tumor suppressor gene with the highest
tumor suppressor rate in humans, and the apoptotic process is
regulated by p53 and other apoptosis-promoting factors (31). Based on this fact, the expression of
p53 protein was also assessed, and the results were consistent with
the results of pro-apoptotic proteins, BAX, cleaved caspase-3 and
cleaved caspase-9. In addition, in a study by Gao et al
(32) the role of FER1L4 in lung
cancer was examined. Their study revealed that FER1L4 inhibited
cell proliferation and metastasis by inhibiting the PI3K/AKT
signaling pathway in lung cancer, which was consistent with our
experimental results. Therefore, we can preliminarily conclude that
overexpression of lncRNA FER1L4 may promote the apoptosis of lung
cancer cells by the PTEN/AKT/p53 pathway.
In summary it was revealed that the expression of
lncRNA FER1L4 was significantly reduced in the plasma of patients
with NSCLC and lung cancer cells, and FER1L4 inhibited the
proliferation of lung cancer cells and promoted the apoptosis of
lung cancer cells through the PTEN/AKT/p53 signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the study. YS and LO drafted the
article. LO was responsible for revising the article. MY and XW
analyzed the data. JF, XL and YZ consulted literature and helped
conduct the experiments. All authors read and approved the
manuscript and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Northern Jiangsu People's Hospital, and all patients
provided written informed consent. All of the procedures were in
compliance with The Declaration of Helsinki and relevant policies
in China.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of lung cancer. Surg Oncol Clin N Am. 25:439–445.
2016. View Article : Google Scholar
|
|
2
|
Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou
XN, Chen R, Gu XY, Wei WW and He J: Report of cancer epidemiology
in China, 2015. Zhonghua Zhong Liu Za Zhi. 41:19–28. 2019.(In
Chinese).
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
4
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Ares LP: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar
|
|
5
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar
|
|
6
|
Xia T, Chen S, Jiang Z, Shao Y, Jiang X,
Li P, Xiao B and Guo J: Long noncoding RNA FER1L4 suppresses cancer
cell growth by acting as a competing endogenous RNA and regulating
PTEN expression. Sci Rep. 5:134452015. View Article : Google Scholar
|
|
7
|
Meng Q, Ren M, Li Y and Song X:
LncRNA-RMRP acts as an oncogene in lung cancer. PLoS One.
11:e01648452016. View Article : Google Scholar
|
|
8
|
Guo F, Cao Z, Guo H and Li S: The action
mechanism of lncRNA-HOTAIR on the drug resistance of non-small cell
lung cancer by regulating wnt signaling pathway. Exp Ther Med.
15:4885–4889. 2018.
|
|
9
|
Wang F, Chen JG, Wang LL, Yan ZZ, Chen SP
and Wang XG: Up-Regulation of LINC00346 inhibits proliferation of
non-small cell lung cancer cells through mediating JAK-STAT3
signaling pathway. Eur Rev Med Pharmacol Sci. 21:5135–5142.
2017.
|
|
10
|
Zhang E, Li W, Yin D, De W, Zhu L, Sun S
and Han L: C-Myc-Regulated long non-coding RNA H19 indicates a poor
prognosis and affects cell proliferation in non-small-cell lung
cancer. Tumour Biol. 37:4007–4015. 2016. View Article : Google Scholar
|
|
11
|
Wu J, Huang J, Wang W, Xu J, Yin M, Cheng
N and Yin J: Long non-coding RNA fer-1-like protein 4 acts as a
tumor suppressor via miR-106a-5p and predicts good prognosis in
hepatocellular carcinoma. Cancer Biomark. 20:55–65. 2017.
View Article : Google Scholar
|
|
12
|
Wang X, Dong K, Jin Q, Ma Y, Yin S and
Wang S: Upregulation of lncRNA FER1L4 suppresses the proliferation
and migration of the hepatocellular carcinoma via regulating
PI3K/AKT signal pathway. J Cell Biochem. 120:6781–6788. 2019.
View Article : Google Scholar
|
|
13
|
Yue B, Sun B, Liu C, Zhao S, Zhang D, Yu F
and Yan D: Long non-coding RNA fer-1-like protein 4 suppresses
oncogenesis and exhibits prognostic value by associating with
miR-106a-5p in colon cancer. Cancer Sci. 106:1323–1332. 2015.
View Article : Google Scholar
|
|
14
|
Liu Z, Shao Y, Tan L, Shi H, Chen S and
Guo J: Clinical significance of the low expression of FER1L4 in
gastric cancer patients. Tumour Biol. 35:9613–9617. 2014.
View Article : Google Scholar
|
|
15
|
Fei D, Zhang X, Liu J, Tan L, Xing J, Zhao
D and Zhang Y: Long noncoding RNA FER1L4 suppresses tumorigenesis
by regulating the expression of PTEN targeting miR-18a-5p in
osteosarcoma. Cell Physiol Biochem. 51:1364–1375. 2018. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Wu J, Li L, Wang Y, Ren X, Lin K and He Y:
The HSP90/AKT pathway may mediate artemether-induced apoptosis of
Cal27 cells. FEBS Open Bio. 9:1726–1733. 2019. View Article : Google Scholar
|
|
18
|
Zhang LX, Ding F, Wang CQ, Bing Q, Zhao Z,
Wang J and Zhang L: MiR-181a affects myocardial
ischemia-reperfusion injury in rats via regulating akt signaling
pathway. Eur Rev Med Pharmacol Sci. 23:6292–6298. 2019.
|
|
19
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar
|
|
20
|
Li Y, Wang Z, Shi H, Li H, Li L, Fang R,
Cai X, Liu B, Zhang X and Ye L: HBXIP and LSD1 scaffolded by lncrna
hotair mediate transcriptional activation by c-myc. Cancer Res.
76:293–304. 2016. View Article : Google Scholar
|
|
21
|
Qi F, Liu X, Wu H, Yu X, Wei C, Huang X,
Ji G, Nie F and Wang K: Long noncoding AGAP2-AS1 is activated by
SP1 and promotes cell proliferation and invasion in gastric cancer.
J Hematol Oncol. 10:482017. View Article : Google Scholar
|
|
22
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar
|
|
23
|
Pan C, Yao G, Liu B, Ma T, Xia Y, Wei K,
Wang J, Xu J, Chen L and Chen Y: Long noncoding RNA FAL1 promotes
cell proliferation, invasion and epithelial-mesenchymal transition
through the PTEN/AKT signaling axis in non-small cell lung cancer.
Cell Physiol Biochem. 43:339–352. 2017. View Article : Google Scholar
|
|
24
|
Lu Q, Shan S, Li Y, Zhu D, Jin W and Ren
T: Long noncoding RNA SNHG1 promotes non-small cell lung cancer
progression by up-regulating MTDH via sponging miR-145-5p. FASEB J.
32:3957–3967. 2018. View Article : Google Scholar
|
|
25
|
Xia L, Nie D, Wang G, Sun C and Chen G:
FER1L4/miR-372/E2F1 works as a ceRNA system to regulate the
proliferation and cell cycle of glioma cells. J Cell Mol Med.
23:3224–3233. 2019. View Article : Google Scholar
|
|
26
|
Qiao Q and Li H: LncRNA FER1L4 suppresses
cancer cell proliferation and cycle by regulating PTEN expression
in endometrial carcinoma. Biochem Biophys Res Commun. 478:507–512.
2016. View Article : Google Scholar
|
|
27
|
Ma L, Zhang L, Guo A, Liu LC, Yu F, Diao
N, Xu C and Wang D: Overexpression of FER1L4 promotes the apoptosis
and suppresses epithelial-mesenchymal transition and stemness
markers via activating PI3K/AKT signaling pathway in osteosarcoma
cells. Pathol Res Pract. 215:1524122019. View Article : Google Scholar
|
|
28
|
Sun X, Zheng G, Li C and Liu C: Long
noncoding RNA fer1like family member 4 suppresses hepatocellular
carcinoma cell proliferation by regulating PTEN in vitro and
in vivo. Mol Med Rep. 19:685–692. 2019.
|
|
29
|
Wang Z and Li C, Li Y, Guo X, Yan Z, Gao F
and Li C: DpdtbA-Induced growth inhibition in human esophageal
cancer cells involved inactivation of the p53/EGFR/AKT pathway.
Oxid Med Cell Longev. 2019:54146702019. View Article : Google Scholar
|
|
30
|
Han Z, Chen F, Ge X, Tan J, Lei P and
Zhang J: MiR-21 alleviated apoptosis of cortical neurons through
promoting PTEN-Akt signaling pathway in vitro after experimental
traumatic brain injury. Brain Res. 1582:12–20. 2014. View Article : Google Scholar
|
|
31
|
Chen J: The cell-cycle arrest and
apoptotic functions of p53 in tumor initiation and progression.
Cold Spring Harb Perspect Med. 6:a0261042016. View Article : Google Scholar
|
|
32
|
Gao X, Wang N, Wu S, Cui H, An X and Yang
Y: Long noncoding RNA FER1L4 inhibits cell proliferation and
metastasis through regulation of the PI3K/AKT signaling pathway in
lung cancer cells. Mol Med Rep. 20:182–190. 2019.
|