Introduction
Liver cancer (LC) is an aggressive disease with high
mortality rate (1). Despite the
considerable efforts made in recent years to increase the
therapeutic arsenal against LC, an efficient cure for advanced LC
continues to be an unmet medical need. In fact, prognosis for
patients with advanced LC remains markedly poor, with a mean
survival estimated between 6 and 20 months (2–5). The
limited response to therapies observed in LC patients, is mainly
due to the resistance of tumor cells to chemotherapeutic substances
(6). Sorafenib, a multiple kinase
inhibitor, FDA-approved since 2007 has exhibited some limited
survival benefits (7–9). However, the majority of LC patients do
not respond to sorafenib and most of initially responsive patients,
subsequently become refractory to this agent (10). A recent study demonstrated that
signaling pathways controlled by EGFR and HER-3 restrict sorafenib
effects both in naïve and sorafenib-resistant LC (11). In fact, it has been proposed that
combination of sorafenib with EGFR inhibitor gefitinib may increase
anti-proliferative response and prevent resistance in LC cellular
models.
HER-3 belongs to the ERBB receptor family, which
includes the epidermal growth factor receptor (EGFR) also known as
HER1, ERBB2/HER2/Neu, and ERBB4/HER4. These tyrosine kinase
receptors are aberrantly activated in multiple cancers and
therefore serve as drug targets and biomarkers in targeted therapy
(12). The therapeutic potential of
HER-3 has been underestimated for a long time, mainly due to its
low kinase activity; however, a large body of evidence has been
collected in recent years revealing a prime role for this receptor
in modulating the sensitivity of targeted therapeutics in several
cancers.
In fact, compensatory upregulation of HER-3
expression and downstream phosphatidylinositol 3-kinase (PI3K)/AKT
signaling is considered as one of the most common mechanisms used
by tumor cells to evade the blockade promoted by targeted therapy
(as gefitinib in lung cancer, PI3K inhibitors in breast cancer,
RAF/MEK inhibitors in melanoma) (12–14).
Moreover, HER-3 somatic oncogenic mutations have been described in
a significant proportion of gastric and colon cancer patients
(15). Therefore, a considerable
effort has recently been made on the development of drugs able to
block the activity of this receptor. In particular, several naked
antibodies have been tested in clinical stages both as mono-therapy
and in combination with several approved anticancer drugs (13,16).
However, results from these studies were not satisfactory.
Recently, the use of antibody-drug conjugates (ADCs)
has emerged as an efficient therapeutic approach to target
HER-3-positive tumor cells. ADCs are an attracting class of novel
anticancer agents in the field of precision oncology, which
preclinical and clinical development has been of increased interest
for the treatment of several tumors, including LC (17–19).
We have previously provided evidence that
EV20/monomethyl auristatin F (MMAF), an ADC generated by coupling
the HER3 targeting antibody EV20 (20–23) to
MMAF via a non-cleavable maleimidocaproyl linker possesses potent
and specific therapeutic activity in melanoma (24) and breast carcinoma (25).
In the present study, we developed a novel
anti-HER-3 targeting ADC [named EV20-sss-valine-citrulline
(vc)/MMAF] by site-specific conjugation of an engineered variant of
EV20 to MMAF via a vc cleavable linker. The antitumor efficacy of
EV20-sss-vc/MMAF was investigated using in vitro and in
vivo approaches.
Materials and methods
Reagents
Antibodies used in the present study were as
follows: phosphorylated (p)-ErbB-3 (Tyr1289; clone 21D3; product
no. 4791), ErbB-3 (clone D22C5; product no. 12708), p-EGFR
(Tyr1068; clone D7A5; product no. 3777), EGFR (clone D38B1; product
no. 4267), GAPDH (clone D16H11; product no. 5174), p-Akt (Ser473;
clone D9E; product no. 4060), Akt (product no. 9272), p-Erk1/2
(Thr202/Tyr204; clone D.13.14.4E; product no. 4370), Erk1/2 (clone
137F5; product no. 4695), all from Cell Signaling Technology, Inc.;
and anti-β-actin (product no. A5441) was purchased from
Sigma-Aldrich; Merck KGaA. Neuregulin-1β (NRG-1β; product no.
5218SC) was purchased Cell Signaling Technology, Inc. Recombinant
human EGF (cat. no. AF-100-15) was purchased from ProSpec-Tany
TechnoGene Ltd. EV20 antibody was produced as previously described
(22,23). Free MMAF (cat. no. HY-15579A) and
sorafenib (cat. no. HY-10201) were purchased from MedChemExpress.
The multidrug resistance-associated protein inhibitors PSC833 (CAS
no. 121584-18-7; product no. SML0572), Reversan (CAS no.
313397-13-6; product no. SML0173) and MK571 (CAS no. 115104-28-4;
product no. MK-571), used in combination with free MMAF in the MTT
assay, were purchased from Sigma-Aldrich; Merck KGaA. T-DM1 was
kindly provided by Professor Atanasio Pandiella from Centro De
Investigaciòn del Càncer (Barcelona, Spain).
Cell lines
A375m human melanoma cell line (CRL3223) and SJSA-1
human osteosarcoma cell line (CRL2098) were purchased from ATCC.
Liver cancer cell lines (HepG2, Hep3B, HuH7, SNU449, and PLC/PRF/5)
were kindly provided by Dr Dituri Francesco from the National
Institute of Gastroenterology ‘S. de Bellis’ Research Hospital
(Castellana Grotte, Bari, Italy). HepG2 cells were authenticated by
ATCC using Short Tandem Repeat (STR) DNA analysis. HepG2SR cells
were obtained from culturing HepG2 parental cells in the presence
of increasing doses of sorafenib up to a final concentration of 2
µM. All cell lines were cultured less than 3 months after
resuscitation. The cells were cultured according to manufacturer's
instructions, using EMEM for HepG2, Hep3B and PLC/PRF/5 cells, DMEM
for HuH7 and A375m cells, and RPMI-1640 medium (all from Thermo
Fisher Scientific, Inc.) for SNU449 and SJSA-1 cells, supplemented
with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.), L-glutamine, 100 U/ml penicillin,
and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA), and
incubated at 37°C in humidified air with 5% CO2.
Generation of EV20-based antibody-drug
conjugates
EV20/MMAF was generated by Levena Biopharma
(http://www.levenabiopharma.com/) as
previously described (24,25). For site-specific conjugation, EV20
was engineered to EV20-sss as previously reported (26). Briefly, the cysteine residues of the
heavy chain in positions 220, 226 and 229 were mutated into serine.
EV20-sss-vc/MMAF was obtained as follows: EV20-sss was reduced
using 60 M excess of Tris(2-carboxyethyl)phosphine (TCEP; Thermo
Fisher Scientific, Inc.) in phosphate-buffered saline (PBS;
Sigma-Aldrich; Meck KGaA), pH 7.4. The reaction was carried out
overnight at room temperature. The reaction was stopped by passing
the EV20-sss/TCEP mixture through a PD10 column (Cytiva)
equilibrated in PBS, pH 7.4. The reduced antibody was then reacted
with 10 M excess of MMAFvc in PBS overnight at room temperature.
The reaction was stopped by adding 500-fold molar excess
iodoacetamide (Sigma-Aldrich; Merck KGaA). To eliminate unreacted
free auristatin, the reaction mixture was passed through a G25
Sephadex column equilibrated in PBS/5% sucrose/10% DMA in an
isocratic way with a flow rate of 1 ml/min. The final concentration
of the ADCs was estimated by UV–VIS spectrophotometry, using an
extinction coefficient ε280 = 1.5 M−1 cm−1.
The auristatin MMAFvc was provided by Levena Biopharma (http://www.levenabiopharma.com/). To evaluate the
drug antibody ratio (DAR), hydrophobic chromatography (HIC)-HPLC
was performed on conjugated and unconjugated antibody sample,
dialyzed in solution containing 1.5 ammonium sulphate, 50 mM sodium
phosphate, isopropanol 5%, pH 7. Subsequently, both samples (0.3
mg/ml) were analyzed in HIC-HPLC (Sol.A: 1.5 ammonium sulphate, 50
mM sodium phosphate, isopropanol 5%, pH 7; Sol.B: 50 mM sodium
phosphate, isopropanol 20%, pH 7) with gradient 0-100% Sol.B in 20
min, flow rate 1 ml/min.
Flow cytometric analysis
For HER receptors surface expression analysis, flow
cytometry was performed as follows. Approximately one million
growing cells were harvested and labeled with 1 µg/ml of primary
antibody for 30 min on ice. For EGFR, HER-2 and HER-3 staining,
primary antibodies used were chimeric anti-EGFR cetuximab (cat. no.
A2000), humanized anti-HER-2 trastuzumab (cat. no. A2007), both
purchased from Selleck Chemicals, and EV20 (humanized anti-HER-3,
developed in our laboratory (Mediapharma srl, University of
Chieti-Pescara, Chieti, Italy) (22,23).
All primary antibodies were used at the dilution of 1
µg/1×106 cells. After washing, the cells were labelled
with PE-conjugated goat anti-Human Fc as secondary antibody at a
dilution of 1:300 (cat. no. H10104; Molecular Probes; Life
Technologies; Thermo Fisher Scientific, Inc.) for 30 min on ice.
Regarding HER-4 analysis, cells were fixed with 1% paraformaldehyde
for 15 min at room temperature and permeabilized with 0,1% Triton
X-100 for 5 min at room temperature, then stained for 30 min on ice
with 1 µg/ml of anti-HER4 antibody (cat. no. MA1-861; Invitrogen;
Thermo Fisher Scientific, Inc.) as a primary antibody followed by
staining for 30 min on ice with goat anti-mouse IgG Alexa-Fluor
488-conjugated at a dilution of 1:300 (cat. no. A11001; Molecular
Probes, Life Technologies; Thermo Fisher Scientific, Inc.). For the
in vivo binding assay of EV20 and EV20-based ADCs, A375m
cells were detached and labelled with 10 µg/ml of EV20 and
EV20-sss-vc/MMAF for 30 min on ice, followed by staining for 30 min
on ice with PE-conjugate goat anti-Human Fc as a secondary antibody
at a dilution of 1:300 (cat. no. H10104). Analysis was performed
using FACSCantoII cytometer (BD Biosciences). Data were analyzed
with FlowJo software V10.7 (FlowJo, LLC).
Cytotoxicity assays
Cell proliferation was assessed by
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck KGaA). Cell lines were seeded into
24-well plates at a density ranging between 4×103 cells/well and
7×103 cells/well in 500 µl of complete culture medium.
Then, cells were treated with drugs at indicated concentrations in
triplicates and further incubated for 120 h. At the end of the
incubation period, cells were incubated with 200 µl of MTT solution
(serum-free medium with 0.5 mg/ml of MTT) for a further 2 h. After
removal of MTT solution, 200 µl of dimethyl sulfoxide (DMSO) was
added to the wells for 10 min and the absorption value at 570 nm
was measured using a multi-plate reader. All experiments were
performed in triplicate and the IC50 values were
calculated using GraphPad Prism 5.0 software (GraphPad Software,
Inc.).
Western Blotting
Lysates from cells in culture were prepared by
washing cells twice in cold PBS followed by lysis with RIPA Buffer
(50 mM Tris-HCl, 1% NP-40, 0.1% SDS, 150 mM NaCl) supplemented with
protease and phosphatase inhibitors (Sigma-Aldrich: Merck KGaA) for
10 min at 4°C. Insoluble materials were removed by centrifugation
(16,000 × g for 10 min at 4°C) and protein concentration was
assessed using a Bradford assay. Equal amounts of protein (30 µg)
were separated by SDS/PAGE on 10% polyacrylamide gel and
transferred to nitrocellulose membranes. Membranes were blocked
with 5% non-fat dry milk in PBS containing 0.1% Tween-20 for 1 h at
room temperature and incubated with following primary antibodies:
p-ErbB-3, ErbB-3, p-EGFR, EGFR, p-Akt, Akt, p-Erk1/2, Erk1/2, all
from Cell Signaling Technology, Inc. and anti-β-actin from
Sigma-Aldrich; Merck KGaA, as aforementioned. All the antibodies
were used at a dilution of 1:1,000 in PBS containing 0.1% Tween-20
overnight at 4°C, except for anti-β-actin, which was used at a
dilution of 1:40,000 in PBS containing 0.1% Tween-20. After
washing, the membranes were hybridized for 1 h at room temperature
with horseradish peroxidase-conjugated secondary antibodies at a
dilution of 1:20,000 [(HRP-conjugated goat anti-mouse IgG; product
code STAR207P) and (HRP-conjugated goat anti-rabbit IgG; product
code STAR208P; both purchased from Bio-Rad Laboratories, Inc.].
Detection was performed with Plus-ECL chemiluminescence kit
(Bio-Rad Laboratories, Inc.). Densitometric analysis of bands was
performed using ImageJ software V1.53 (National Institutes of
Health).
HER-3 expression in tumor and
peritumor samples
For the evaluation of HER-3 protein expression in
human specimens, tumor and peritumor samples, as well as normal
liver samples, were collected, snap-frozen, and analyzed by western
blotting. Male (n=9) and female (n=2) patients aged between 48 and
80 years were included. Samples were collected at the Department of
Emergencies and Organ Transplant of the Policlinic Hospital of Bari
(Bari, Italy), between September 2016 and September 2017. All
patients provided written consent for the use of their specimens
for research purposes; none were identifiable. Frozen specimens of
tissues were homogenized with a Polytron homogenizer in a lysis
buffer T-PER Tissue Protein Extraction (cat. no. 78510; Thermo
Fisher Scientific, Inc.) supplemented with proteinases/phosphatases
inhibitor cocktail (cat. no. 1861280; Thermo Fisher Scientific,
Inc.). The homogenates were then centrifuged at 16,000 × g for 10
min at 4°C, and the protein concentration was determined using a
Bio-Rad assay kit according to the manufacturer's instructions
(cat. no. 131947; Bio-Rad Laboratories, Inc.). Equal amounts of
proteins (20 µg) were separated by SDS/PAGE on 10% polyacrylamide
gel and transferred to a nitrocellulose membrane. The membrane was
probed overnight at 4°C with the HER-3 (product no. 12708) and
GAPDH (product no. 5174; both 1:1000; both from Cell Signaling
Technology, Inc.) antibodies, washed with TBS-T (TBS 1X + 0.05%
Tween-20), and then incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibody
(HRP-conjugated goat anti-rabbit IgG; cat. no. STAR208P; Bio-Rad
Laboratories, Inc.) at the dilution of 1:20,000 in TBB buffer
(TBS-T + 5% nonfat milk). Detection was performed with Clarity Max
Western ECL Substrate (Bio-Rad Laboratories, Inc.). This study was
approved by the Local Ethics Committee, Azienda Ospedaliero
Universitaria Consorziale Policlinico di Bari (Bari, Italy);
protocol no. 254; date of release, February 2012.
Internalization assays
For flow cytometric quantification, A375m and HepG2
cells were plated in 60 mm plates and grown in DMEM containing 10%
FBS, for 24 h. Cells were then incubated with 10 µg/ml of EV20 in
complete medium on ice for 30 min before returning the plates in
the incubator at 37°C for 1 h, maintaining control cells on ice in
the presence of the antibody. Finally, the cells were detached and
stained with PE-conjugate goat anti-Human Fc at a dilution of 1:300
(cat. no. H10104) for 30 min on ice. Analysis was performed using
FACSCantoII cytometer (BD Biosciences). Data were analyzed with
FlowJo software V10.7 (FlowJo, LLC).
For confocal microscopy, A375m and HepG2 cells were
seeded on round cover slips in 12-well plates to 70% confluence in
complete medium for 24 h. Cells were then incubated with 10 µg/ml
of EV20 in complete medium on ice for 30 min, after which they were
then incubated again at 37°C for 2 h. The antibody was washed away
and the cells were fixed for 15 min at room temperature with 4%
paraformaldehyde (pH 7.4). Cells were then permeabilized for 5 min
at room temperature with 0.5% Triton X-100 and labeled with goat
anti-human IgG Alexa-Fluor 488-conjugated at a dilution of 1:200
(cat. no. A11013; Molecular Probes, Life Technologies; Thermo
Fisher Scientific, Inc.) and Draq5 (product no. 4084; Cell
Signaling Technologies, Inc.) to visualize nuclei. Images were
acquired at a magnification of ×63 with a Zeiss LSM 510
meta-confocal microscope (Zeiss AG) using 488- and 633-nm
lasers.
ELISA
Recombinant HER-3 extracellular domain (ECD) (cat.
no. ER3-H5223; AcroBiosystems) (1 µg/ml) was pre-coated overnight
at 4°C on 96 well-plates NUNC Maxisorp modules. After blocking with
1% BSA in PBS for 1 h at room temperature, increasing
concentrations (ranging between 0.05 nM and 6.6 nM) of EV20-sss or
EV20-sss-vc/MMAF were incubated for 1 h at room temperature. After
several washes with PBS + 0,05% Tween-20, a goat anti-human IgG-HRP
solution at a dilution of 1:5,000 (product no. A0170;
Sigma-Aldrich; Merck KGaA) was added to each well and incubated for
1 h at room temperature. After washing, stabilized chromogen was
added to each well for at least 10 min in the dark, then the
reaction was stopped with the addition of
H2SO4 1N and the absorbance was read at 450
nm with an ELISA reader.
Animal studies
Homozygous Balb/c nu/nu athymic female mice
(4-6-weeks old) were purchased from Charles River Laboratories and
maintained at 22-24°C and relative humidity (40-60%) under
pathogen-limiting conditions as required. Cages, bedding, and food
were autoclaved before use. Mice were provided with a standard diet
and water ad libitum and acclimatized for 2 weeks before the
start of the experiments. Housing and all procedures involving the
mice were performed according to the protocol approved by the
Institutional Animal Care and Use Committee of the Italian Ministry
of Health (authorization no. 292/2017-PR).
Five million of exponentially growing HepG2 cells
were implanted subcutaneously (s.c.) into the right flank of the
mice in a ratio of 1:6 with Matrigel (Cultrex Basement Membrane
Matrix; cat. no. 3432-001-01; Trevigen, Inc.). When tumors became
palpable (approximately 150 mm3), animals were randomly
divided into two groups and treated intravenously via tail vein
with vehicle (PBS, once a week for a total of 4 injections), or
EV20-sss-vc/MMAF (10 mg/kg, once a week for a total of 4
injections), respectively. The tumor volume was monitored weekly by
a caliper and calculated using the following formula: tumor volume
(mm3) = (length × width2)/2. A tumor volume
of 1.5 cm3 was selected as the endpoint for all
experiments after which mice were sacrificed using CO2
inhalation (20-70%).
Statistical analysis
For in vivo xenograft curves and HER-3
expression level in tumor tissues, P-values were determined by a
paired Student's t-test and considered significant for P<0.05.
Statistical analysis of PI3K/AKT activation was performed using
one-way ANOVA, followed by Tukey's multiple comparisons test.
Experimental sample numbers (n) are indicated in the figure
legends. All statistical analysis was performed with GraphPad Prism
5.0 software (GraphPad Software, Inc.). Survival curves were
evaluated by Kaplan-Meier and analyzed by the log-rank test with
GraphPad Prism 5.0 software (GraphPad Software, Inc.).
Results
HER expression and signaling pathway
in human LC
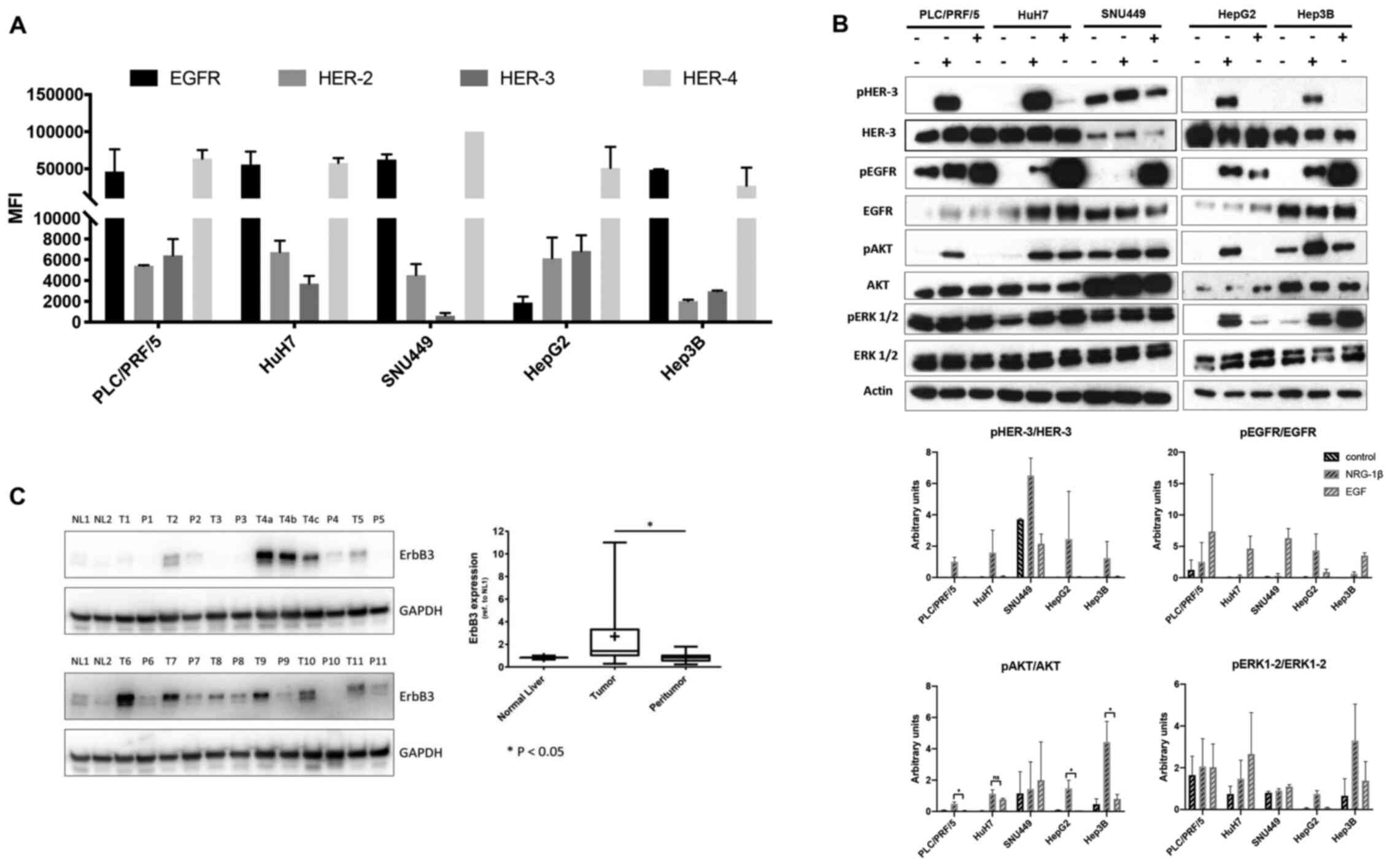
A panel of human LC cell lines for surface
expression of HER-3 and its preferred partner HER-2 were screened
by flow cytometry. The HER-3 and HER-2 receptors were expressed in
all the LC cell lines assessed except for SNU449 cells, where HER-3
expression was found to be markedly low (Fig. 1A and B). Next, whether the
downstream signalling of HER-3 was activated in these cells was
evaluated. To this end, western blot analysis was performed of
lysates prepared from this panel of LC cells stimulated with either
HER-3 ligand neuregulin1b (NRG-1b) or epidermal growth factor
(EGF), the ligand for the other member of the receptor family,
EGFR. Notably, despite the fact that EGFR was highly expressed in
this panel of cell lines (Fig. 1A),
NRG-1b activated the PI3K/AKT the survival signaling pathway more
potently than EGF (Fig. 1B). ERK
activation was more pronounced upon NRG-1b stimulation in HepG2
cells. By contrast, a stronger activation upon EGF stimulation was
observed in HuH7 and Hep3B cells, while PLC/PRF/5 and SNU449
exhibited high basal, but no inducible ERK phosphorylation
(Fig. 1B).
Next, HER-3 receptor expression levels were
evaluated by western blotting in 11 tumoral and corresponding
peritumoral LC tissues. HER-3 expression was revealed to be
significantly higher in tumoral than corresponding peritumoral
tissues. Notably, HER-3 expression was barely detectable in normal
liver tissues (Fig. 1C). All
together these data indicated that HER-3 receptor was expressed in
LC and that the NRG-1b/HER-3/Akt signalling axis was activated in
these cancers, thus reinforcing the hypothesis that HER-3 may
represent a suitable target for an ADC-based therapy.
Cytotoxic activity of EV20-based ADCs
in LC cell lines
The antitumor activity of EV20/MMAF, an anti-HER-3
ADC, which we have recently revealed to possess a potent and
specific therapeutic activity in melanoma and breast cancer models
(24,25) was evaluated. Surprisingly, the
activity of EV20/MMAF in LC cell lines was revealed to be
significantly lower in comparison to the activity observed in
non-LC cell lines. In fact, IC50 values ranging between
~25 and ~70 nM were observed for HuH7 and PLC/PRF/5 cells,
respectively, whereas an IC50 >100 nM was observed
for the other three cell lines assessed (Fig. 2A).
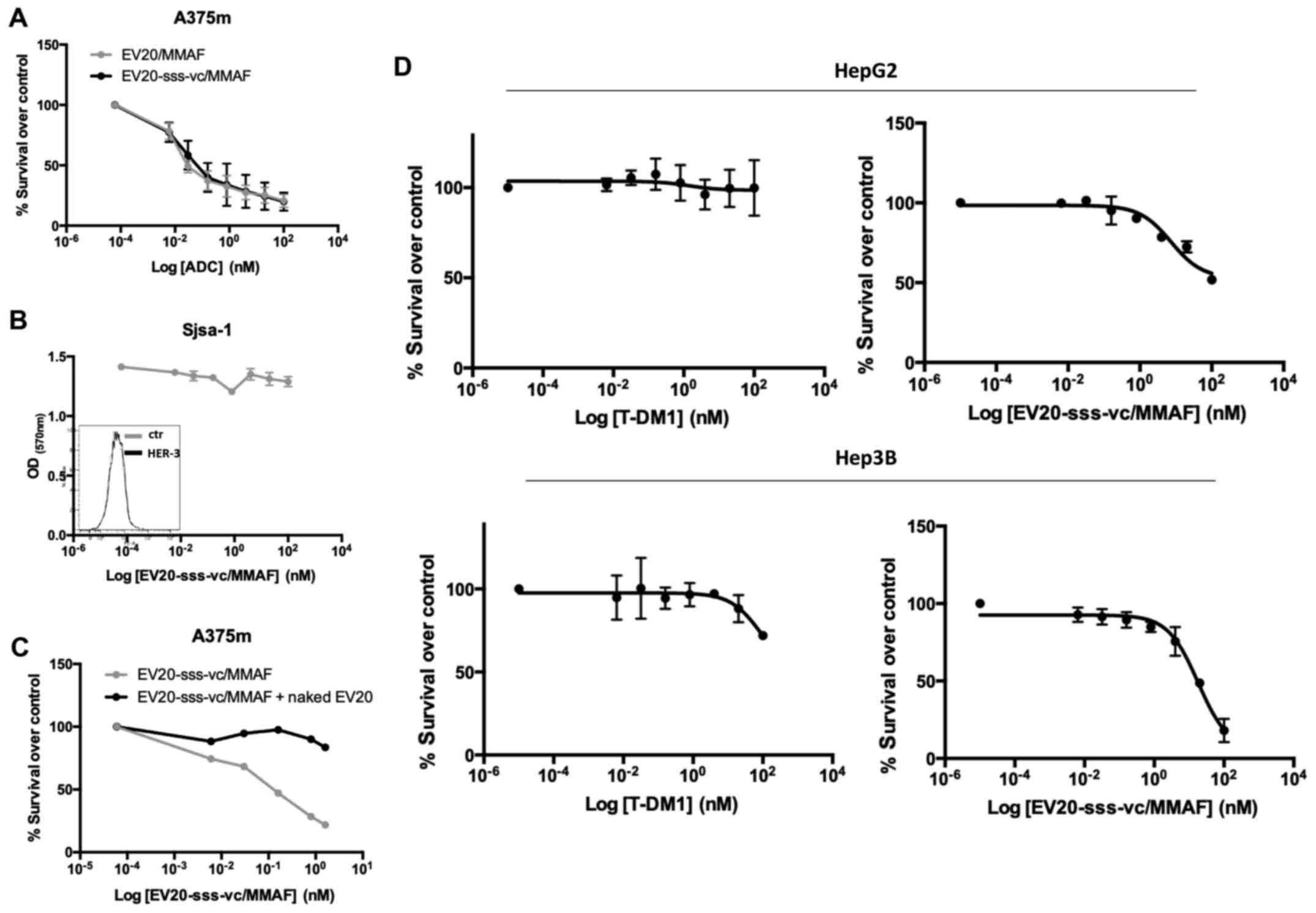
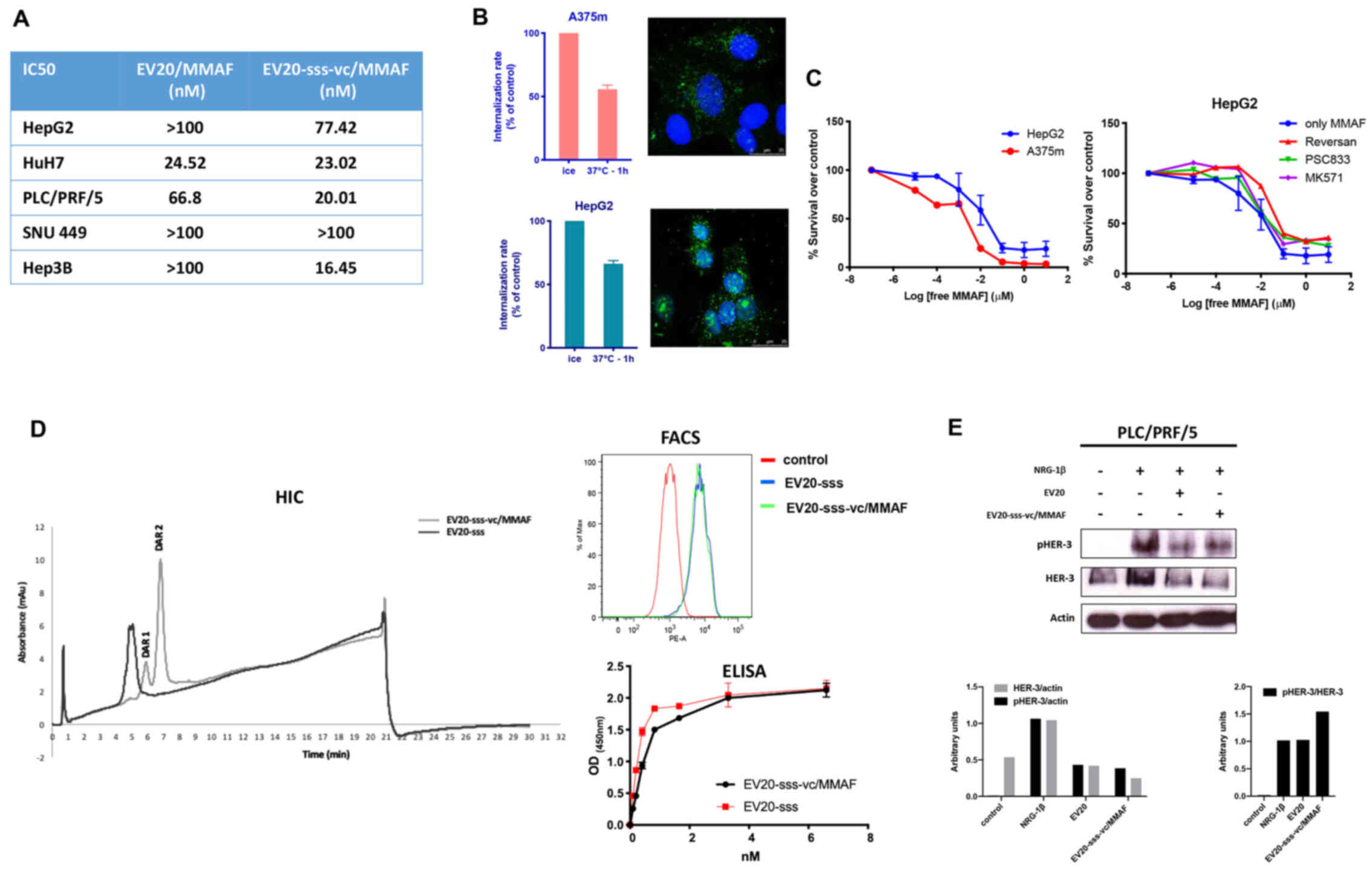 | Figure 2.In vitro antitumor activity of
EV20-based ADCs. (A) The cytotoxic response of LC cells to
EV20-based ADCs treatment was evaluated by MTT after 120 h of
treatment with increasing doses ranging between 0.006 nM and 100
nM. The IC50 values were calculated with GraphPad Prism
5.0 software and reported. (B) A375m and HepG2 cells were
maintained for 30 min on ice in the presence of 10 µg/ml EV20 and
placed again in the incubator at 37°C for 1 h. The internalization
rate of the antibody was evaluated by flow cytometry and by
confocal microscopy imaging. The histogram represents the
percentage of MFI referred to control (cells maintained on ice).
Plotted results are an average ± SD of three independent
experiments. For confocal microscopy imaging, EV20 and nuclei were
visualized on green and blue channels, respectively. (C) A375m and
HepG2 cells were incubated for 72 h with eight increasing
concentrations of free MMAF, diluted from 10 µM to 10 pM, in 1:10
dilution increments. Proliferation was evaluated by MTT assay (left
panel). HepG2 cells were incubated for 72 h with the same
increasing doses of free MMAF used in (C) in absence or presence of
MK571 (25 µM), PSC833 (3 µM) and Reversan (15 µM) and proliferation
was evaluated by MTT assay (right panel). (D) EV20-sss-vc/MMAF
characterization. HIC was used for DAR calculation; melanoma A375m
HER3+ cells were used for cell binding by flow
cytometry. ELISA was performed for in vitro binding with
naked EV20-sss mAb used as control. (E) PLC/PRF/5 LC cells were
incubated for 2 h or not with naked or conjugated EV20 mAb, at a
dose of 10 µg/ml, before NRG-1β stimulation (10 min, 10 ng/ml).
Total and phosphorylated HER-3 receptor was analysed by western
blotting and bands were quantified using actin as loading control.
Histograms represent densitometric analysis of a single experiment,
expressed as arbitrary units. MFI, mean fluorescence intensity;
ADCs, antibody-drug conjugates; LC, liver cancer; MTT,
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide; MMAF,
monomethyl auristatin F; vc, valine-citrulline; HIC, hydrophobic
chromatography; DAR, drug antibody ratio. |
To rule out the possibility that low efficacy of
EV20/MMAF in LC cells was due to the impairment of an
internalization process of the antibody/receptor complex, the EV20
internalization rate in HepG2 (LC, low responders) vs. A375m cells,
which in our previous work were revealed to be markedly sensitive
to this ADC (24), were compared.
As revealed in Fig. 2B, no
significant differences were observed between the two cell lines,
indicating that the internalization process was functional in LC
cells. Notably, HepG2 sensitivity to free MMAF was not
significantly different from that of high-responder A375m melanoma
cells (Fig. 2C, left panel) neither
was it increased by multidrug resistance-associated protein
inhibitors (such as Reversan, PSC833 and MK571 as reported in other
systems (27,28) (Fig.
2C, right panel).
To improve the ADC activity, we generated a novel
EV20-based ADC (named EV20-sss-vc/MMAF) maintaining the same
cytotoxic payload coupled to an engineered variant of EV20 (named
EV20-sss) generated by means of a cleavable linker, as described in
the method section and in our previous work (26). The results obtained are presented in
HIC in Fig. 2D, by detecting at
ε280 nm: with naked antibody (DAR =0) as a negative control,
eluting with a retention time of 5 min, and present only in the
sample of the unconjugated but not in the ADC chromatogram (0%);
the antibody conjugated with DAR =1 is present in a small
percentage (18%) (retention time 6 min); the antibody conjugated
with DAR =2 is present at 7 min (82%). Percentages were obtained by
integration of the peak area. Due to the site-specific conjugation
process, this ADC had a fixed DAR of 2 or 1 (Fig. 2D). Moreover, EV20-sss-vc/MMAF was
revealed to possess the same in vitro and cell binding
ability of that observed with the naked EV20-sss (Fig. 2D) as well as receptor downregulatory
capacity, while inhibition of ligand-induced HER-3 phosphorylation
appeared slightly reduced in conjugated vs. naked EV20 antibody
(Fig. 2E).
Notably, EV20-sss-vc/MMAF displayed higher cell
killing activity than EV20/MMAF (Fig.
2A), although the two ADCs exhibited superimposable cell
killing activity in A375m melanoma cells (Fig. 3A).
Additionally, EV20-sss-vc/MMAF cell killing activity
was revealed to be to be strictly target-dependent. This was
demonstrated by using HER-3 negative osteosarcoma SjSa-1cells, in
which no cell killing could be observed (Fig. 3B) and by a competition assay with a
500-fold molar excess of naked EV20 antibody (Fig. 3C). Finally, the HER-3 low-expressing
SNU 449 cells were revealed to be nearly insensitive to the ADC
(Fig. 2A).
As HER-2 was revealed to be highly expressed in LC
cells, the activity of EV20-sss-vc/MMAF was compared to the
activity of the clinically approved anti-HER-2 ADC (T-DM1) in HepG2
cells. Despite the cells expressing similar levels of HER-2 and
HER-3 (Fig. 1A), EV20-sss-vc/MMAF
exhibited a significantly increased cell killing activity,
suggesting this ADC was more effective in comparison to T-DM1
(Fig. 3D).
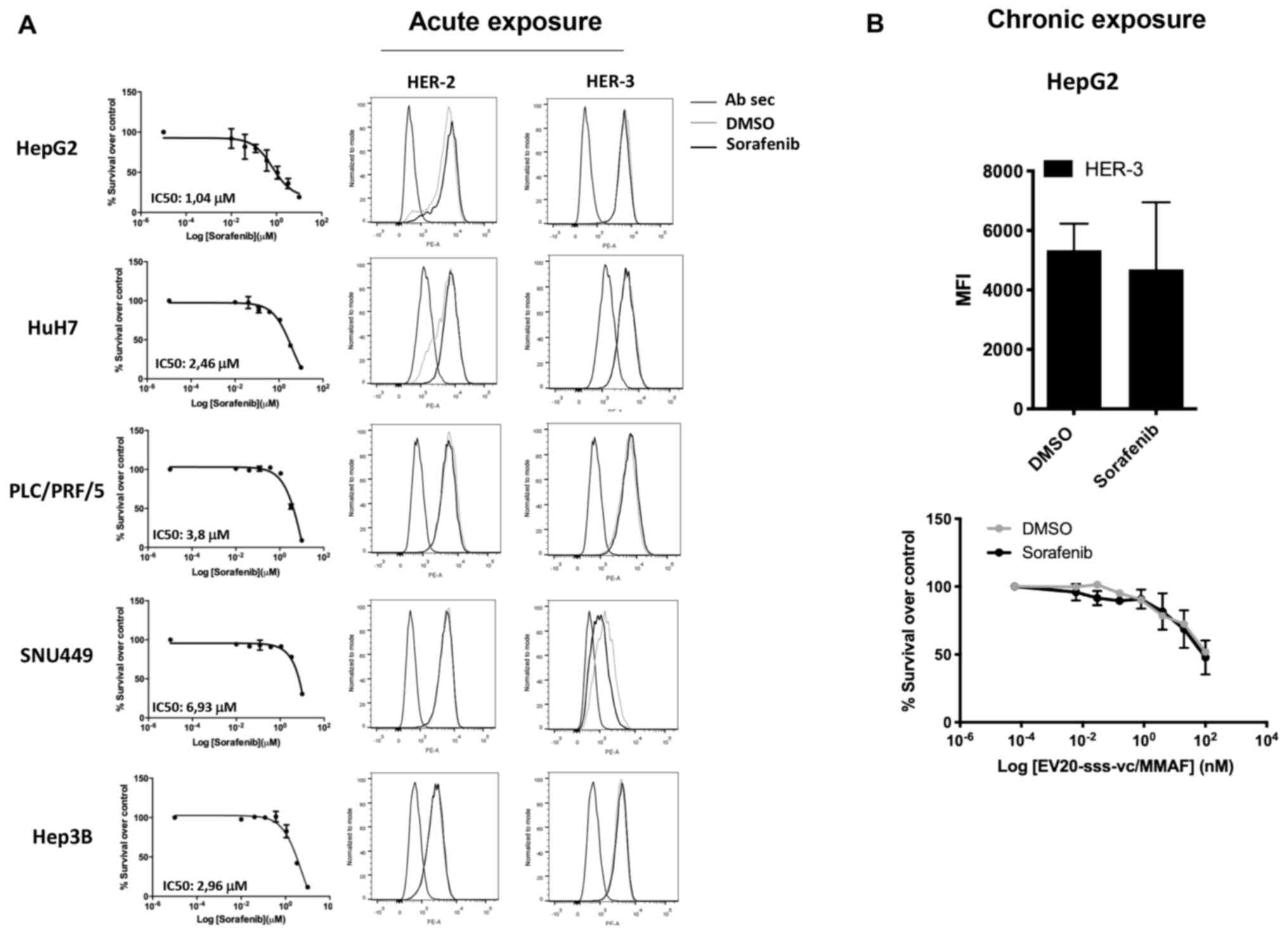
As HER-3 expression/activation is upregulated in
response to several drugs, including sorafenib (11,20,29,30),
it was investigated whether the activity of EV20-sss-vc/MMAF could
be potentiated by combination with this agent. In line with a
previous study (9), treatment of LC
cells with sorafenib induced a potent cell killing activity with an
IC50 ranging between ~7 and 1 µM (Fig. 4A, left panels). However, cell
killing activity was not associated with the upregulation of either
HER-3 or HER-2 receptors (Fig. 4A,
right panels). Similarly, no upregulation of HER-3 expression was
detected in HepG2 cells grown under chronic exposure (up to 5
months) of 2 µM of sorafenib, (Fig.
4B, upper panel) and no increase of EV20-sss-vc/MMAF cell
killing activity was observed in these cells (Fig. 4B, lower panel).
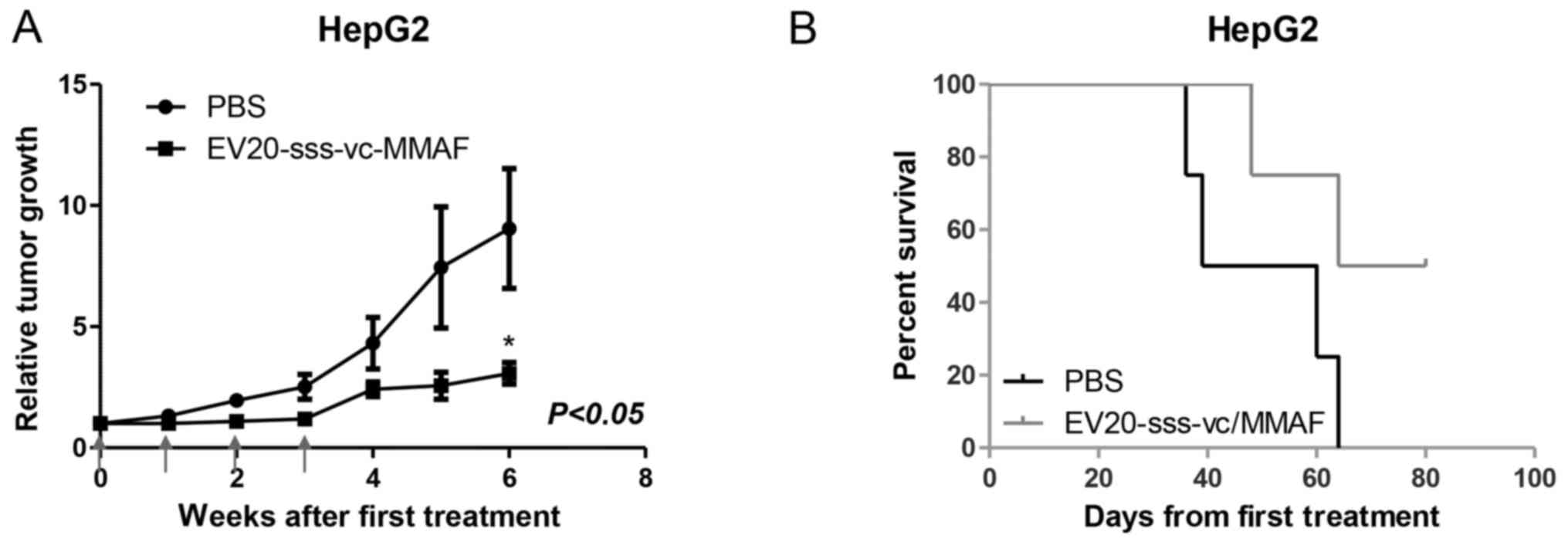
Antitumor activity in vivo
The therapeutic activity of EV20-sss-vc/MMAF ADC as
a single agent in LC-derived xenografts was evaluated in xenografts
assays. Although different methodological approaches were assessed,
only mice harbouring tumours derived from subcutaneous injection of
HepG2 were available for therapeutic study. As revealed in Fig. 5A, within 6 weeks after the start of
administration, the tumor volumes in the EV20-sss-vc/MMAF-treated
group were significantly smaller compared with the vehicle-treated
group. The growth suppression effect was accompanied by increased
survival (Fig. 5B).
Discussion
Primary LC is one of the most common types of cancer
worldwide and is currently the third leading cause of
cancer-related deaths (1). The
therapeutical agent sorafenib, a tyrosine kinase inhibitor, has a
limited effect on survival rate, leaving patients with a markedly
poor prognosis (8,9).
The number of additional treatment options has
recently increased with supplemental FDA approvals of small
molecule tyrosine kinase inhibitors (lenvatinib, regorafenib, and
cabozantinib) (31), as well as
immunotherapies such as immune check point inhibitors (nivolumab
and pembrolizumab) (32–35) and the monoclonal IgG1 antibody,
ramucirumab (36). These novel
therapeutic agents are systemically administered in patients with
advanced unresectable tumors either as a single agent or in
combination therapy, depending on decisional criteria based on the
Barcelona Clinic Liver Cancer (BCLC) staging system, Performance
Status (PS) or Child-Pugh system (37). Sorafenib and lenvatinib are used in
first-line therapy, whereas regorafenib, cabozantinib and
ramucirumab in second-line treatment regimens (31,36).
ADCs represent an emerging class of therapeutics
which potentially improve the therapeutic index of cytotoxic agents
through a selective targeting mechanism (38). Currently, nine ADCs are already
available for treatment but more than 80 different ADCs are in
clinical testing and numerous others in preclinical development
(38–40).
In the present study, it was revealed that the HER-3
receptor was highly expressed in a panel of patient tumor samples.
Moreover, the NRG-1β/HER-3/Akt signalling axis was activated in LC
cell lines, thus reinforcing our hypothesis that this receptor
represents a suitable target for an ADC. HER-3 is known to potently
induce the PI3K/Akt signaling pathway (12), therefore analysis of Akt
phosphorylation may be used as a suitable readout for receptor
activation. Surprisingly, EV20/MMAF, our previously developed ADC
with potent and durable therapeutic activity in melanoma (24) and breast carcinoma (25) displayed only a modest cell killing
activity in LC cells.
The reason for the lack of sensitivity of LC cells
to EV20/MMAF is presently unknown as these cells express HER-3 and
receptor/antibody internalization occurs to the same extent as
observed in melanoma and breast cancer cells.
We therefore generated a novel EV20-based ADC with
the same cytotoxic payload (i.e. the tubulin inhibitors MMAF) site
specifically conjugated to an engineered variant of EV20 (EV20-sss)
through a vc cleavable linker. Notably, it was revealed that cell
killing induced by EV20-sss-vc/MMAF (DAR ~2 and a cleavable linker)
was identical to that induced by EV20/MMAF (DAR ~4.5 and a
non-cleavable linker) in melanoma cells, but superior in LC cells.
This indicated that, at least in LC, the mechanism of cell killing
by EV20-sss-vc/MMAF, which is generated with a cleavable linker
occurs through cleavage of the cytotoxic payload by the lysosomal
cysteine cathepsins rather than via antigen/antibody degradation,
which typically occurs when the payload is released in ADCs
generated with a non-cleavable linker. However, cell killing
activity was not analyzed using assays which directly reflect tumor
proliferation and therefore this could have limited our
observations. Studies are ongoing to better elucidate this
important aspect. Notably, EV20-sss-vc/MMAF in vitro
antitumor activity was revealed to be higher compared to that of
the clinical approved T-DM1, although receptor expression (HER-3
and HER-2) were similar in LC cells.
It was also investigated whether HER-3 is involved
in primary or acquired resistance to sorafenib and we did not
observe HER-3 upregulation in response to acute or chronic exposure
to sorafenib in HepG2 cells. Accordingly, no increase in cell
killing activity was detected in combination treatment, i.e.
EV20-sss-vc/MMAF plus sorafenib in comparison to single agents.
Finally, the therapeutic activity of
EV20-sss-vc/MMAF at 10 mg/kg in a model of HepG2 ×enograft revealed
a significant inhibition of tumor growth rate after four doses. A
trend for increased survival in treated animals was observed,
although this was not significant possibly due to the low number of
available mice for this study.
To the best of our knowledge, thus far only another
ADC targeting LC has been implemented. This ADC, at the preclinical
stage targets glypican-3 (GPC3), a protein found on the surface of
LC cells in >70 percent of LC cases (17). The ADC, called hYP7-PC has exhibited
potency at picomolar concentrations against a panel of
GPC3-positive cancer cell lines (17).
In summary, the present study suggests HER-3 as a
potential therapeutic target in LC and fosters further development
to increase the activity of EV20-based ADC for LC therapy.
Acknowledgements
We thank Dr Annalisa Di Risio and Dr Annalisa
Nespoli (from the University ‘G. D'Annunzio’ of Chieti-Pescara) for
technical assistance. We are indebted to Dr Caroline Pellet-Many
(from Royal Veterinary College, London, UK) for English revision of
the manuscript.
Funding
This project was funded by Fondazione AIRC (Italian
Association for Cancer Research) (GS ID:18467; VDL ID: 20043). EC
is the recipient of an AIRC fellowship. The PhD program of SP is
funded by the Italian Ministry of Instruction, University, and
Research under the national project PON ricerca e innovazione
2014-2020.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DDA performed in vitro and in vivo
work. RG performed in vitro work and confocal imaging. SP
and GDV performed purification of the antibody, conjugation and
analytic characterization of the ADC. FD analyzed HER-3 expression
in LC tumor samples. GG revised the manuscript critically for
important intellectual content. CR supervised the in vivo
work on the subcutaneous xenografts. LM analyzed the results and
revised the manuscript critically for important intellectual
content. FG supervised ADC generation, read the manuscript and
suggested ideas. VDL analyzed the results and revised the
manuscript critically for important intellectual content. SI
provided substantial contributions to design of the work and wrote
the manuscript. RI provided substantial contributions to the
conception of the work and analyzed the results. EC performed and
supervised in vitro and in vivo work and wrote the
paper. GS conceived the study, analyzed the results and wrote the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Local Ethics
Committee, Azienda Ospedaliero Universitaria Consorziale
Policlinico di Bari (Bari, Italy); protocol no. 254; date of
release, February 2012. All patients provided written consent for
the use of their specimens for research purposes; none were
identifiable. All procedures involving the mice were performed
according to the protocol approved by the Institutional Animal Care
and Use Committee of the Italian Ministry of Health (Authorization
no. 292/2017-PR).
Patient consent for publication
Not applicable.
Competing interests
GS and SI are shareholders of Mediapharma SRL. The
other authors have no potential competing interests to
disclose.
References
|
1
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–2800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang BG, Wang N, Huang J, Yang Y, Sun LL,
Pan ZY and Zhou WP: Tumor SOCS3 methylation status predicts the
treatment response to TACE and prognosis in HCC patients.
Oncotarget. 8:28621–28627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perini MV, Starkey G, Fink MA, Fink MA,
Bhandari R, Muralidharan V, Jones R and Christophi C: From minimal
to maximal surgery in the treatment of hepatocarcinoma: A review.
World J Hepatol. 7:93–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oda K, Uto H, Mawatari S and Ido A:
Clinical features of hepatocellular carcinoma associated with
nonalcoholic fatty liver disease: A review of human studies. Clin J
Gastroenterol. 8:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizuguchi T, Kawamoto M, Meguro M, Okita
K, Ota S, Ishii M, Ueki T, Nishidate T, Kimura Y, Furuhata T, et
al: Impact of aging on morbidity and mortality after liver
resection: A systematic review and meta-analysis. Surg Today.
45:259–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo XL, Li D, Hu F, Song J, Zhang S, Deng
W, Sun K, Zhao Q, Xie X, Song Y, et al: Targeting autophagy
potentiates chemotherapy-induced apoptosis and proliferation
inhibition in hepatocarcinoma cells. Cancer Lett. 320:171–179.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abou-Alfa GK: Sorafenib use in
hepatocellular carcinoma: More questions than answers. Hepatology.
60:15–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu AX: Beyond sorafenib: Novel targeted
therapies for advanced hepatocellular carcinoma. Expert Opin
Investig Drugs. 19:663–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palmer DH: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:2498, author reply
2498–2499. 2008.
|
|
10
|
Niu L, Liu L, Yang S, Ren J, Lai PBS and
Chen GG: New insights into sorafenib resistance in hepatocellular
carcinoma: Responsible mechanisms and promising strategies. Biochim
Biophys Acta Rev Cancer. 1868:564–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blivet-Van Eggelpoel MJ, Chettouh H,
Fartoux L, Aoudjehane L, Barbu V, Rey C, Priam S, Housset C,
Rosmorduc O and Desbois-Mouthon C: Epidermal growth factor receptor
and HER-3 restrict cell response to sorafenib in hepatocellular
carcinoma cells. J Hepatol. 57:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishra R, Patel H, Alanazi S, Yuan L and
Garrett JT: HER3 signaling and targeted therapy in cancer. Oncol
Rev. 12:3552018.PubMed/NCBI
|
|
13
|
Gaborit N, Lindzen M and Yarden Y:
Emerging anti-cancer antibodies and combination therapies targeting
HER3/ERBB3. Hum Vaccin Immunother. 12:576–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capone E, Prasetyanti PR and Sala G:
HER-3: Hub for escape mechanisms. Aging (Albany NY). 7:899–900.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaiswal BS, Kljavin NM, Stawiski EW, Chan
E, Parikh C, Durinck S, Chaudhuri S, Pujara K, Guillory J, Edgar
KA, et al: Oncogenic ERBB3 mutations in human cancers. Cancer Cell.
23:603–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aurisicchio L, Marra E, Roscilli G,
Mancini R and Ciliberto G: The promise of anti-ErbB3 monoclonals as
new cancer therapeutics. Oncotarget. 3:744–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Y, Urban DJ, Nani RR, Zhang YF, Li N,
Fu H, Shah H, Gorka AP, Guha R, Chen L, et al: Glypican-3 specific
antibody drug conjugates targeting hepatocellular carcinoma.
Hepatology. 70:563–576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagayama A, Ellisen LW, Chabner B and
Bardia A: Antibody-drug conjugates for the treatment of solid
tumors: Clinical experience and latest developments. Target Oncol.
12:719–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moek KL, de Groot DJ, de Vries EG and
Fehrmann RS: The antibody-drug conjugate target landscape across a
broad range of tumour types. Ann Oncol. 28:3083–3091. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prasetyanti PR, Capone E, Barcaroli D,
D'Agostino D, Volpe S, Benfante A, van Hooff S, Iacobelli V, Rossi
C, Iacobelli S, et al: ErbB-3 activation by NRG-1beta sustains
growth and promotes vemurafenib resistance in BRAF-V600E colon
cancer stem cells (CSCs). Oncotarget. 6:16902–16911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghasemi R, Rapposelli IG, Capone E, Rossi
C, Lattanzio R, Piantelli M, Sala G and Iacobelli S: Dual targeting
of ErbB-2/ErbB-3 results in enhanced antitumor activity in
preclinical models of pancreatic cancer. Oncogenesis. 3:e1172014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sala G, Rapposelli IG, Ghasemi R, Piccolo
E, Traini S, Capone E, Rossi C, Pelliccia A, Di Risio A, DEgidio M,
et al: EV20, a novel anti-ErbB-3 humanized antibody, promotes
ErbB-3 down-regulation and inhibits tumor growth in vivo. Transl
Oncol. 6:676–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sala G, Traini S, D'Egidio M, Vianale G,
Rossi C, Piccolo E, Lattanzio R, Piantelli M, Tinari N, Natali PG,
et al: An ErbB-3 antibody, MP-RM-1, inhibits tumor growth by
blocking ligand-dependent and independent activation of ErbB-3/Akt
signaling. Oncogene. 31:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Capone E, Lamolinara A, D'Agostino D,
Rossi C, De Laurenzi V, Iezzi M, Iacobelli S and Sala G:
EV20-mediated delivery of cytotoxic auristatin MMAF exhibits potent
therapeutic efficacy in cutaneous melanoma. J Control Release.
277:48–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gandullo-Sanchez L, Capone E, Ocana A,
Iacobelli S, Sala G and Pandiella A: HER3 targeting with an
antibody-drug conjugate bypasses resistance to anti-HER2 therapies.
EMBO Mol Med. 12:e114982020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giansanti F, Capone E, Ponziani S, Piccolo
E, Gentile R, Lamolinara A, Di Campli A, Sallese M, Iacobelli V,
Cimini A, et al: Secreted Gal-3BP is a novel promising target for
non-internalizing antibody-drug conjugates. J Control Release.
294:176–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen R, Hou J, Newman E, Kim Y, Donohue C,
Liu X, Thomas SH, Forman SJ and Kane SE: CD30 Downregulation, MMAE
resistance, and MDR1 upregulation are all associated with
resistance to brentuximab vedotin. Mol Cancer Ther. 14:1376–1384.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Warmann S, Gohring G, Teichmann B,
Geerlings H and Fuchs J: MDR1 modulators improve the chemotherapy
response of human hepatoblastoma to doxorubicin in vitro. J Pediatr
Surg. 37:1579–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enhanced ERBB3 Signaling promotes
resistance in melanoma. Cancer Discov. 3:4792013.
|
|
30
|
Chakrabarty A, Sanchez V, Kuba MG,
Rinehart C and Arteaga CL: Feedback upregulation of HER3 (ErbB3)
expression and activity attenuates antitumor effect of PI3K
inhibitors. Proc Natl Acad Sci USA. 109:2718–2723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deeks ED: Cabozantinib: A review in
advanced hepatocellular carcinoma. Target Oncol. 14:107–113. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zongyi Y and Xiaowu L: Immunotherapy for
hepatocellular carcinoma. Cancer Lett. 470:8–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang T, Zhang L, Xu Y, Lu X, Zhao H, Yang
H and Sang X: Neoadjuvant therapy and immunotherapy strategies for
hepatocellular carcinoma. Am J Cancer Res. 10:1658–1667.
2020.PubMed/NCBI
|
|
34
|
Llovet JM, Montal R and Villanueva A:
Randomized trials and endpoints in advanced HCC: Role of PFS as a
surrogate of survival. J Hepatol. 70:1262–1277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Luca E, Marino D and Di Maio M:
Ramucirumab, a second-line option for patients with hepatocellular
carcinoma: A review of the evidence. Cancer Manag Res.
12:3721–3729. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marrero JA, Fontana RJ, Barrat A, Askari
F, Conjeevaram HS, Su GL and Lok AS: Prognosis of hepatocellular
carcinoma: Comparison of 7 staging systems in an American cohort.
Hepatology. 41:707–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ponziani S, Di Vittorio G, Pitari G,
Cimini AM, Ardini M, Gentile R, Iacobelli S, Sala G, Capone E,
Flavell DJ, et al: Antibody-drug conjugates: The new frontier of
chemotherapy. Int J Mol Sci. 21:55102020. View Article : Google Scholar
|
|
39
|
Coats S, Williams M, Kebble B, Dixit R,
Tseng L, Yao NS, Tice DA and Soria JC: Antibody-drug conjugates:
Future directions in clinical and translational strategies to
improve the therapeutic index. Clin Cancer Res. 25:5441–5448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Birrer MJ, Moore KN, Betella I and Bates
RC: Antibody-drug conjugate-based therapeutics: State of the
science. J Natl Cancer Inst. 111:538–549. 2019. View Article : Google Scholar : PubMed/NCBI
|