Introduction
Non-small cell lung cancer (NSCLC) is the most
common type of lung cancer and remains the leading cause of global
cancer-related mortality and morbidity (1). As a highly diverse form of cancer, the
heterogeneity of NSCLC is attributed to different histological
origins as well as genomic and epigenetic abnormalities (2–4).
NSCLC is divided into two primary subtypes,
adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC),
accounting for about 40 and 30% of all lung cancer cases,
respectively (3,5). Anatomically, the tracheobronchial tree
lined with respiratory epithelium is divided into central and
peripheral compartments (6). LUAD and
LUSC arise from different epithelial cell types with distinct
genomic abnormalities and functional variability and thus require
extremely different therapeutic strategies (2,3,5,7). For
instance, adenocarcinomas predominantly originate from cells of the
peripheral airways secreting mucus and expressing biomarkers
consistent with its distal bronchial origin, while squamous cell
carcinomas mainly arise from the epithelium of the larger proximal
airway (3,7). In contrast to the development of
targeted therapies for lung adenocarcinoma, only a few oncogenic
drivers have been identified in LUSC that limit the availability of
targetable molecules for clinical trial (2,3,7). Also, it remains inconclusive whether
these two tumor types stem from diverse cell types or from common
precursor cells (3,5,8). Moreover,
there is a substantial proportion of NSCLC lacking clear
histological identity and biomarkers for subtyping (2,3,5,7,9). There are increasing reports showing
acquired resistance to epidermal growth factor receptor-tyrosine
kinase inhibitors (EGFR-TKIs) due to transformation of EGFR-mutant
lung adenocarcinoma to squamous cell carcinoma (10,11).
Therefore, more definitive markers are needed to distinguish the
heterogeneity between LUSC and LUAD for precision treatment,
especially for various resistance to targeted therapy.
Mucins (MUCs) are main components of the bronchiolar
mucosal barrier, consisting of a family of high-molecular-weight
glycoproteins expressed by specialized epithelial cells in secreted
or membrane-bound forms (6,12). Among 21 mucins identified so far, most
are expressed in the respiratory tract or lung parenchyma,
including the secreted MUC2, MUC5AC, MUC5B, MUC7, MUC8 and MUC19
and membrane-bound MUC1, MUC3A, MUC4, MUC12, MUC13, MUC15, MUC16,
MUC17, MUC20, MUC21 and MUC22 (6,12–15). Membrane-bound MUCs are present on
epithelial cells serving as receptors and sensors to mediate signal
transduction (12,13,16).
Aberrant expression of MUCs is associated with the degree of lung
cancer malignancy via multiple pathways (12,14,16).
Therefore, MUCs are used as tumor-associated antigens (TAAs) and as
immunotherapeutic targets for NSCLC (14,15,17).
Genetic and epigenetic profiling of lung cancer
reveals differential expression of MUCs in diverse tumor
microenvironments (14,18). In the present study, we examined the
differential expression and epigenetic alterations of MUCs in LUSC
and LUAD as potential tumor biomarkers. We found that MUC22,
a new MUC family member, was decreased in the cells and tissues of
LUSC (MUC22Low) but increased in LUAD
(MUC22High) due to diverse epigenetic
alterations. Distinct expression of MUC22 in NSCLC was
associated with varied outcome of patients. Our results suggest the
potential of MUC22 expression and its epigenetic alterations
to distinguish NSCLC subtypes important for precision
treatment.
Materials and methods
Patients and specimens
This study was approved by the Institutional Ethical
Review Board for Human Investigation at Xuchang Central Hospital,
Henan, China. Paired specimens including 24 LUSC and 24 LUAD tumors
as well as tumor-adjacent tissues were obtained from 2002 to 2007
and stored at the Tissue Bank in accordance with the Standard
Operating Procedures of the Ethics Committee of Xuchang Central
Hospital (Table SI). The cohort
included 33 males and 15 females (mean age, 60.38±12.19 years;
range, 20–84 years). All patients signed informed consents.
Cell culture
Human NSCLC cell lines and human bronchial
epithelial cell line BEAS-2B were obtained from ATCC (ATCC number:
NCI-H1703, CRL-5889; NCI-H2170, CRL-5928; SK-MES-1, HTB-58;
NCI-H226, CRL-5826; NCI-H1975, CRL-5908; NCI-H522, CRL-5810;
NCI-H1395, CRL-5868; and HCC-827, CRL-2868). Cell lines were split
to low density (30% confluence) and grown in 90% RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). Before collection,
cells were also treated with 5-aza-2′-deoxycytidine (5-Aza) (5 µM;
Sigma-Aldrich; Merck KGaA) for 96 h with the growth medium being
changed every 24 h, or TSA (5 µM, Sigma-Aldrich; Merck KGaA) for 24
h as previously described (19).
Knockdown of MUC22 using small
interfering RNA (siRNA)
Two siRNA oligonucleotides for MUC22
(siMUC22-1 and-2) and RNAi Negative Control (siNC) were used in
this study (Table SII). SiMUC22s
were obtained from Beijing AUGCT Biotechnology Co. and siNC
(siN0000002-1-5) were purchased from RiboBio, and were transfected
into SK-MES-1, NCI-H522 and BEAS-2B cells using Lipofectamine 3000
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc.). After adequate knockdown efficiency was
confirmed by RT-qPCR, the transfected cells were used for
subsequent analyses.
RNA isolation, reverse transcription
(RT) and polymerase chain reaction (PCR)
RNA isolation, RT and PCR were performed as previous
described (20,21). Cells were harvested for RNA isolation
using RNeasy Mini Kit (Qiagen) and first-strand cDNA was
synthesized with the Superscript First-Strand Synthesis System
(Invitrogen; Thermo Fisher Scientific, Inc.). PCR was performed
using primers listed in Table SII.
qPCR was performed using 2X SYBR-Green-based qPCR reagent on ABI
7500 qPCR machine (Applied Biosystems). The relative expression
level of each mRNA was normalized against β-actin using
2−ΔΔCq method presented as ‘relative expression (% of
control)’, or further compared to its own baseline control
presented as ‘normalized fold expression’ (22).
DNA extraction, bisulfite
modification, methylation-specific PCR (MSP-PCR) and bisulfite
sequencing (BS)
DNA extraction, bisulfite modification and MSP-PCR
were performed as previously described (19,21).
Genomic DNA was extracted from tissues using the QIAamp DNA mini
Kit (Qiagen) followed by quantitative analysis using NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.). Bisulfite
modification of DNA was performed using Zymo DNA Methylation Kit
(Zymo Research). The positive and negative control were the Human
Methylated & Non-methylated DNA Set (Zymo Research). MSP-qPCR
was performed by using primer pairs specifically for either
methylated or unmethylated sequences of the MUC22 (Table SII). The relative level of
methylation and unmethylation of MUC22 promoter region was
normalized to β-actin using the 2−ΔΔCq method (19,22). MSP
products were analyzed using a 2% agarose gel electrophoresis.
Western blot analysis
Total cell protein was prepared using RIPA Lysis
Buffer (Beyotime Institute of Biotechnology). Protein was measured
using a BCA protein Assay Kit (CWBIO). Proteins were resolved by
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto PVDF membranes using a Bio-Rad Mini
PROTEAN 3 system (Bio-Rad Laboratories, Inc.). The membranes were
blocked with PBS containing 5% milk and 0.1% Tween-20 at room
temperature for 1 h. The primary antibodies were as follows:
β-actin (mouse monoclonal, dilution 1:10,000; A4551) was from
Sigma-Aldrich; Merck KGaA. Lamin A (mouse monoclonal, dilution
1:1,000; sc-71481) was obtained from Santa Cruz Biotechnology, Inc.
Anti-NF-κB p65 (rabbit polyclonal, dilution 1:1,000; ab16502),
anti-Histone H3 acetyl K9 (rabbit polyclonal, dilution 1:5,000,
ab4441), anti-HDAC1(rabbit polyclonal, dilution 1:5,000, ab109411)
and anti-IκB-α (rabbit polyclonal, dilution 1:5,000, ab32518) were
purchased from Abcam. Horseradish peroxidase-conjugated anti-mouse
(1:2,500 dilution) or anti-rabbit (1:2,500 dilution) secondary
antibodies were purchased from Bioworld Technology, Inc.
Immunoreactive bands were visualized by using the Amersham ECL
Western Blotting Detection Kit (Cytiva) according to the
manufacturer's instructions. β-actin served as a loading control
(19,23).
Chromatin immunoprecipitation
(ChIP)
ChIP assay was performed by following EpiTech
ChIPOneDay kit protocol (Qiagen) (19–21).
SK-MES-1 cells with different treatments were fixed with 1%
formaldehyde. Chromatin was prepared by sonication of cell lysate
and pre-clearing with protein A beads. Aliquots of pre-cleared
chromatin solution (named as ‘IP fractions’) were incubated with 2
µg of specific rabbit anti-Histone H3 acetyl K9 or preimmune rabbit
IgG on a rotation platform at 4°C overnight, and 1% of the IP
fraction served as the ‘Input control’ for each ChIP assay. Protein
A beads were added to precipitate the antibody-enriched protein-DNA
complexes from the IP fractions. After washing, the immune
complexes were subjected to reversal crosslink to release DNA
fragments. Immunoprecipitated DNA fractions were purified by using
a QIA quick purification kit (Qiagen) and analyzed by qPCR using
0.05% immunoprecipitated DNA as template.
Cell viability
SK-MES-1, NCI-H522 and BEAS-2B cells were seeded
into 96-well plates at 2×103 cells/well, and cell
viability was determined every day using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit (Thermo Fisher Scientific, Inc.). The absorbance for MTT
at 490 nm/570 nm wavelength was detected using a microplate reader
(Thermo Multiskan MK3, Thermo Fisher Scientific Inc.) (23).
Migration assay
Transwell apparatus was inserted with an 8-µm pore
membrane (Corning Inc.). The upper chambers were seeded with
serum-free medium containing 2×104 tumor cells in 200
µl. The lower chambers were filled with 500 µl of 10%
FBS-RPMI-1640. After 24 h, the cells that migrated across the
membrane were fixed and stained with 0.2% crystal violet (Beyotime
Institute of Biotechnology). Images were then acquired using Leica
microsystems (Leica DMi8 Inverted Microscope, GE) (23).
Statistical analysis
The data are expressed as the means ± standard
deviation (SD) of at least three independent experiments. The
differences in MUC22 expression and its epigenetic
alterations were analyzed by using the two-tailed Student's t-test
or one-way analysis of variance (ANOVA) with Tukey's post hoc test.
The relationship between MUC22 and clinical pathologic
characteristics was assessed by χ2 tests. The difference
of overall survival curve based on Kaplan-Meier plot was assessed
for statistical significance with the Cramer-von Mises test using R
package. All statistical analyses were performed using SPSS version
23.0 (IBM Corp.).
Results
Differential expression of MUC22 in
LUSC vs. LUAD
We first analyzed the RNA-Seq data of LUSC and LUAD
in TCGA (The Cancer Genome Atlas) database (https://www.cancer.gov/tcga) (24) for the expression of membrane-bound
mucins. After data consolidation, a total of 16,393 differentially
expressed genes (DEGs) were identified, including 9,168 in LUSC
(55.93%) and 7,225 in LUAD (44.07%). A total of 10,339 DEGs was
upregulated (52.86% in LUSC and 47.14% in LUAD) and 6,054 DEGs were
downregulated (61.17% in LUSC and 38.83% in LUAD) (Tables SIII and SIV). These results suggest a
transcriptional heterogeneity within NSCLC.
Among DEGs graphically presented in a volcano plot
according to the P-values and fold changes, we found differentially
expressed mucin genes in NSCLC tissues (Fig. 1A). MUC22 expression was
significantly lower in LUSC (MUC22Low) but higher
in LUAD (MUC22High) tissues (Fig. 1B) in association with lung
adenocarcinoma-associated MUC21, whereas MUCL1, MUCL4,
MUCL13 and MUCL20 were highly expressed in both LUAD and
LUSC tissues, with consistently normal expression of MUC12
(Table I). The distinct expression
pattern of MUC22 between LUSC and LUAD was further verified
using GEPIA database data (http://gepia.cancer-pku.cn/) (25), showing MUC22Low in
LUSC (n=486) and MUC22High in LUAD (n=483) as
compared with normal tissues (Fig.
1C).
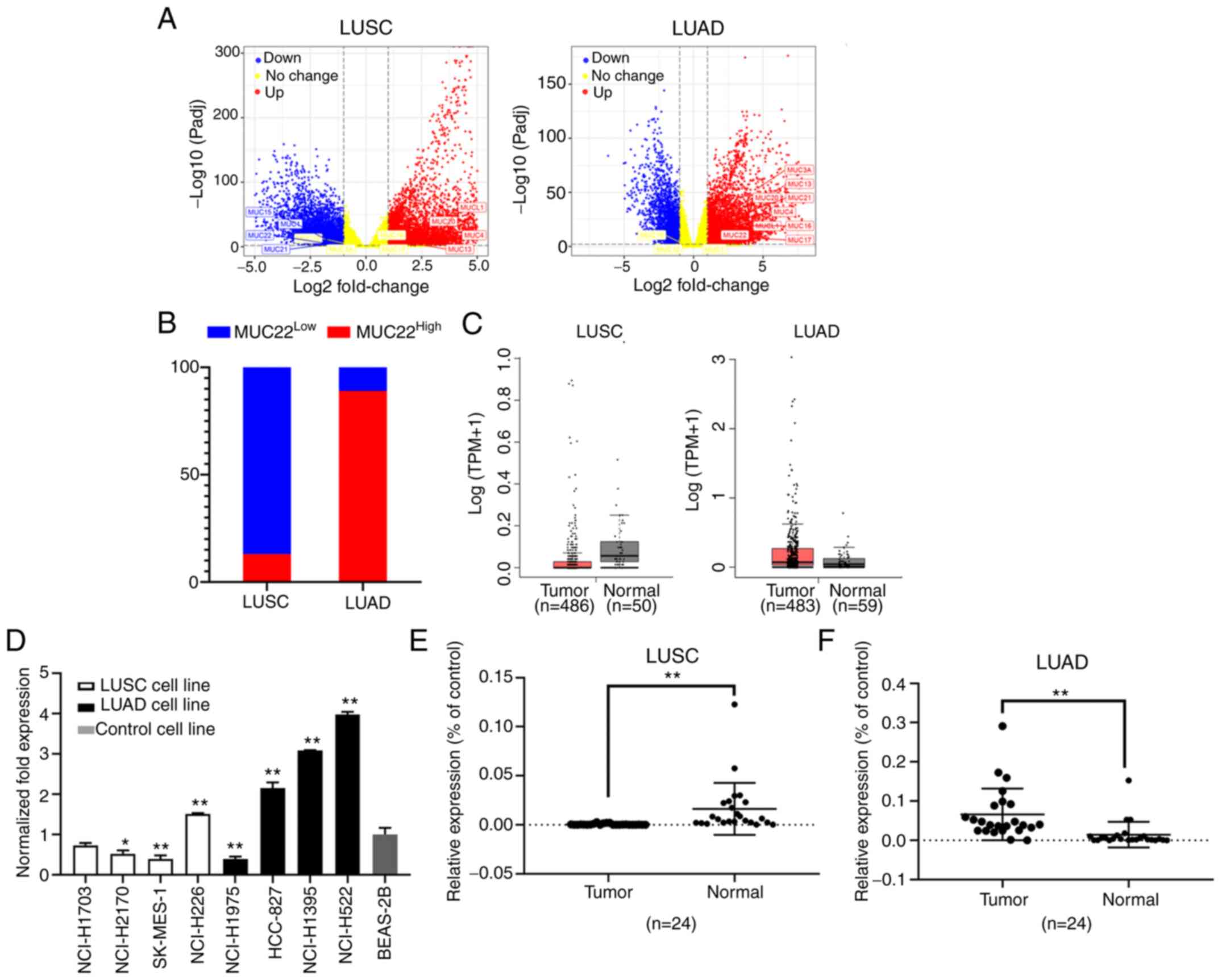 | Figure 1.Differential expression of
MUC22 in human LUSC and LUAD cells and tissues. (A and B)
Data were extracted from the TCGA RNA-Seq database (https://www.cancer.gov/tcga). Differentially
expressed genes (DEGs) in TCGA-LUSC (Tumor=545, Normal=49) and
TCGA-LUAD (Tumor=583, Normal=59) were analyzed by using iBio Tools
v5.0. (A) Volcano plots showing DEGs ranked according to their
statistical P-value for normalized log10 transformed read counts of
RNA-seq data (y-axis) and the absolute value of log2 fold change
(x-axis) (data shown in Tables SIII
and SIV). Colored spots represent
signification upregulated (red) or downregulated (blue), or no
change (yellow). Membrane-bound mucins are marked. (B) Shown is a
100% stacked bar chart showing differential expression of
MUC22 in LUSC (n=545) and LUAD (n=583) as compared with the
normal controls. MUC2LLow or
MUC22High group was classified based on the
default cutoff value used in the TCGA data portal (https://www.cancer.gov/tcga). (C) GEPIA portal
analysis of TCGA RNA-sequencing results of MUC22 mRNA in
tumor tissues and normal lung tissues. Left: LUSC (Tumor=486,
Normal=50); Right: LUAD (Tumor=483, Normal=58). The images were
derived from GEPIA (http://gepia.cancer-pku.cn/). (D) RT-qPCR of
MUC22 mRNA in LUSC cell lines (NCI-H1703, NCI-H2170,
SK-MES-1 and NCI-H226), LUAD cell lines (NCI-H1975, HCC-827,
NCI-H1395 and NCI-H522) and an immortalized human bronchial
epithelial BEAS-2B cell line. The relative expression of
MUC22 mRNA normalized with β-actin, and as fold of BEAS-2B
is presented as the mean ± SD. (E and F) RT-qPCR of MUC22
mRNA in tumor tissues in comparison with paired normal control
tissues of NSCLC patients. Detailed clinicopathologic parameters of
patients are shown in Table SI. E,
LUSC (n=24); F, LUAD (n=24). *P<0.05 and **P<0.01, vs.
relevant control (D, one-way ANOVA with Tukey's post hoc test; E
and F, two-tailed Student's t-test). MUC22, mucin 22; LUSC,
lung squamous cell carcinoma; LUAD, lung adenocarcinoma, NSCLC,
non-small cell lung cancer. |
 | Table I.Differential expression of
membrane-bound mucins (MUCs) between LUSC and LUAD. |
Table I.
Differential expression of
membrane-bound mucins (MUCs) between LUSC and LUAD.
| Type | Upregulated | Downregulated | Normal |
|---|
| LUSC |
UCL1a, MUC4a, MUC13a, MUC20a |
MUC21a, MUC22b, MUC1a, MUC15a | MUC12, MUC3A,
MUC16, MUC17 |
| LUAD |
MUCL1a, MUC4a, MUC13a, MUC20a |
| MUC12, MUC1,
MUC15 |
|
|
MUC21a, MUC22b, MUC3Aa, MUC16a, MUC17a |
|
|
We then investigated the expression of MUC22 in LUSC
cell lines (NCI-H1703, NCI-H2170, SK-MES-1 and NCI-H226) and LUAD
cell lines (NCI-H1975, NCI-H522, NCI-H1395 and HCC-827). As shown
in Fig. 1D, MUC22 mRNA
expression was relatively lower in the LUSC cell lines but higher
in the LUAD cell lines compared to the lung epithelial cell line
BEAS-2B, except for the reversed expression pattern of MUC22
in LUSC cell line NCI-H226 and LUAD cell line NCI-H1975,
respectively. Paired specimens for further validation showed that
the mRNA level of MUC22 was significantly lower in LUSC, but
enhanced in LUAD compared to matched adjacent normal lung tissues
(Table II, Fig. 1E and F). Thus, MUC22 is
differentially expressed in NSCLC with distinction between LUSC and
LUAD.
 | Table II.Association of MUC22
expression with clinicopathologic parameters of the NSCLC patients
(N=48). |
Table II.
Association of MUC22
expression with clinicopathologic parameters of the NSCLC patients
(N=48).
|
Characteristics | No. of cases |
MUC22High n (%) |
MUC22Low n (%) | P-value |
|---|
| Total | 48 | 27 (56.3) | 21 (43.7) |
|
| Sex |
|
|
|
|
|
Male | 33 | 16 (48.5) | 17 (51.5) |
|
|
Female | 15 | 11 (73.3) | 4 (26.7) | 0.107 |
| Age (years) |
|
|
|
|
|
≥60 | 25 | 13 (52.0) | 12 (48.0) |
|
|
<60 | 23 | 14 (60.9) | 9 (39.1) | 0.539 |
| Pathological
type |
|
|
|
|
|
LUAD | 24 | 21 (87.5) | 3 (12.5) |
|
|
LUSC | 24 | 6 (25.0) | 18 (75.0) |
1.3×10−5a |
| Tumor invasive
depth |
|
|
|
|
| 1 | 12 | 8 (66.7) | 4 (33.3) |
|
| 2 | 31 | 17 (54.8) | 14 (45.2) | 0.481 |
| 3 | 5 | 2 (40.0) | 3 (60.0) | 0.309 |
| Lymph node
metastasis |
|
|
|
|
| 0 | 40 | 24 (60.0) | 16 (40.0) |
|
| 1 | 8 | 3 (37.5) | 5 (62.5) | 0.242 |
| Clinical stage |
|
|
|
|
| I | 3 | 2 (66.7) | 1 (33.3) |
|
| II | 31 | 19 (61.3) | 12 (38.7) | 0.856 |
|
III | 14 | 6 (42.9) | 8 (57.1) | 0.453 |
Epigenetic regulation of MUC22 in
human lung cancer cells and tissues
Given that epigenetic alterations, such as DNA
methylation, can non-genetically modify gene expression resulting
in functional disruption in cancer (19) or other disorders (26), we examined epigenetic contribution to
the differential expression of MUC22 in human lung cancer.
MSP-qPCR was performed with lung cancer cell lines and tissues with
primers covering the promoter region of MUC22 (Fig. 2A). As shown in Fig. 2B, compared to BEAS-2B cells, the
promoter region of MUC22 was partially methylated in the
LUSC cell lines, except NCI-H226 cells in which a more
hypomethylated status of MUC22 promoter was detected.
Conversely, MUC22 promoter was hypomethylated in LUAD cell
lines, but heavily methylated in NCI-1975 cells. Further analysis
showed that the promoter methylation of MUC22 was associated
reversely with its expression in the lung cancer cells (Fig. 2C). Consistently, the MUC22
promoter was moderately or heavily methylated in 96% (23 of 24) of
LUSC tissues, but unmethylated in 70% (16 of 23) of LUAD tissues
(P<0.001) associated with its expression in the tumors (100%
MUC22Low with methylation, P<0.001) (Figs. 2D and S1 and Table
SV). These results suggested a closed correlation of
MUC22 promoter methylation with its expression.
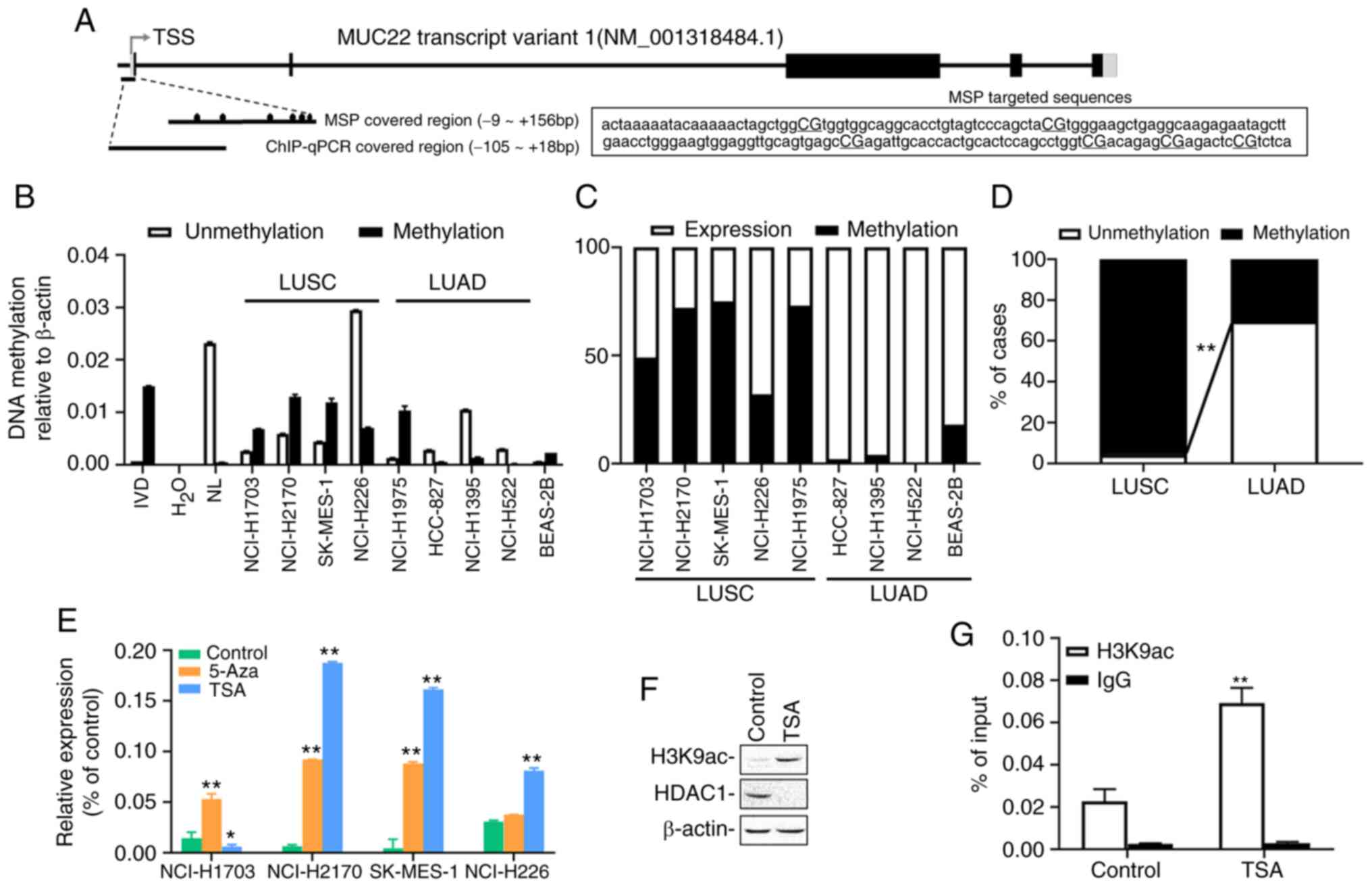 | Figure 2.Epigenetic alterations of
MUC22 expression in LUSC and LUAD. (A) Schematic
representation of MUC22 transcript structure. The exons and
introns are represented as boxes and lines. The regions targeted by
primer pairs for methylation-specific PCR (MSP) and ChIP-PCR
analysis are shown. Fragment sequences of MUC22 for MSP are
displayed with six CG sites capitalized and underlined. TSS,
transcription start site. Black boxes, coding regions (CDS); gray
boxes, untranslated regions (UTR); black ovals, CpG sites. (B)
MS-qPCR analysis of MUC22 methylation in LUSC cell lines
(NCI-H1703, NCI-H2170, SK-MES-1 and NCI-H226), LUAD cell lines
(NCI-H1975, HCC-827, NCI-H1395 and NCI-H522) and BEAS-2B cells.
IVD, in vitro methylated DNA served as an MSP positive
control; NL, normal blood lymphocyte DNA as negative control. (C)
Comparative association between methylation and the expression of
MUC22 in LUSC and LUAD cell lines is visualized by 100%
stacked bar graph. (D) The methylation frequency of MUC22
promoter determined using MSP in LUSC (n=24) and LUAD (n=23) tissue
samples. The frequency of methylation (M) and unmethylation (U) in
the samples is presented as the percentage of cases. MUC22
methylation in LUSC vs. LUAD. (E) RT-qPCR analysis of MUC22
expression in cancer cells treated with epigenetic reagents,
5-aza-2′-deoxycytidine (5-Aza) or trichostatin A (TSA). The
relative expression of MUC22 mRNA normalized with β-actin is
presented as the mean ± SD. (F) Western blotting for H3K9ac and
HDAC1 in SK-MES-1 cell lysates upon TSA treatment. (G) ChIP
performed with SK-MES-1 cells after TSA treatment using antibody
against H3K9ac or control IgG. Precipitated ChIP DNA fractions were
analyzed by qPCR for the enrichment of H3K9ac in MUC22
promoter region. Results are expressed as the percentage of input
quantity. 5-Aza: 5 µM, 96 h; TSA: 5 µM, 24 h. *P<0.05 and
**P<0.01, vs. the untreated control cells (D and G, two-tailed
Student's t-test; E, one-way ANOVA with Tukey's post hoc test).
MUC22, mucin 22; LUSC, lung squamous cell carcinoma; LUAD,
lung adenocarcinoma. |
We further investigated the epigenetic regulation of
MUC22 expression by treating LUSC cells with epigenetic
modifiers, the DNA methyltransferase inhibitor 5-Aza or histone
deacetylase inhibitor TSA. As shown in Fig. 2E, 5-Aza treatment markedly increased
mRNA expression of MUC22 in NCI-H1703, NCI-H2170 and
SK-MES-1 cells, but not in NCI-H226 cells, whereas TSA
significantly upregulated MUC22 in NCI-H2170, SK-MES-1 and
NCI-H226 cells, but not in NCI-H1703 cells. Moreover, TSA treatment
of SK-MES-1 cells resulted in reduction in the global protein level
of histone deacetylase 1 (HDAC1) accompanied by a marked
upregulation of acetylation of histone 3 at lysine 9 (H3K9ac) as
analyzed by western blotting of whole cell extract (Fig. 2F). ChIP-qPCR analysis of genomic DNA
immunoprecipitated with anti-H3K9ac antibody in SK-MES-1 cells
showed that H3K9ac was significantly enriched in the MUC22
promoter region upon the treatment (Fig.
2A and G). These results demonstrate that coordinated
epigenetic modifications regulate MUC22 expression in LUSC
cells, in which epigenetic silent MUC22 differentially
responded to 5-Aza or TSA, suggesting heterogeneity of NSCLC cells
subject to epigenetic regulation.
Knockdown of MUC22 promotes lung
cancer cell growth and migration via NF-κB activation
To explore the functional role of MUC22 in lung
cancer cells, siRNAs targeting MUC22 (siMUC22-1 and 2) were
transfected into SK-MES-1, NCI-H522 and BEAS-2B cells, and the
knockdown efficiency was evaluated by RT-qPCR (Fig. 3A). As shown in Fig. 3B, knockdown of MUC22 promoted
the proliferation of both SK-MES-1 and NCI-H522 cell lines.
Transwell migration assay showed significantly increased number of
migrating SK-MES-1 and NCI-H522 cells after MUC22 knockdown
(Fig. 3C). These results suggest that
MUC22 inhibits the proliferation and migration of lung cancer
cells. We further observed suppressive effect of MUC22 on lung cell
malignancy in an immortalized bronchial epithelial cell line
BEAS-2B, in which MUC22 knockdown promoted cell growth and
motility (Fig. 3A-C).
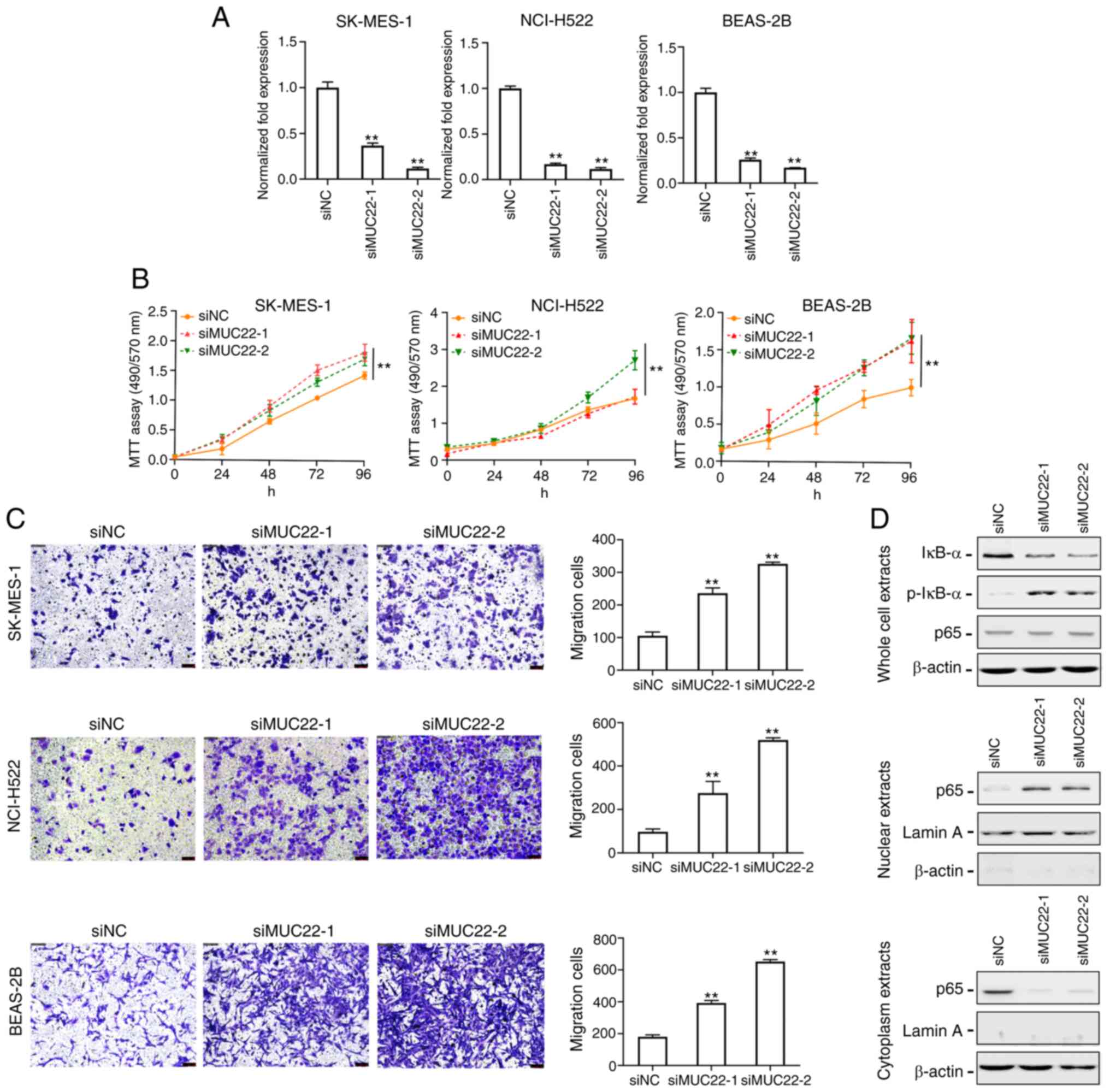 | Figure 3.MUC22 knockdown promotes lung
cancer cell proliferation and migration via nuclear factor (NF)-κB
activation. (A) RT-qPCR analysis of the RNA interference
efficiency. SK-MES-1, NCI-H522 and BEAS-2B cells were transfected
with siRNA oligonucleotides (siMUC22-1 and −2) or with RNAi
Negative Control (siNC). The expression of MUC22 was then
examined. (B) MTT assays showing the viability of SK-MES-1,
NCI-H522 and BEAS-2B cells transfected with siMUC22s or siNC. Data
are presented as the mean ± SD. (C) The motility of SK-MES-1,
NCI-H522 and BEAS-2B cells after transfection was assessed using
Transwell assay shown with representative images (left panels) or
quantification (right panels). (D) Western blot analysis of NF-κB
in SK-MES-1 cells upon MUC22 knockdown. The protein levels
of IκB-α, p-IκB-α and NF-κB p65 subunit are shown in whole cell
extracts (upper panel), nuclear extracts (middle panel) and
cytoplasmic extracts (lower panel). β-actin serves as cytoplasmic,
and Lamin A as nuclear protein loading controls, respectively.
**P<0.01, vs. siNC. One-way ANOVA with Tukey's post hoc test was
performed. MUC22, mucin 22; p-, phosphorylated. |
Nuclear factor (NF)-κB as a key inflammatory
regulator plays a critical role in the transformation of chronic
inflammation towards carcinogenesis, which is preceded by aberrant
expression of MUCs (12–14). We thus investigated the effect of
MUC22 on the NF-κB pathway in lung cancer cells. As shown in
Fig. 3D, transfection of SK-MES-1
cells with MUC22 siRNAs resulted in a decrease in total
IκB-α, but an increase in the phosphorylated IκB-α (p-IκB-α)
protein. However, there was no apparent changes in total p65
subunit of NF-κB in whole cell extracts. Because both increased
IκB-α and reduced p-IκB-α expression contribute to NF-κB
inactivation by trapping NF-κB in the cytoplasm, the distribution
of NF-κB p65 subunit in lung cancer cells was examined, which
showed a diminution of p65 in the cell cytoplasm with an
augmentation in the nuclei upon siMUC22s transfection. Blockade of
NF-κB p65 subunit translocation indicates the ability of MUC22 to
inactivate NF-κB. These results suggest that epigenetic silencing
of MUC22 facilitates lung cancer cell growth and motility
via NF-κB activation.
Prognostic prediction value of
distinct MUC22 in LUSC and LUAD
The potential prognostic prediction value of
MUC22 expression in LUSC (n=494) and LUAD (n=499) was
assessed by Kaplan-Meier analysis of overall survival (OS) of
MUC22Low and MUC22High lung
cancer patients using the dataset available from the Human Protein
Atlas (http://www.proteinatlas.org)
(27) (Table SVI). As shown in Fig. 4A-E, Cramer-von Mises test analysis of
the survival curves revealed that MUC22High was
significantly associated with favorable OS in LUSC patients but
with worse OS in LUAD patients (Fig.
4A). Further analyses revealed the association of
MUC22High with more favorable outcome of LUSC
patients at stages I and III cancer (Fig.
4B and D) but not at stages II and IV (Fig. 4C and E). By contrast, compared to
MUC22Low, MUC22High LUAD patients had
a significantly worse OS at stages I and II (Fig. 4B and C), but with a reversed outcome
in patients with stage III cancer (Fig.
4D). The diverse association of MUC22 expression with
patient survival with different stages of LUSC and LUAD reveals a
complicated role of MUC22 in tumor heterogeneity during
cancer progression.
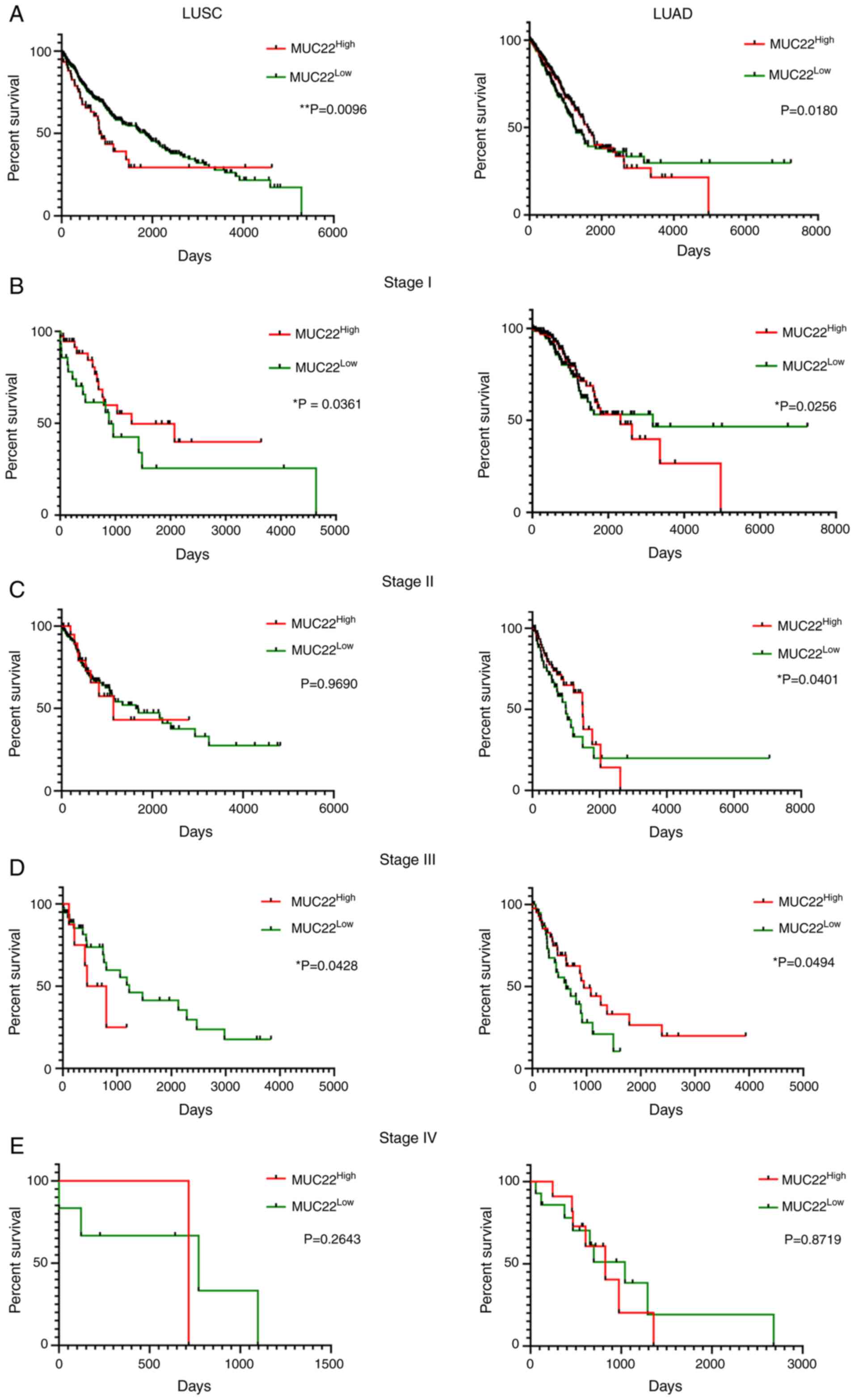 | Figure 4.Prognostic prediction value of
MUC22 expression in LUSC and LUAD patients. RNA-sequencing
data was obtained from the Human Protein Atlas (HPA, http://www.proteinatlas.org). Detailed
clinicopathologic parameters are summarized in Table SVI. Kaplan-Meier survival analyses of
overall survival of LUSC (left panels) and LUAD (right panels)
patients with cancer at different stages. Patients with
MUC22Low or MUC22High tumors at
different stages were classified based on the cutoff value of 0.02,
which is a default parameter also used in the Human Protein Atlas
(sample sizes: LUSC: n=494; LUAD: n=499). Results are stratified in
accordance with the expression patterns of MUC22 in cancers
of patients without staging (A), or with stage I (B), II (C), III
(D), and IV (E). *P<0.05, **P<0.01,
MUC22Low vs. MUC22High
(Cramer-von Mises test). MUC22, mucin 22; LUSC, lung
squamous cell carcinoma; LUAD, lung adenocarcinoma. |
Discussion
In the present study, we revealed the biological
significance and prognostic values of distinct expression and
epigenetic alterations of mucin 22 (MUC22) in lung
adenocarcinoma (LUAD) vs. squamous cell carcinoma (LUSC). Thus,
MUC22 may serve as a potential biomarker for subtyping
non-small cell lung cancer (NSCLC). Our research also provides
evidence for mucins to contribute to the heterogeneous development
of malignancy of lung cancer.
Mucins (MUCs) as a group of large glycoproteins
expressed by various epithelial cells not only control the local
environment but also contribute to cellular signal transduction in
response to external stimuli (12,13,16).
Abnormal MUC expression has been reported in various cancer types
including lung cancer (12–15,18,19,28–30).
In addition to several well-known mucins serving as
tumor-associated antigens and cancer biomarkers particularly in
adenocarcinomas (13,16,29,30), some
MUCs are preferentially distributed in the respiratory tract under
normal conditions. Their disruption was reported in lung cancer and
this has been used as biomarkers (13,16,30) as
well as therapeutic targets (13,16,31–34).
MUC22 is a novel membrane-bound mucin with previously unknown
pathophysiological roles (35,36). We
herein identified a distinct pattern of MUC22 expression,
MUC22Low in LUSC vs. MUC22High
in LUAD, in multiple lung cancer cell lines and tissues. We also
found that MUC22 expression was significantly associated
with MUC21 in both LUAD and LUSC tissues, differing from the
higher expression of MUCL1, MUCL4, MUCL13 and MUCL20.
Recent studies have shown aberrant expression of MUCs in LUAD
including MUC1, MUC3A, MUC5B, MUC6, MUC7 and MUC21, in bronchial
neoplasm (MUC4, MUC5AC, MUC5B, MUC6), or in small cell lung cancer
(MUC1, MUC5AC, MUC3A, MUC6) (37–42). MUC21
is preferentially expressed in normal lung tissues particularly in
the epithelia of bronchi and bronchioli (43). The divergence of MUC21 expression was
shown in LUAD, but not in LUSC (39,40). MUC21
is known as an epiglycanin-like glycoprotein involved in the
incohesive growth pattern in LUAD, and MUC21-expressing malignant
bronchial epithelial cells may be the origin of LUADs (39,40,43). Since
MUC22 was co-localized with MUC21 in a mucin gene
cluster on chromosome 6p21.3 (36),
co-expression of MUC22 with MUC21 may indicate a link
between MUC21 and MUC22 in lung cancer progression contributing to
lung cancer heterogeneity (14,16,31,44).
Epigenetic regulation of the expression of MUC1,
MUC2, MUC4, MUC5AC and MUC17 has been reported under
control by an interplay between DNA methylation and histone
modifications in a variety of cancer cells (18,19,30,45–52).
Our study demonstrated a more complicated epigenetic regulation of
MUC22 in lung cancer. Although the differential expression
of MUC22 was negatively correlated with MUC22
methylation status in multi-lung cancer cell lines and tissues and
MUC22 silencing in LUSC is under the coordinated epigenetic
modifications between DNA hypermethylation and histone
deacetylation, increased MUC22 expression in LUAD was
associated with the hypomethylation status in the promoter region.
Promoter hypomethylation associated with mucin expression was also
shown in several types of cancer cells and tissues, including lung
cancer (45–48). Interestingly, instead of impacting
gene transcription by methylation of CpG islands (CpGIs), we found
there were six CpG sites in the MUC22 promoter region with
their methylation status affecting MUC22 expression.
Consistent with this, some MUCs, such as MUC1, MUC4, MUC5AC,
MUC6 and MUC17, tend to have fewer CpG sites in their
promoter regions or have distal CpG sites such as ‘CpG island
shore’, or have a differentially methylated region (DMR), the
methylation status of which is correlated with transcriptional
regulation (18,19,31,46–51).
Considering tissue-specific gene expression regulated by scattered
or distal CpG sites, such as DMR, rather than CpG islands in the
proximal promoter (53), the
differential methylation status in the MUC22 promoter may
determine the specificity of MUC22 expression during lung
carcinogenesis. Therefore, the methylation status of the
MUC22 promoter may serve as a noninvasive cancer biomarker
to monitor the cell origin in lung carcinogenesis as well as
therapy-resistant adenocarcinoma undergoing transformation to
squamous cell carcinoma (10,11). Additionally, the pattern of epigenetic
controlling of MUC22 expression was opposite to what was
predicted in LUSC NCI-H226 (MUC22High with
hypomethylation) and LUAD NCI-H1975 cells
(MUC22Low with hypermethylation), indicating that
mucins contribute to inter- or intra-tumor heterogeneity in the
context of epigenetic regulation (14,16,31,50).
The structural and functional link of MUC22 to lung
cancer remains uncertain (12,14). We
found that MUC22 knockdown promoted the growth and motility
of lung cancer cells as well as an immortalized bronchial
epithelial BEAS-2B cell line. The suppressive role of MUC22 in lung
cancer cells was supported by the favorable prognostic prediction
value of MUC22 in early stage LUSC, but not in LUAD. In support of
our results, a recent report showed decreased MUC22 as one
of three TP53-related prognostic signatures for LUSC (51). However, despite diverse effects of
MUC22 expression on patient survival with different stages,
the survival analysis showed that MUC22Low in
LUSC and MUC22High in LUAD were associated with
unfavorable outcome for patients at stage I but converted into
protective factors at stage III. Since the prognosis in association
with MUC22 expression in LUSC or LUAD became reversed in
advanced lung cancer, a dynamic change in the role of MUC22
through the malignant process of LUSC or LUAD may be responsible
for a functional heterogeneity in lung carcinogenesis (12,14,16).
Therefore, it is important to delineate the precise molecular
mechanisms underlying epigenetic regulation of MUC22 that
contributes to phenotypic differences within NSCLC.
In conclusion, our research is the first to report
the distinct expression and function of MUC22 in LUSC and
LUAD. Epigenetic silencing of MUC22 may provide a molecular
model for dissecting mucin-associated lung cancer heterogeneity,
thus having clinical implication in distinguishing NSCLC subtypes
for precision treatment.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the Beijing Municipal
Administration of Hospitals Incubating Program (PX2021063), the
Intramural Research Funding Program from Beijing Tuberculosis and
Thoracic Tumor Research Institute/Beijing Chest Hospital. CT, KC,
WG, JH and JMW were also funded in part by federal funds from the
National Cancer Institute, National Institutes of Health, under
contract no. HHSN261200800001E and were supported in part by the
Intramural Research Program of the NCI, CCR, LCIM, NIH.
Availability of data and materials
All data are publicly released from TCGA, GEPIA and
CCLE databases and hyperlinks including citations have been
included in the ‘Results’ section. Some of the data are also
provided in the Electronic Supplementary Material.
Authors' contributions
Conceptualization of the research was conducted by
SL, CT and JH. Methodology was designed by SL, CT, JL, BL, TM, KC,
and WG. Formal analysis and investigation were conducted by SL, CT,
JMW, and JH. Writing of the original draft preparation was
conducted by SL and JH; writing of the review and editing were
performed by SL, JMW, and JH. Funding acquisition was provided by
JMW and JH; resources were provided by JL and WG. Supervision was
carried out by JH. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was conducted with the approved of the
Institutional Ethical Review Board for Human Investigation at
Xuchang Central Hospital (Xuchang, Henan, China) and with informed
consent from the patients.
Patient consent for publication
Not applicable.
Authors' information
Shuye Lin: ORCID: 0000-0002-4292-0302. Jiaqiang
Huang: ORCID: 0000-0002-6610-8159.
Competing interests
The authors declare no competing interests.
Glossary
Abbreviations
Abbreviations:
|
5-Aza
|
5-aza-2′-deoxycytidine
|
|
TAA
|
tumor- associated antigen
|
|
BS
|
bisulfite sequencing
|
|
ChIP
|
chromatin immunoprecipitation
|
|
DEGs
|
differentially expressed genes
|
|
EGFR
|
epidermal growth factor receptor
|
|
EGFR-TKI
|
epidermal growth factor
receptor-tyrosine kinase inhibitor
|
|
H3K9ac
|
histone H3 lysine 9 acetylation
|
|
HDAC1
|
histone deacetylase 1
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
MSP
|
methylation specific-PCR
|
|
MSP-qPCR
|
methylation-specific real-time
PCR
|
|
MUCs
|
mucins
|
|
MUC22
|
mucin 22
|
|
MUC22High
|
high expression of mucin 22
|
|
MUC22Low
|
low expression of mucin 22
|
|
NSCLC
|
non-small cell lung carcinoma
|
|
OS
|
overall survival
|
|
RT-PCR
|
reverse transcription-PCR
|
|
TSA
|
trichostatin A
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skoulidis F and Heymach JV: Co-occurring
genomic alterations in non-small-cell lung cancer biology and
therapy. Nat Rev Cancer. 19:495–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quintanal-Villalonga Á and Molina-Pinelo
S: Epigenetics of lung cancer: A translational perspective. Cell
Oncol (Dordr). 42:739–756. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travis WD: Lung cancer pathology: Current
concepts. Clin Chest Med. 41:67–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bustamante-Marin XM and Ostrowski LE:
Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol.
9:a0282412017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Relli V, Trerotola M, Guerra E and Alberti
S: Abandoning the notion of non-small cell lung cancer. Trends Mol
Med. 25:585–594. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbosh C, Birkbak NJ, Wilson GA,
Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA,
Veeriah S, Rosenthal R, et al: Phylogenetic ctDNA analysis depicts
early-stage lung cancer evolution. Nature. 545:446–451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niederst MJ, Sequist LV, Poirier JT,
Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN,
Moran T, et al: RB loss in resistant EGFR mutant lung
adenocarcinomas that transform to small-cell lung cancer. Nat
Commun. 6:63772015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roca E, Pozzari M, Vermi W, Tovazzi V,
Baggi A, Amoroso V, Nonnis D, Intagliata S and Berruti A: Outcome
of EGFR-mutated adenocarcinoma NSCLC patients with changed
phenotype to squamous cell carcinoma after tyrosine kinase
inhibitors: A pooled analysis with an additional case. Lung Cancer.
127:12–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhanisha SS, Guruvayoorappan C, Drishya S
and Abeesh P: Mucins: Structural diversity, biosynthesis, its role
in pathogenesis and as possible therapeutic targets. Crit Rev Oncol
Hematol. 122:98–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu M, Wang DC, Wang X and Zhang Y:
Correlation between mucin biology and tumor heterogeneity in lung
cancer. Semin Cell Dev Biol. 64:73–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lucchetta M, da Piedade I, Mounir M,
Vabistsevits M, Terkelsen T and Papaleo E: Distinct signatures of
lung cancer types: Aberrant mucin O-glycosylation and compromised
immune response. BMC Cancer. 19:8242019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jamal-Hanjani M, Wilson GA, McGranahan N,
Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R,
Rosenthal R, et al: Tracking the evolution of non-small-cell lung
cancer. N Engl J Med. 376:2109–2121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada N, Kitamoto S, Yokoyama S, Hamada
T, Goto M, Tsutsumida H, Higashi M and Yonezawa S: Epigenetic
regulation of mucin genes in human cancers. Clin Epigenetics.
2:85–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin S, Zhang Y, Hu Y, Yang B, Cui J, Huang
J, Wang JM, Xing R and Lu Y: Epigenetic downregulation of MUC17 by
H. pylori infection facilitates NF-κB-mediated expression of
CEACAM1-3S in human gastric cancer. Gastric Cancer. 22:941–954.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan Y, Lin S, Xing R, Zhu M, Lin B, Cui J,
Li W, Gao J, Shen L, Zhao Y, et al: Epigenetic upregulation of
metallothionein 2A by diallyl trisulfide enhances chemosensitivity
of human gastric cancer cells to docetaxel through attenuating
NF-κB activation. Antioxid Redox Signal. 24:839–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin B, Zhou X, Lin S, Wang X, Zhang M, Cao
B, Dong Y, Yang S, Wang JM, Guo M and Huang J: Epigenetic silencing
of PRSS3 provides growth and metastasis advantage for human
hepatocellular carcinoma. J Mol Med (Berl). 95:1237–1249. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin S, Wang X, Pan Y, Tian R, Lin B, Jiang
G, Chen K, He Y, Zhang L, Zhai W, et al: Transcription factor
myeloid zinc-finger 1 suppresses human gastric carcinogenesis by
interacting with metallothionein 2A. Clin Cancer Res. 25:1050–1062.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghandi M, Huang FW, Jané-Valbuena J,
Kryukov GV, Lo CC, McDonald ER III, Barretina J, Gelfand ET,
Bielski CM, Li H, et al: Next-generation characterization of the
cancer cell line encyclopedia. Nature. 569:503–508. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masser DR, Hadad N, Porter H, Stout MB,
Unnikrishnan A, Stanford DR and Freeman WM: Analysis of DNA
modifications in aging research. Geroscience. 40:11–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jonckheere N and Van Seuningen I:
Integrative analysis of the cancer genome atlas and cancer cell
lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20
signature is associated with poor survival in human carcinomas. J
Transl Med. 16:2592018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atanasova KR and Reznikov LR: Strategies
for measuring airway mucus and mucins. Respir Res. 20:2612019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yonezawa S, Higashi M, Yamada N, Yokoyama
S, Kitamoto S, Kitajima S and Goto M: Mucins in human neoplasms:
Clinical pathology, gene expression and diagnostic application.
Pathol Int. 61:697–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Quoix E, Lena H, Losonczy G, Forget F,
Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A,
Kazarnowicz A, et al: TG4010 immunotherapy and first-line
chemotherapy for advanced non-small-cell lung cancer (TIME):
Results from the phase 2b part of a randomised, double-blind,
placebo-controlled, phase 2b/3 trial. Lancet Oncol. 17:212–223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hall PE, Ready N, Johnston A, Bomalaski
JS, Venhaus RR, Sheaff M, Krug L and Szlosarek PW: Phase II study
of arginine deprivation therapy with pegargiminase in patients with
relapsed sensitive or refractory small cell lung cancer. Clin Lung
Cancer. 21:527–533. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taherali F, Varum F and Basit AW: A
slippery slope: On the origin, role and physiology of mucus. Adv
Drug Deliv Rev. 124:16–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fini ME, Jeong S, Gong H,
Martinez-Carrasco R, Laver NMV, Hijikata M, Keicho N and Argüeso P:
Membrane-associated mucins of the ocular surface: New genes, new
protein functions and new biological roles in human and mouse. Prog
Retin Eye Res. 75:1007772020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hijikata M, Matsushita I, Tanaka G,
Tsuchiya T, Ito H, Tokunaga K, Ohashi J, Homma S, Kobashi Y,
Taguchi Y, et al: Molecular cloning of two novel mucin-like genes
in the disease-susceptibility locus for diffuse panbronchiolitis.
Hum Genet. 129:117–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim YK, Shin DH, Kim KB, Shin N, Park WY,
Lee JH, Choi KU, Kim JY, Lee CH, Sol MY and Kim MH: MUC5AC and
MUC5B enhance the characterization of mucinous adenocarcinomas of
the lung and predict poor prognosis. Histopathology. 67:520–528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee HK, Kwon MJ, Seo J, Kim JW, Hong M,
Park HR, Min SK, Choe JY, Ra YJ, Jang SH, et al: Expression of
mucins (MUC1, MUC2, MUC5AC and MUC6) in ALK-positive lung cancer:
Comparison with EGFR-mutated lung cancer. Pathol Res Pract.
215:459–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kai Y, Amatya VJ, Kushitani K, Kambara T,
Suzuki R, Tsutani Y, Miyata Y, Okada M and Takeshima Y: Mucin 21 is
a novel, negative immunohistochemical marker for epithelioid
mesothelioma for its differentiation from lung adenocarcinoma.
Histopathology. 74:545–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshimoto T, Matsubara D, Soda M, Ueno T,
Amano Y, Kihara A, Sakatani T, Nakano T, Shibano T, Endo S, et al:
Mucin 21 is a key molecule involved in the incohesive growth
pattern in lung adenocarcinoma. Cancer Sci. 110:3006–3011. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lakshmanan I, Rachagani S, Hauke R, Krishn
SR, Paknikar S, Seshacharyulu P, Karmakar S, Nimmakayala RK,
Kaushik G, Johansson SL, et al: MUC5AC interactions with integrin
β4 enhances the migration of lung cancer cells through FAK
signaling. Oncogene. 35:4112–4121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qu J, Yu H, Li F, Zhang C, Trad A, Brooks
C, Zhang B, Gong T, Guo Z, Li Y, et al: Molecular basis of antibody
binding to mucin glycopeptides in lung cancer. Int J Oncol.
48:587–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itoh Y, Kamata-Sakurai M, Denda-Nagai K,
Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M and
Irimura T: Identification and expression of human
epiglycanin/MUC21: A novel transmembrane mucin. Glycobiology.
18:74–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang DC, Wang W, Zhu B and Wang X: Lung
cancer heterogeneity and new strategies for drug therapy. Annu Rev
Pharmacol Toxicol. 58:531–546. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamada N, Nishida Y, Tsutsumida H, Hamada
T, Goto M, Higashi M, Nomoto M and Yonezawa S: MUC1 expression is
regulated by DNA methylation and histone H3 lysine 9 modification
in cancer cells. Cancer Res. 68:2708–2716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yokoyama S, Higashi M, Kitamoto S, Oeldorf
M, Knippschild U, Kornmann M, Maemura K, Kurahara H, Wiest E,
Hamada T, et al: Aberrant methylation of MUC1 and MUC4 promoters
are potential prognostic biomarkers for pancreatic ductal
adenocarcinomas. Oncotarget. 7:42553–42565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okudaira K, Kakar S, Cun L, Choi E, Wu
Decamillis R, Miura S, Sleisenger MH, Kim YS and Deng G: MUC2 gene
promoter methylation in mucinous and non-mucinous colorectal cancer
tissues. Int J Oncol. 36:765–775. 2010.PubMed/NCBI
|
|
48
|
Yokoyama S, Higashi M, Tsutsumida H,
Wakimoto J, Hamada T, Wiest E, Matsuo K, Kitazono I, Goto Y, Guo X,
et al: TET1-mediated DNA hypomethylation regulates the expression
of MUC4 in lung cancer. Genes Cancer. 8:517–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kitamoto S, Yamada N, Yokoyama S, Houjou
I, Higashi M, Goto M, Batra SK and Yonezawa S: DNA methylation and
histone H3-K9 modifications contribute to MUC17 expression.
Glycobiology. 21:247–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vincent A, Perrais M, Desseyn JL, Aubert
JP, Pigny P and Van Seuningen I: Epigenetic regulation (DNA
methylation, histone modifications) of the 11p15 mucin genes (MUC2,
MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene.
26:6566–6576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu F, Lin H, He P, He L, Chen J, Lin L and
Chen Y: A TP53-associated gene signature for prediction of
prognosis and therapeutic responses in lung squamous cell
carcinoma. Oncoimmunology. 9:17319432020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamada N, Hamada T, Goto M, Tsutsumida H,
Higashi M, Nomoto M and Yonezawa S: MUC2 expression is regulated by
histone H3 modification and DNA methylation in pancreatic cancer.
Int J Cancer. 119:1850–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lakshminarasimhan R and Liang G: The role
of DNA methylation in cancer. Adv Exp Med Biol. 945:151–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|


















