Introduction
The terpenoid polyphenol pinosylvin
(trans-3,5-dihydroxystilbene) is a stilbene present in the
heartwood of coniferous trees of the genus Pinus (1). Many studies have demonstrated that
biological characteristics of pinosylvin include antibacterial and
antifungal activity (2) and
protection against oxidative stress in human cells (3). Pinosylvin regulates Src/ERK and
GSK-3/β-catenin signaling to inhibit tumor cell growth (4). Pinosylvin has been shown to inhibit the
expression of MMP-2 and MMP-9 in human fibrosarcoma HT1080 cells
(5).
Nasopharyngeal carcinoma (NPC) is a tumor located in
the nasopharynx and is caused by epithelial cells covering the
nasopharyngeal surface. Unlike other head and neck epithelial
cancers, NPC is highly invasive and metastatic (6). NPC is particularly prevalent in Southern
China, Southeast Asia, North Africa and the Arctic region, which is
a unique geographical distribution (7). Four primary causes of nasopharyngeal
carcinoma have been identified, including Epstein-Barr and human
papillomavirus infection, genetic susceptibility and consumption of
salted fish (8). NPC occurs adjacent
to cervical lymph nodes, which increases the risk of metastasis in
other parts of the body, thereby causing difficulties in surgical
treatment (8). Currently,
chemotherapy and radiotherapy can improve the survival rate of
patients with advanced NPC (9).
Preventing distant metastasis is key to treatment, and more
effective systemic drugs should be investigated (10).
The metastasis of NPC occurs in two stages:
Translocation to distant tissue and colonization (11). The initial step degrades and
penetrates the extracellular matrix of surrounding tissue (12). Among the involved proteolytic enzymes,
zinc-dependent MMPs contribute substantially to proteolytic
degradation and intercellular interaction damage (13). Research has indicated that MMP-2 and
MMP-9 are key treatment targets for regulation of tumor metastasis
in NPC (14), cervical cancer
(15) and retinoblastoma (16). Lyu et al (17) reported that liposome-containing
thermosensitive liposomes can deliver MMP inhibitors, decreasing
the activity of MMP-2 and MMP-9 by 50 and 43%, respectively, to
inhibit metastasis and angiogenesis. Huang et al (18) demonstrated that exosomes with low
expression levels of microRNA-34c-3p affect expression of integrin
α2β1 and promote the invasion and migration of non-small cell lung
cancer cells.
Epithelial-mesenchymal transition (EMT) is a key
process involved in tumor metastasis and recurrence (19,20).
Research has indicated that the expression of mesenchymal markers,
such as vimentin and N-cadherin, increases during EMT, whereas
epithelial marker E-cadherin, a powerful tumor cell invasion
inhibitor, is downregulated (21,22). The
MAPK pathway is an important intracellular signal transduction
pathway that serves a key role in regulating tumor metastasis, as
well as regulating cell proliferation, differentiation, apoptosis
and angiogenesis (23). The ERK
subfamily (typical ERK 1/2/5 and atypical ERK 3/4/7/8) of proteins
is known for its contributions to EMT (23,24).
PI3K/AKT and MAPK pathways contribute to TGF-β2-induced
upregulation of Jagged-1, which mimics TGF-β2-induced EMT in
retinal pigment epithelium cells (25). TGF-β, in addition to its role in cell
differentiation, migration and adhesion, also induces EMT via both
Smad and MAPK pathways (26). A
previous study indicated that pinosylvin exerts antimetastatic
effects on human oral cancer cells (27). However, the antimigratory effect of
pinosylvin on NPC cells remains unknown. Therefore, the present
study investigated the effect of pinosylvin on NPC cell metastasis
and regulation of its signaling.
Materials and methods
Chemicals
Pinosylvin (≥97% purity) was purchased from
ChemFaces. DMSO was used to prepare 100 mM storage solution of
pinosylvin, which was stored at −20°C. The maximum concentration of
DMSO used for treatment in medium was <0.2%. MTT, ERK1/2, p38
and JNK1/2 specific inhibitors (U0126, SB203580 and SP600125) were
obtained from Sigma-Aldrich (Merck KGaA).
Cell culture
Nasal cavity cancer cells (RPMI 2650) were obtained
from Japanese Collection of Research Bioresources Cell Bank (Osaka,
Japan). Human nasopharyngeal cancer cell lines (NPC-039 and NPC-BM)
were provided by Dr Jen-Tsun Lin, Department of Hematology and
Oncology, Changhua Christian Hospital (Changhua, Taiwan). RPMI-2650
cells were cultured in Eagle's Minimum Essential Medium (Gibco;
Thermo Fisher Scientific, Inc.); NPC cell lines were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). All
culture media were supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 1 mM glutamine, 1%
penicillin/streptomycin (10,000 U/ml penicillin and 10 mg/ml
streptomycin), 1.5 g/l sodium bicarbonate and 1 mM sodium pyruvate.
All cell cultures were maintained at 37°C in a humidified
atmosphere of 5% CO2.
In vitro cytotoxicity assay (MTT
assay)
Cytotoxicity was assessed via MTT (0.1%,) assay. All
cells were cultured in 96-well plates (1×104/well) at
37°C in 5% CO2 overnight. Subsequently, supernatant was
removed and cultures were treated with different concentrations of
pinosylvin (0, 20, 40 and 80 µM) at 37°C for 24 h. Following
treatment, the medium containing pinosylvin was removed and MTT
reagent (1 mg/ml) was added to each well at 37°C in 5%
CO2. After 4 h, the supernatant containing MTT reagent
was removed and DMSO was added to dissolve the formed blue formazan
crystals. Absorbance was measured at 595 nm using
spectrophotometry. A total of three independent experimental
replicates was performed.
Gap closure assay
Gap closure assay was used to measure migration of
NPC-039 and NPC-BM cells over a certain distance. NPC-039 and
NPC-BM cells (3×104) were grown onto each side of a
culture insert (Ibidi GmbH) at 37°C overnight. After reaching 90%
confluence, culture inserts were removed and gap closure assay was
performed. Cultures were treated with pinosylvin (0, 20, 40 and 80
µM) in serum-free RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C for 24 h. The cell migration distance was observed and
photographed after 0, 3 and 6 h. Migration was measured using
ImageJ 1.47 version software (National Institutes of Health) and
expressed as a percentage using the following formula: (Initial gap
width of the experimental group-remaining width of the experimental
group)/(initial gap width of the control group-remaining width of
the untreated control group) ×100. Images were captured under a
light microscope (Lecia GmbH). The entire procedure was repeated
three times and the values are indicated as mean ± SD.
Cell migration and invasion assay
NPC-039 and NPC-BM cell migration and invasion
assays were performed as described by Yang et al (28). Briefly, NPC cells (3×104)
were placed on the upper well of a Transwell insert (Greiner
Bio-One International GmbH) with serum-free medium (RPMI-1640) and
10% FBS-containing medium (RPMI-1640 medium) (600 µl) was added to
the lower chamber for 24 h at 37°C. For the invasion assay,
Matrigel (25 mg/50 ml; 60 µl; BD Biosciences) was coated on the
upper Transwell at 37°C, overnight. Migrated or invaded cells were
fixed with 99% methanol at room temperature for 15 min and stained
with Giemsa (1X) at room temperature for 2 h. Images were captured
and number of cells was counted under an optical light microscope
(Lecia Germany) at 100× magnification using ImageJ 1.47 version
cell count software (National Institutes of Health). A total three
fields of view was randomly selected for each concentration. Data
are presented as the mean ± SD (n=3).
Gelatin zymography
Enzyme activity of MMP-2 was analyzed via gelatin
zymography. Briefly, after plating NPC-039 and NPC-BM cells
(5×104 cells/well) in 24-well plates at 37°C for 16 h,
cells were treated with different concentrations (0, 20, 40 and 80
µM) of pinosylvin at 37°C for 24 h. Culture medium was collected
and subjected to 8% SDS-PAGE with 0.1% gelatin as described
previously (29).
Western blot analysis
Following treatment with different concentrations of
pinosylvin, cells were lysed with 1X RIPA buffer (EMD Millipore)
containing protease and phosphatase inhibitor cocktails and
subjected to BCA (Thermo Fisher Scientific, Inc.) protein
concentration assay. All samples were separated using 10.0 or 12.5%
SDS-PAGE and proteins were transferred onto a PVDF membrane (EMD
Millipore). Membranes were blocked with 5% non-fat milk in TBST
(0.05% Tween-20) at room temperature for 1 h. Detection was
performed with a primary antibody overnight at 4°C followed by a
horseradish peroxidase (HRP)-conjugated secondary antibody
(Anti-rabbit IgG, #7074, 1:3,000; Anti-mouse IgG, #7076, 1:3,000,
Cell Signaling Technology, Inc.) at room temperature for 1 h. The
following antibodies (all 1:1,000; all Cell Signaling Technology,
Inc. unless otherwise indicated) were used: Anti-ERK1/2 (cat. no.
#4695; 42, 44 kDa), anti-JNK1/2 (cat. no. #9252; 46, 54 kDa),
anti-p38 (cat. no. #8690; 40 kDa), anti-phosphorylated
(phospho-)ERK1/2 (cat. no. #4370; 42, 44 kDa), anti-phospho-JNK1/2
(cat. no. #4668; 46, 54 kDa), anti-phospho-p38 (cat. no. #4511; 43
kDa), anti-MMP-2 (cat. no. #87809; 64 kDa), anti-N-cadherin (cat.
no. #13116; 140 kDa), anti-E-cadherin (cat. no. #3195; 135 kDa),
anti-zonula occludens (ZO)-1 (cat. no. #8193; 220 kDa),
anti-vimentin (cat. no. #5741; 57 kDa), anti-MMP-9 (cat. no.
#AB19016; 92 kDa; EMD Millipore) and anti-b-actin (1:5,000; cat.
no. NB600-501; 42 kDa; Novus Biologicals). Immunoblotting was
observed using HRP chemiluminescent substrates (EMD Millipore).
Images were captured using ImageQuant LAS 4000 mini (GE Healthcare)
and relative density was quantitated by ImageJ 1.47 version
software (National Institutes of Health).
Proteome profiler human protease
array
The Proteome Profiler Human Protease Array kit (cat.
no. ARY021B; R&D Systems, Inc.) was used according to the
manufacturer's instructions. Array buffer 6 was added into each
well of the 4-well Multi-dish and incubated at room temperature for
1 h. Then, 15 µl reconstituted protease detection antibody cocktail
was added at room temperature for 1 h. Sample mixtures were
incubated with membrane overnight at 4°C. Each membrane was washed
with wash buffer for 10 min. Streptavidin-HRP was added into each
well and incubated for 30 min at room temperature. Immunoblotting
was observed using HRP chemiluminescent substrate (EMD Millipore).
Images were captured using ImageQuant LAS 4000 mini (GE Healthcare)
and relative density was quantitated by ImageJ 1.47 version
software (National Institutes of Health).
Statistical analysis
The experimental data are expressed as the mean ± SD
(n≥3). Comparisons between >2 groups were analyzed by one-way
ANOVA followed by post hoc Tukey's test. Paired student's t-test
was used to analyze differences between two groups. All statistical
analyses were performed using GraphPad Prism Software Version 5.0
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
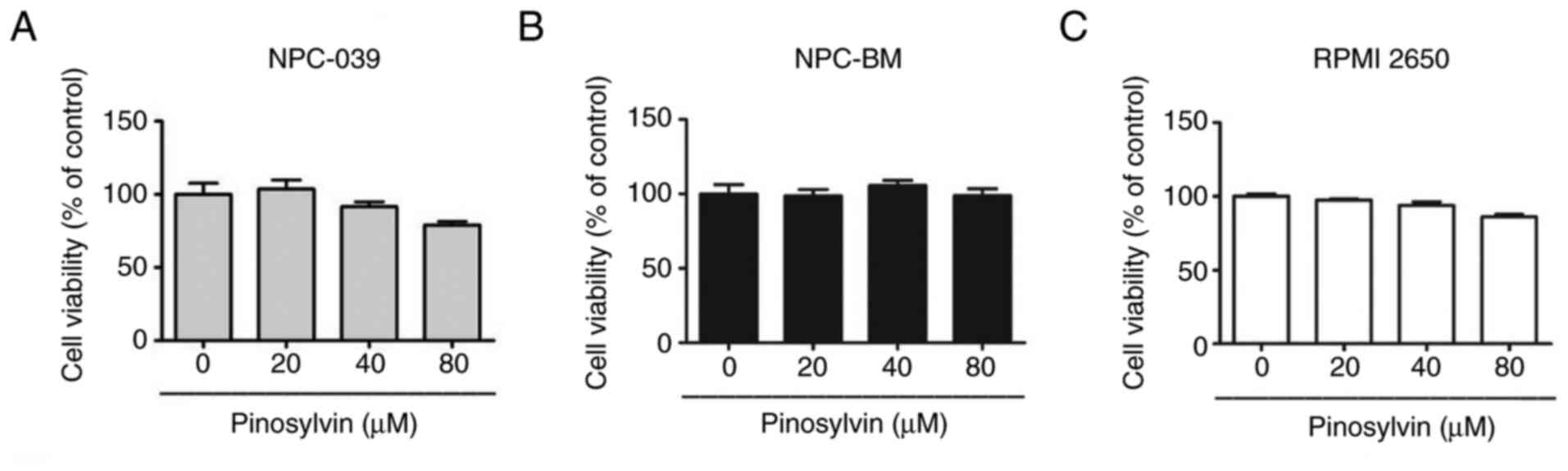
Pinosylvin does not induce
cytotoxicity in three cell lines
The cytotoxic effects of various concentrations of
pinosylvin (0, 20, 40 and 80 µM) on cell lines were assessed using
MTT assay for 24 h (Fig. 1A-C).
Pinosylvin did not exert significant cytotoxic effects on the
viability of NPC-039, NPC-BM and RPMI-2650 cell lines. All
subsequent experiments examined antimetastatic properties of
pinosylvin at non-cytotoxic concentrations.
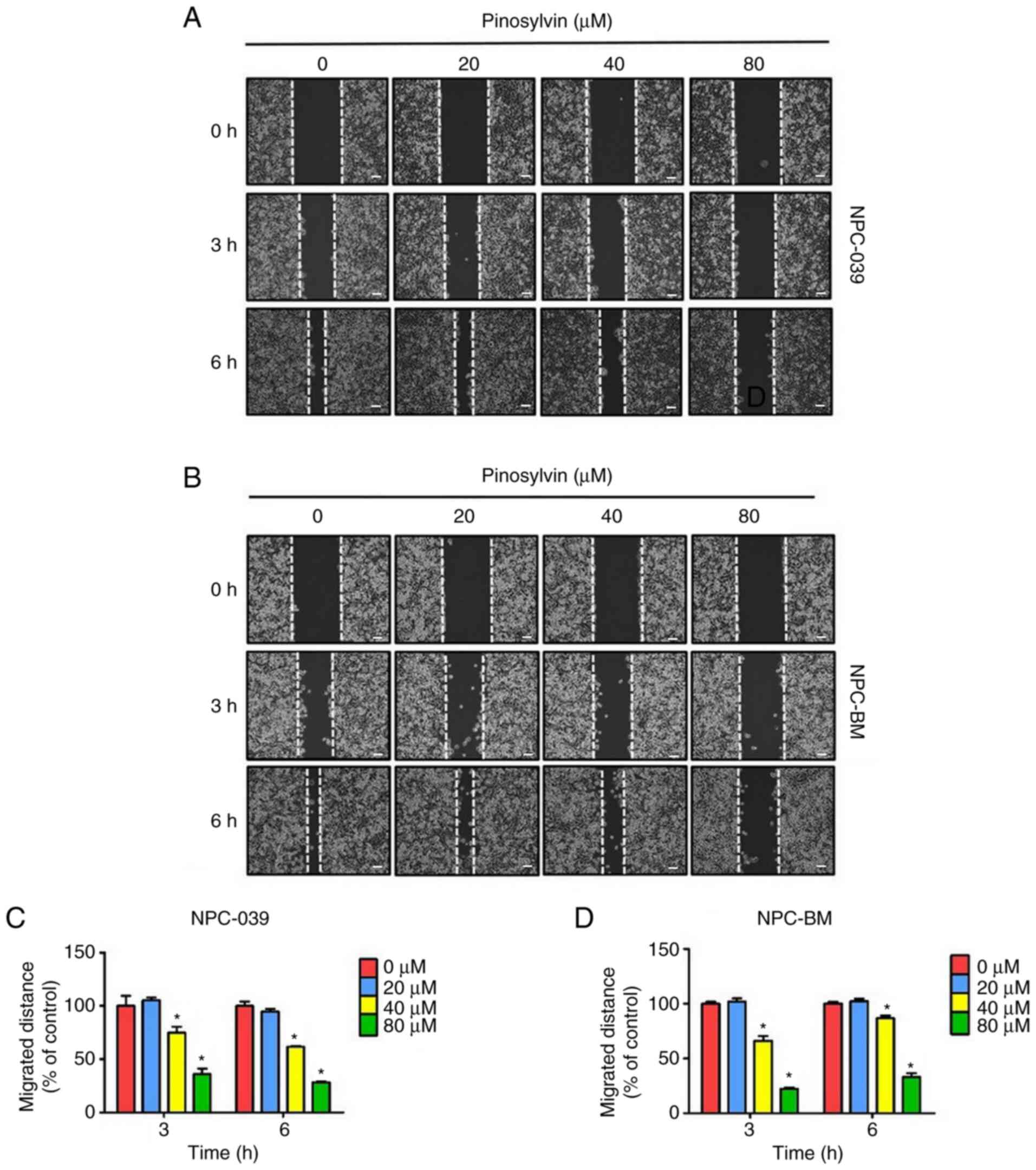
Pinosylvin inhibits migration and
invasion in NPC cell lines
Gap closure assay was performed to assess the effect
of pinosylvin on the mobility of NPC cells treated with 0–80 µM
pinosylvin for 0, 3 and 6 h (Fig. 2).
Compared with the control group, the migrated distance of the cell
monolayers was significantly decreased at high concentrations (80
µM) of pinosylvin. In addition, the effect of pinosylvin on the
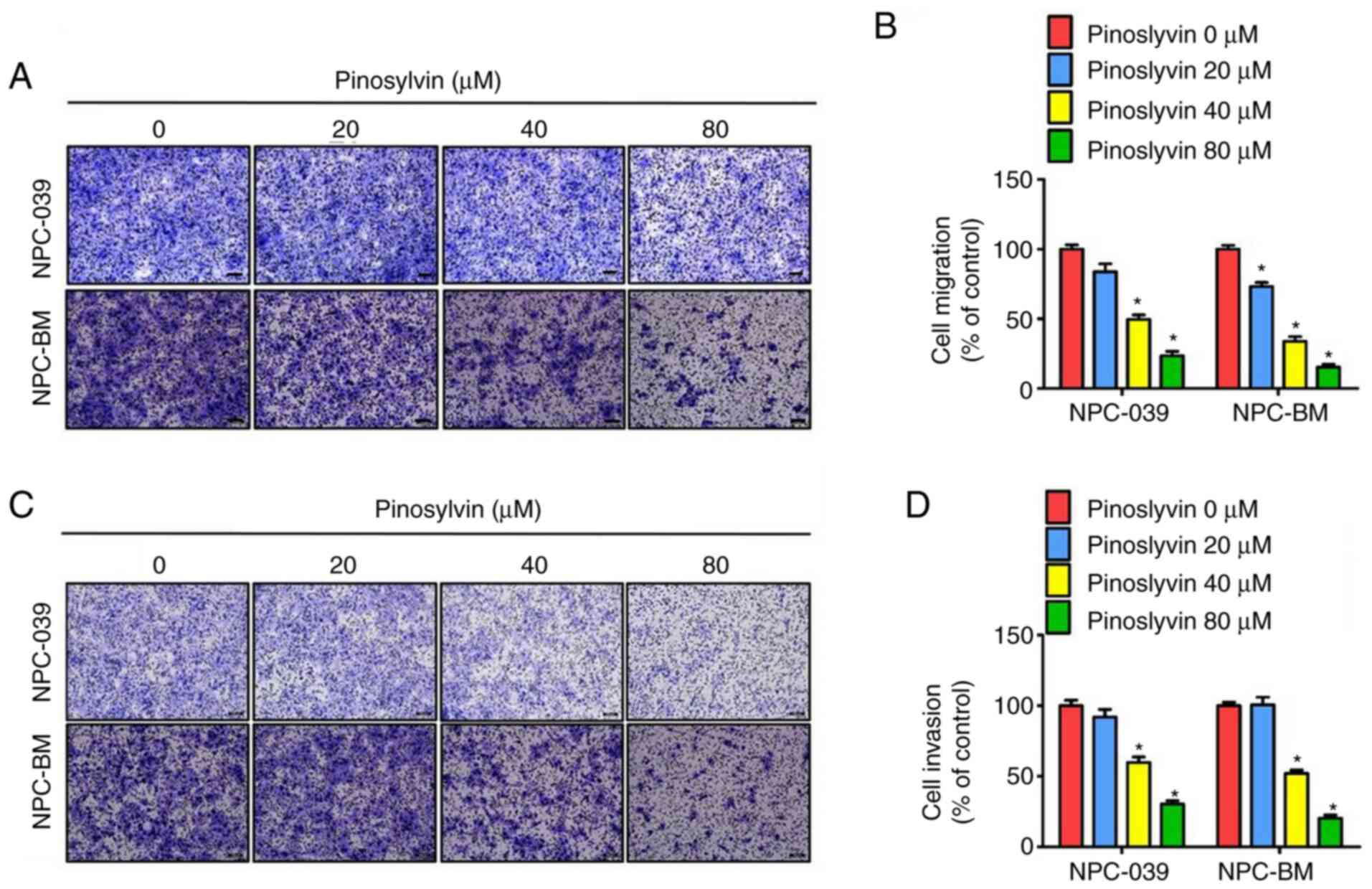
migration and invasion ability in NPC cells was assessed by
Transwell assay (Fig. 3A-D);
pinosylvin significantly decreased the migration and invasion
abilities of two NPC cell lines.
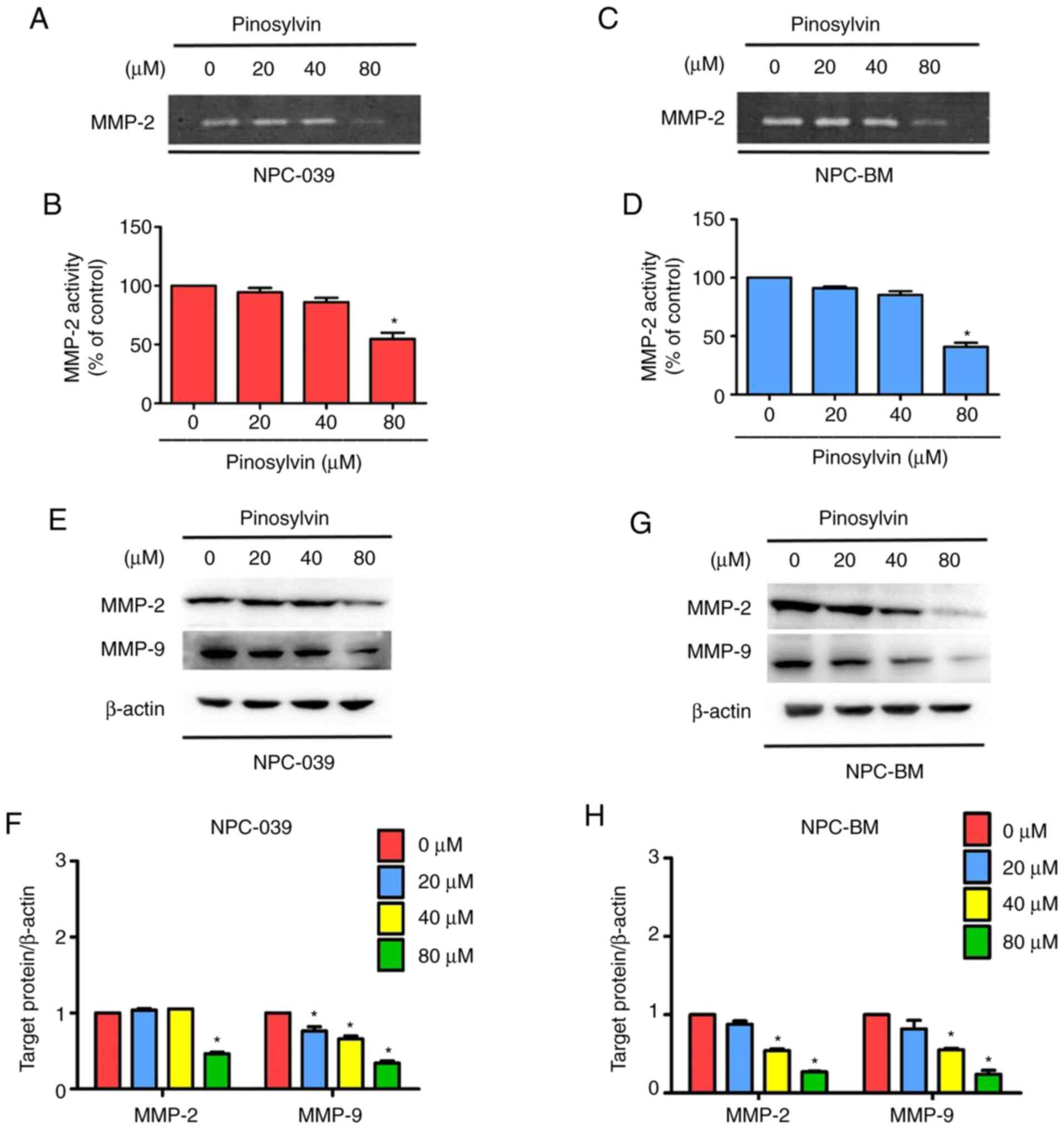
Pinosylvin changes migration of NPC
cell line and inhibits MMP-2 activity
According to the results of Proteome Profiler Human
Protease Array (Fig. S1), to lack of
observed differences. MMPs regulate cancer cell migration and
invasion (13). In order to determine
whether MMP-2 and MMP-9 are regulated by pinosylvin in two NPC cell
lines, zymography and western blotting were performed to analyze
enzyme activity and protein concentration. Pinosylvin at the
highest concentration significantly decreased enzymatic activity of
MMP-2 in two NPC cell lines (Fig.
4A-D). Following 24 h treatment, a high pinosylvin
concentration (80 µM) decreased expression levels of MMP-2 and
MMP-9 to 54 and 66 in NPC-039 and 52 and 41% in NPC-BM cells,
respectively (Fig. 4E-H).
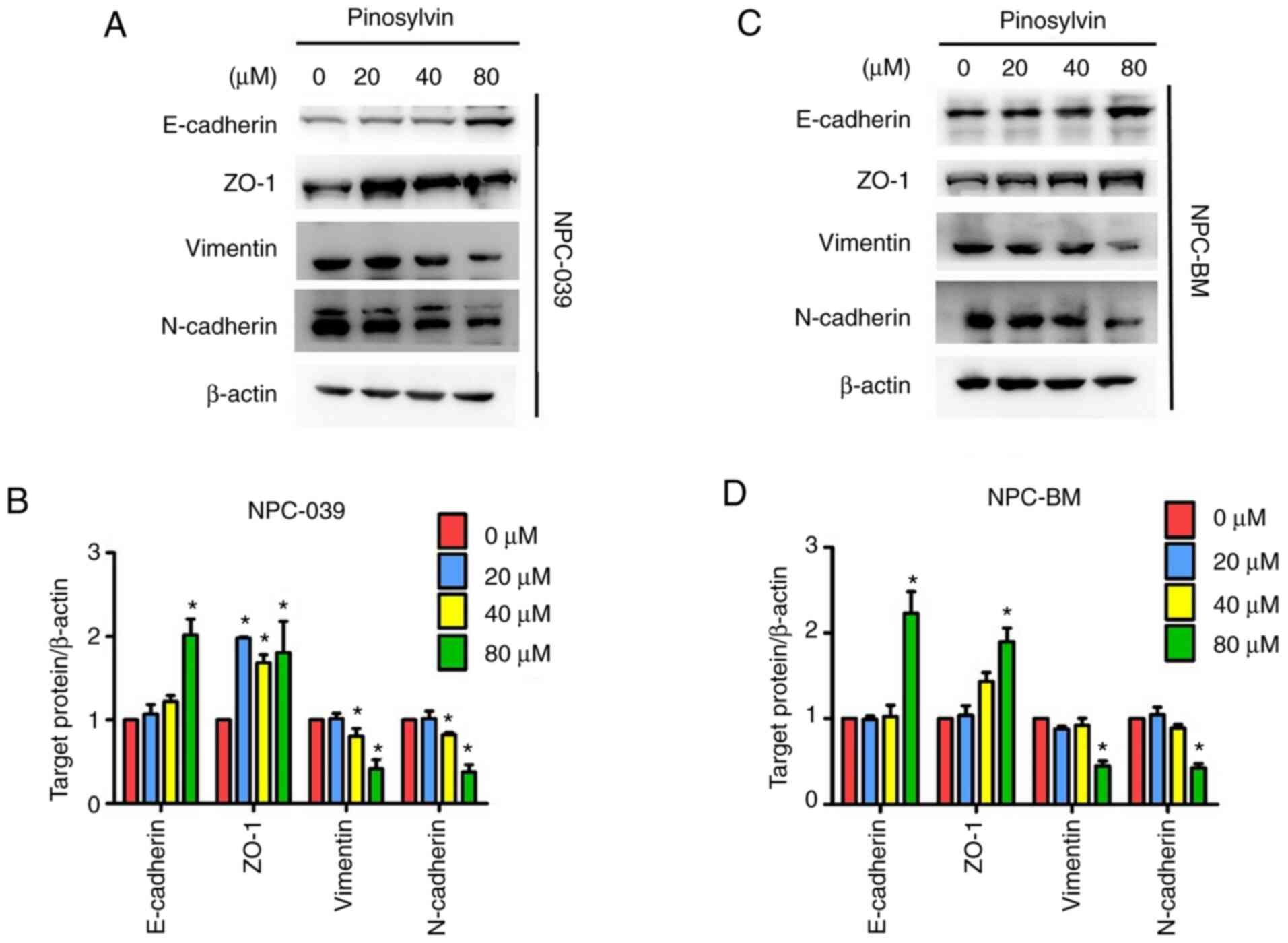
Pinosylvin affects EMT-associated
protein expression in NPC cell lines
When wound healing occurs, organ fibrosis and the
initiation of metastasis in cancer progression prompt EMT (30). Analysis of expression levels of
EMT-specific proteins (Fig. 5A-D)
demonstrated that pinosylvin at a high concentration significantly
decreased expression of vimentin and N-cadherin to 58.5 and 62.5 in
NPC-039 and 55.0 and 58.5% in NPC-BM, respectively, and
significantly increased expression of ZO-1 and E-cadherin to 80.0
and 101.5 in NPC-039 and 90.0 and 123.0% in NPC-BM,
respectively.
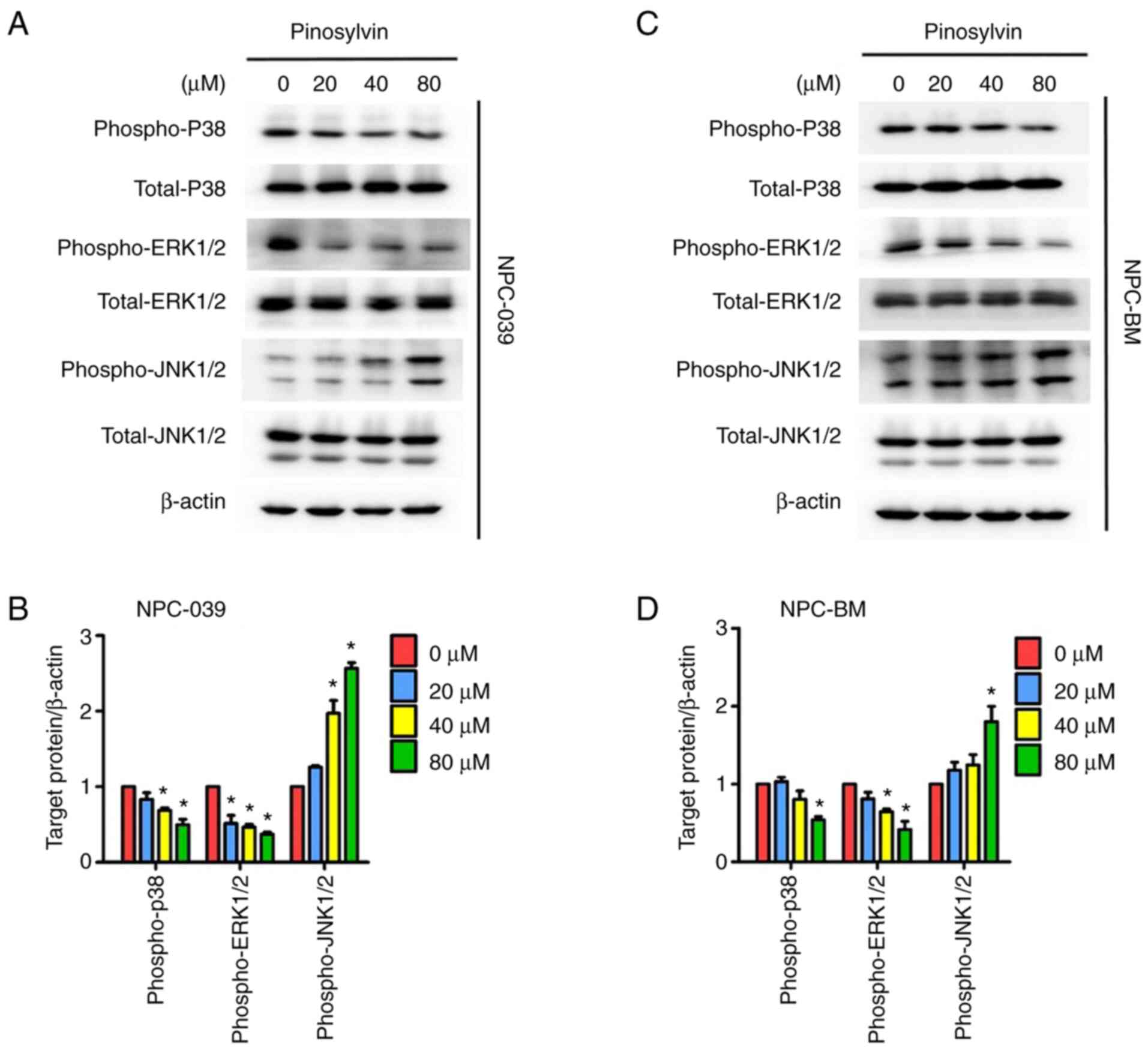
Pinosylvin decreases invasion and
migration ability of NPC cell lines via MAPK pathways
Western blotting was performed to detect changes in
the molecular mechanisms of MAPK pathways in response to treatment
with pinosylvin (Fig. 6A-D). As the
concentration of pinosylvin increased, phosphorylation of ERK1/2
and p38 decreased significantly. According to ImageJ analysis of
blots, treatment with 80 µM pinosylvin decreased the
phosphorylation of ERK1/2 and p38 to 63 and 51 in NPC-039 cells and
59 and 46% in NPC-BM cells, respectively, at 24 h compared with
untreated controls. By contrast, phosphorylation of JNK1/2 was
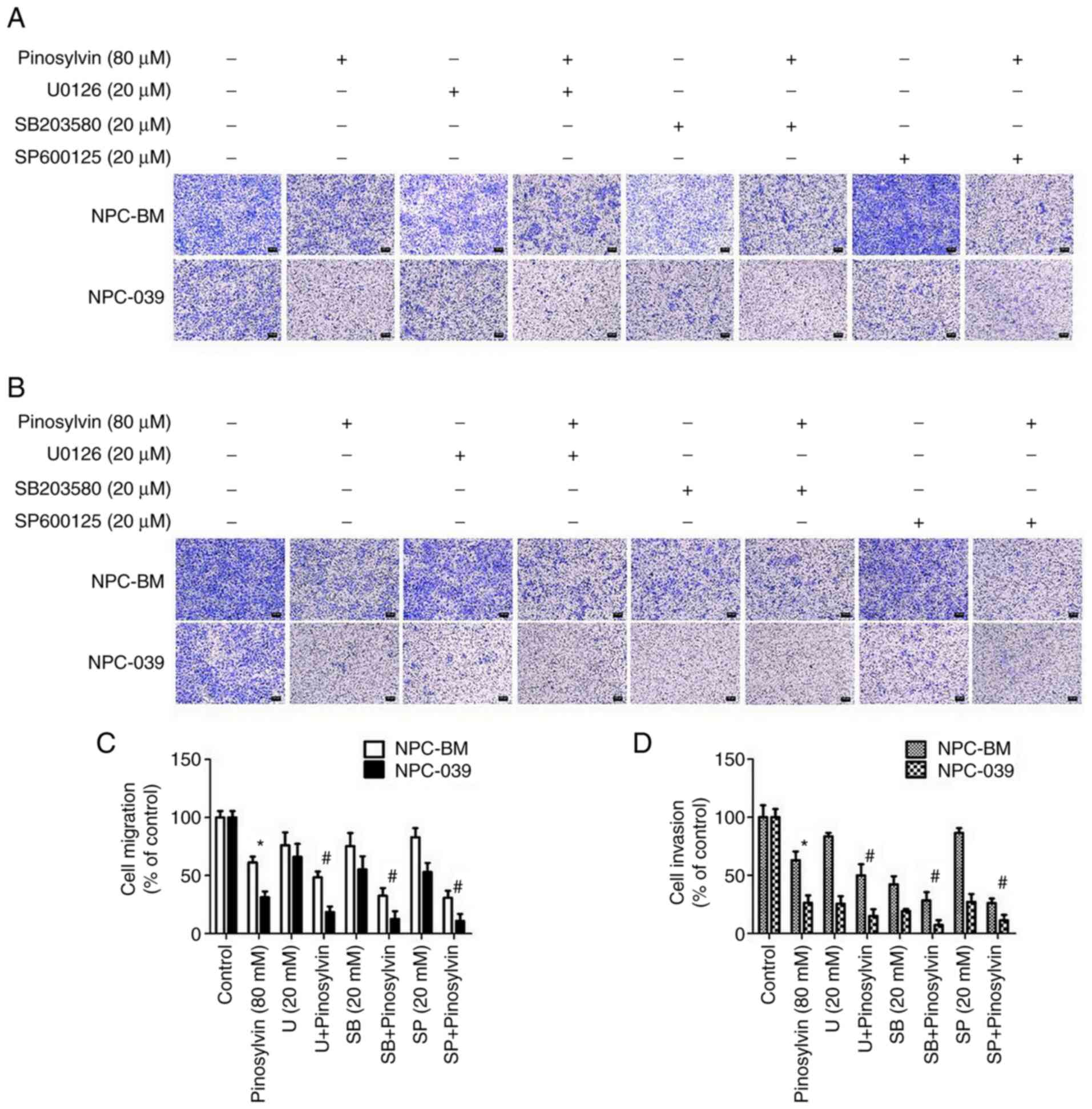
significantly increased in the two NPC cell lines. In order to
confirm the molecular mechanism underlying pinosylvin-induced
inhibition of NPC cell migration, cells were pre-treated with
specific inhibitors of ERK1/2, p38 and JNK1/2; following
pre-treatment with specific inhibitors, pinosylvin-inhibited cell
migration and invasion ability were significantly improved
(Fig. 7A-D). Taken together, these
findings indicate that pinosylvin exerted anti-metastatic effects
via p38, ERK1/2 and JNK1/2 signaling pathways in human NPC
cells.
Discussion
In an analysis of metastasis patterns of 629
patients with NPC, Huang et al (31) found that 95% of distant metastases
occurred <3 years after completion of radiotherapy. Hence,
determining effective methods of suppressing distant metastasis is
important in the treatment of NPC. Plant polyphenols are important
plant secondary metabolites with biological functions (such as
countering infection by pathogens or mitigating environmental
stresses), as well as antioxidant, anticancer and anti-inflammatory
properties (1,3,5,7). Studying compounds with such biochemical
activity is beneficial for drug development in the pharmaceutical
industry (32). Pinosylvin and
resveratrol are terpenoid polyphenols with similar structures
(2). Research has indicated that
pinosylvin inhibits growth of human colorectal cancer cells
(4), suppresses MMP-2 and MMP-9
activity in HT1080 cells (5) and
suppresses migration and invasion in SCC-9, SAS and HSC-3 cell
lines (27). In the present study,
pinosylvin did not decrease the viability of the two NPC cell lines
or a nasal cavity cancer cell line (RPMI 2650), however, high
concentrations of pinosylvin inhibited the migration and invasion
of NPC-039 and NPC-BM cells. Furthermore, the present results
indicated that pinosylvin inhibited NPC cell metastatic effects by
downregulating MMP-2/MMP-9 expression levels and modifying the
regulation of EMT markers.
MMPs serve important roles in mediating cancer cell
growth, differentiation, apoptosis, migration, invasion and
angiogenesis (33). Di Carlo et
al (34) performed zymography
analysis and demonstrated that the ratio of MMP-9/MMP-2 in patients
with cancer was increased compared with that in patients with
benign disease and healthy individuals. High expression of MMP-2
and MMP-9 is significantly correlated with local and distant
metastatic tumor recurrence and poor prognosis in head and neck
squamous cell carcinoma (35–37). In the present study, gelatin
zymography and western blotting were performed to analyze the
effects of pinosylvin on MMPs in two NPC cell lines; pinosylvin
significantly inhibited expression of MMP-2 and MMP-9 as well as
MMP2 activity. Tissue inhibitors of metalloproteinase (TIMPs)
control proteolytic activity and are a specific endogenous
inhibitor of MMPs (38). Western
blotting here showed that pinosylvin did not increase TIMP-1 or −2
protein levels in the two NPC cell lines (data not shown). This
indicated that pinosylvin decreased MMP-2 protein expression levels
and activity, via regulated the activation of zymogen at the
post-transcriptional level.
EMT is a key step in tumor cell migration and
invasion in various types of human cancer (39–41).
Upregulation of N-cadherin induces EMT (40); another regulator of EMT is E-cadherin,
which inhibits the occurrence of EMT and serves as a tumor
suppressor (41). Vimentin is the
primary cytoskeletal component of mesenchymal cells (42). ZO-1 and ZO-2 are required for tight
junction formation and function (43,44);
mutations in ZO-1 and claudin-1 induce EMT (45). In the present study,
pinosylvin-treated NPC-BM and NPC-039 cells exhibited significantly
induced E-cadherin and ZO-1 expression, but decreased expression of
N-cadherin and vimentin. These findings suggest that pinosylvin
inhibited EMT at the initiation step of tumor metastasis.
Compared with other intracellular signal
transduction pathways (23), the MAPK
pathway serves a more important role in cell proliferation,
differentiation, apoptosis, angiogenesis and tumor metastasis
(23,24). A study indicated that TBL-12, a sea
cucumber extract, inhibits migration and invasion of human PCa
cells by inhibiting MMP-2 and MMP-9 via decreased phosphorylation
of p38 (46). Additionally,
18β-glycyrrhetinic acid inhibits migration and invasion of gastric
cancer cells via the reactive oxygen species/protein kinase C-α/ERK
signaling pathway (47). Therefore,
the present study investigated whether the MAPK pathway is altered
by pinosylvin treatment. Western blot analysis revealed that
pinosylvin suppressed ERK1/2 and p38 protein phosphorylation but
induced JNK protein phosphorylation in both NPC cell lines. This
result is consistent with previously reported inhibition of Huh7
cell proliferation and metastasis by cucurbitacin E via suppression
of MAPKs (48). A previous study
showed that pinosylvin inhibits the growth of human colorectal
cancer cells via suppression of Src/ERK and GSK-3/β-catenin
signaling (4). In our previous
research, pinosylvin inhibited migration and invasion of oral
cancer cells by suppressing the expression and activity of MMP-2
and ERK1/2 signaling (27). The
present results suggest that pinosylvin was involved in MMP-2/MMP-9
regulation in NPC cells and that the MAPK pathway may serve a key
role.
Identifying effective methods for treating distant
metastases resulting from NPC is crucial. In summary, the present
results demonstrated that pinosylvin decreased activity of MMP-2
and expression of MMP-2/MMP-9 in both NPC-BM and NPC-039 cell
lines. Pinosylvin significantly inhibited both cell migration and
invasion. The expression levels of epithelial markers increased,
while those of mesenchymal markers decreased following treatment
with pinosylvin. Following pre-treatment with specific inhibitors
of ERK1/2, p38 and JNK1/2, pinosylvin-inhibited cell migration and
invasion significantly improved. However, the lack of activator
experiments is a potential limitation to the present study. A
recent study suggested that pinosylvin is mostly metabolized in
vivo and may provide a material basis for studying the
pharmacological action of pinosylvin, thus providing information
for the clinical treatment of chronic gastritis and gastric ulcers
using Radix Linderae Reflexae (49).
The short half-life and limited systemic exposure of pinosylvin
prompt caution in its therapeutic application (50). However, the lack of in vivo
experiments is a potential limitation to the present study. The
present results suggested that pinosylvin may be useful in the
development of drugs for treating NPC and preventing migration and
invasion of NPC cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Changhua
Christian Hospital (grant no. 109-CCH-IRP-013).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MCH, MJH and JTL conceptualized and designed the
study. CCL, YCC, YSL and HYH. acquired, analyzed and interpreted
data. MCH, YCC and MJH drafted and revised the manuscript. MJH and
JTL had overall responsibility for the published work. MJH and CCL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviation
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
References
|
1
|
Riviere C, Pawlus AD and Merillon JM:
Natural stilbenoids: Distribution in the plant kingdom and
chemotaxonomic interest in vitaceae. Nat Prod Rep. 29:1317–1333.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SK, Lee HJ, Min HY, Park EJ, Lee KM,
Ahn YH, Cho YJ and Pyee JH: Antibacterial and antifungal activity
of pinosylvin, a constituent of pine. Fitoterapia. 76:258–260.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koskela A, Reinisalo M, Hyttinen JM,
Kaarniranta K and Karjalainen RO: Pinosylvin-mediated protection
against oxidative stress in human retinal pigment epithelial cells.
Mol Vis. 20:760–769. 2014.PubMed/NCBI
|
|
4
|
Park EJ, Chung HJ, Park HJ, Kim GD, Ahn YH
and Lee SK: Suppression of Src/ERK and GSK-3/β-catenin signaling by
pinosylvin inhibits the growth of human colorectal cancer cells.
Food Chem Toxicol. 55:424–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park EJ, Park HJ, Chung HJ, Shin Y, Min
HY, Hong JY, Kang YJ, Ahn YH, Pyee JH and Lee SK: Antimetastatic
activity of pinosylvin, a natural stilbenoid, is associated with
the suppression of matrix metalloproteinases. J Nutr Biochem.
23:946–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen AW, Tseng YS, Lin CC, His YT, Lo YS,
Chuang YC, Lin SH, Yu CY, Hsieh MJ and Chen MK: Norcantharidin
induce apoptosis in human nasopharyngeal carcinoma through caspase
and mitochondrial pathway. Environ Toxicol. 33:343–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng WT, Lee MC, Hung WM, Choi CW, Lee KC,
Chan OS and Lee AW: Clinical outcomes and patterns of failure after
intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int
J Radiat Oncol Biol Phys. 79:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Overall CM and Lopez-Otin C: Strategies
for MMP inhibition in cancer: Innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chien SY, Hsieh MJ, Chen CJ, Yang SF and
Chen MK: Nobiletin inhibits invasion and migration of human
nasopharyngeal carcinoma cell lines by involving ERK1/2 and
transcriptional inhibition of MMP-2. Expert Opin Ther Targets.
19:307–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun YS, Thakur K, Hu F, Cespedes-Acuna CL,
Zhang JG and Wei ZJ: Icariside II suppresses cervical cancer cell
migration through JNK modulated matrix metalloproteinase-2/9
inhibition in vitro and in vivo. Biomed Pharmacother.
125:1100132020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC Cancer.
17:4342017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyu Y, Xiao Q, Yin L, Yang L and He W:
Potent delivery of an MMP inhibitor to the tumor microenvironment
with thermosensitive liposomes for the suppression of metastasis
and angiogenesis. Signal Transduct Target Ther. 4:262019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Yan Y, Liu Y, Lin M, Ma J, Zhang
W, Dai J, Li J, Guo Q, Chen H, et al: Exosomes with low miR-34c-3p
expression promote invasion and migration of non-small cell lung
cancer by upregulating integrin α2β1. Signal Transduct Target Ther.
5:392020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Floor S, van Staveren WC, Larsimont D,
Dumont JE and Maenhaut C: Cancer cells in epithelial-to-mesenchymal
transition and tumor-propagating-cancer stem cells: Distinct,
overlapping or same populations. Oncogene. 30:4609–4621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wan FZ, Chen KH, Sun YC, Chen XC, Liang
RB, Chen L and Zhu XD: Exosomes overexpressing miR-34c inhibit
malignant behavior and reverse the radioresistance of
nasopharyngeal carcinoma. J Transl Med. 18:122020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin K, Baritaki S, Militello L, Malaponte
G, Bevelacqua Y and Bonavida B: The role of B-RAF mutations in
melanoma and the induction of EMT via dysregulation of the
NF-kappaB/Snail/RKIP/PTEN Circuit. Genes Cancer. 1:409–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura M and Tokura Y:
Epithelial-mesenchymal transition in the skin. J Dermatol Sci.
61:7–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
24
|
Olea-Flores M, Zuñiga-Eulogio MD,
Mendoza-Catalán MA, Rodríguez-Ruiz HA, Castañeda-Saucedo E,
Ortuño-Pineda C, Padilla-Benavides T and Navarro-Tito N:
Extracellular-signal regulated kinase: A central molecule driving
epithelial-mesenchymal transition in cancer. Int J Mol Sci.
20:28852019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu
M, Ye S and Liu Y: Blockade of Jagged/Notch pathway abrogates
transforming growth factor β2-induced epithelial-mesenchymal
transition in human retinal pigment epithelium cells. Curr Mol Med.
14:523–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balogh P, Katz S and Kiss AL: The role of
endocytic pathways in TGF-β signaling. Pathol Oncol Res.
19:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen MK, Liu YT, Lin JT, Lin CC, Chuang
YC, Lo YS, His YT and Hsieh MJ: Pinosylvin reduced migration and
invasion of oral cancer carcinoma by regulating matrix
metalloproteinase-2 expression and extracellular signal-regulated
kinase pathway. Biomed Pharmacother. 117:1091602019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang SF, Chu SC, Liu SJ, Chen YC, Chang YZ
and Hsieh YS: Antimetastatic activities of Selaginella tamariscina
(Beauv.) on lung cancer cells in vitro and in vivo. J
Ethnopharmacol. 110:483–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho HY, Lin CW, Chien MH, Reiter RJ, Su SC,
Hsieh YH and Yang SF: Melatonin suppresses TPA-induced metastasis
by downregulating matrix metalloproteinase-9 expression through
JNK/SP-1 signaling in nasopharyngeal carcinoma. J Pineal Res.
61:479–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang CJ, Leung SW, Lian SL, Wang CJ, Fang
FM and Ho YH: Patterns of distant metastases in nasopharyngeal
carcinoma. Kaohsiung J Med Sci. 12:229–234. 1996.PubMed/NCBI
|
|
32
|
Marienhagen J and Bott M: Metabolic
engineering of microorganisms for the synthesis of plant natural
products. J Biotechnol. 163:166–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rahimi Z, Yari K and Rahimi Z: Matrix
metalloproteinase-9-1562T allele and its combination with MMP-2-735
C allele are risk factors for breast cancer. Asian Pac J Cancer
Prev. 16:1175–1179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 type IV collagenases in serum of patients with
pleural effusions. Int J Oncol. 26:1363–1368. 2005.PubMed/NCBI
|
|
35
|
Yoshizaki T, Maruyama Y, Sato H and
Furukawa M: Expression of tissue inhibitor of matrix
metalloproteinase-2 correlates with activation of matrix
metalloproteinase-2 and predicts poor prognosis in tongue squamous
cell carcinoma. Int J Cancer. 95:44–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Riedel F, Gotte K, Schwalb J, Bergler W
and Hormann K: Expression of 92-kDa type IV collagenase correlates
with angiogenic markers and poor survival in head and neck squamous
cell carcinoma. Int J Oncol. 17:1099–1105. 2000.PubMed/NCBI
|
|
37
|
Hong SD, Hong SP, Lee JI and Lim CY:
Expression of matrix metalloproteinase-2 and −9 in oral squamous
cell carcinomas with regard to the metastatic potential. Oral
Oncol. 36:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shrestha B, Bajracharya D, Byatnal AA,
Kamath A and Radhakrishnan R: May High MMP-2 and TIMP-2 expressions
increase or decrease the aggressivity of oral cancer? Pathol Oncol
Res. 23:197–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bagheri M, Fazli M, Saeednia S, Gholami
Kharanagh M and Ahmadiankia N: Sulforaphane modulates cell
migration and expression of β-catenin and epithelial mesenchymal
transition markers in breast cancer cells. Iran J Public Health.
49:77–85. 2020.PubMed/NCBI
|
|
40
|
Lopez-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Techasen A, Loilome W, Namwat N, Khuntikeo
N, Puapairoj A, Jearanaikoon P, Saya H and Yongvanit P: Loss of
E-cadherin promotes migration and invasion of cholangiocarcinoma
cells and serves as a potential marker of metastasis. Tumour Biol.
35:8645–8652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chernoivanenko IS and Minin AA and Minin
AA: Role of vimentin in cell migration. Ontogenez. 44:186–202.
2013.(In Russian). PubMed/NCBI
|
|
43
|
Helfand BT, Chang L and Goldman RD:
Intermediate filaments are dynamic and motile elements of cellular
architecture. J Cell Sci. 117:133–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shin K, Fogg VC and Margolis B: Tight
junctions and cell polarity. Annu Rev Cell Dev Biol. 22:207–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oliveira SS and Morgado-Diaz JA: Claudins:
Multifunctional players in epithelial tight junctions and their
role in cancer. Cell Mol Life Sci. 64:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan L, Huang X, Zhou K, Zhu X, Huang B,
Qiu S, Cao K and Xu L: Sea cucumber extract TBL-12 inhibits the
proliferation, migration, and invasion of human prostate cancer
cells through the p38 mitogen-activated protein kinase and
intrinsic caspase apoptosis pathway. Prostate. 79:826–839. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai H, Chen X, Zhang J and Wang J:
18β-glycyrrhetinic acid inhibits migration and invasion of human
gastric cancer cells via the ROS/PKC-α/ERK pathway. J Nat Med.
72:252–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Yang H, Guo Q, Liu T, Jiang Y, Zhao
M, Zeng K and Tu P: Cucurbitacin E inhibits Huh7 hepatoma carcinoma
cell proliferation and metastasis via suppressing MAPKs and
JAK/STAT3 pathways. Molecules. 25:5602020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fu Y, Sun X, Wang L and Chen S:
Pharmacokinetics and tissue distribution study of pinosylvin in
rats by Ultra-High-Performance liquid chromatography coupled with
linear trap quadrupole orbitrap mass spectrometry. Evid Based
Complement Alternat Med. 2018:41810842018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yeo SC, Luo W, Wu J, Ho PC and Lin HS:
Quantification of pinosylvin in rat plasma by liquid
chromatography-tandem mass spectrometry: Application to a
pre-clinical pharmacokinetic study. J Chromatogr B Analyt Technol
Biomed Life Sci. 931:68–74. 2013. View Article : Google Scholar : PubMed/NCBI
|