Introduction
Liver cancer is an aggressive tumor, and its
incidence is increasing globally (1). Hepatocellular carcinoma (HCC),
accounting for more than 90% of the total cases of primary liver
cancer, is the sixth most common type of cancer, and the fourth
most common cause of cancer-associated deaths worldwide (2). The majority of patients are diagnosed
at an advanced stage, at which time they have lost the opportunity
of operable (surgical) therapy. For inoperable patients, the drugs
that are available as molecular-targeting therapies include
sorafenib and lenvatinib. However, sorafenib only extends the
survival of patients by 3 months on average, and the objective
response rate is only 2–3% (3);
the extension of survival time of patients when administered
lenvatinib are similar to those of sorafenib (4). The majority of patients with HCC
already display metastasis at the time of diagnosis, even if their
tumors are small. Complications due to tumor metastasis are the
leading cause of cancer-associated deaths (5). Previous research published recently
has shown that changes in gene expression, the tumor
microenvironment and epithelial-to-mesenchymal transition (EMT) are
associated with tumor metastasis (6), although the complexity of the
underlying mechanism has only been partially elucidated for certain
types of cancer. Consequently, a better understanding of the
molecular mechanisms of HCC metastasis is urgently required in
order to provide new opportunities for therapeutic
interventions.
The extracellular matrix (ECM) is composed of
interstitial collagens and basement membrane. In addition to
providing tissue structural integrity and scaffolding, ECM also
adheres to the integrin family on cell membranes forming focal
adhesions that serve to promote tumor metastasis (7). The interaction between laminin-332,
one of the main proteins of the ECM, and integrin α6β4 (ITGA6 and
ITGB4) plays a key role in mediating the recognition and adhesion
of cells with the ECM, and also with respect to signal transmission
(8). Upon ligation, the
integrin-mediated pathway is activated, and the integrin-mediated
phosphorylation of focal adhesion kinase (FAK) activates downstream
signaling (9). In particular, the
hyperactivation of FAK, phosphoinositide 3-kinase (PI3K) and AKT
has been shown to be a common occurrence in human malignancies,
including esophageal squamous cell carcinoma, breast cancer and
HCC, and is involved with tumor metastasis (10–12).
Spindle pole body component 25 homolog (SPC25) is a
mitosis-associated spindle-assembly checkpoint regulatory protein
(13) that is involved with
genomic instability (14). SPC25
has been shown to be highly expressed in tumors, including
prostate, breast and non-small cell lung cancer (NSCLC), and is
associated with tumor progression (15–17).
In previously published studies on HCC, the results revealed that
SPC25 acts as an oncogene in HCC progression (18–20).
However, the underlying mechanism has yet to be fully elucidated.
In the present study, it was demonstrated that overexpression of
SPC25 in HCC is associated with poor prognosis, and both in
vitro and in vivo experiments were used to demonstrate
the promotion of metastasis by SPC25. The results of Agilent cDNA
microarray analysis showed that SPC25 silencing was
significantly correlated with ‘ECM receptor interactions’ which
were mainly mediated by the interaction of laminin-332 and integrin
α6β4 (ITGA6 and ITGB4). ITGB4 and SPC25 had the
strongest positive correlation based on the TCGA data and PCR and
had a similar role in promoting HCC metastasis. Ectopic
overexpression of ITGB4 with simultaneous silencing of SPC25
partially mitigated the reduction in cell invasion and migration
capability caused by SPC25 silencing. In the KEGG enrichment
analysis, we also found that the PI3K/AKT signaling pathway was
significantly altered. Previous study has shown that integrin
laminin binding increased FAK phosphorylation, which induces
activation of the PI3K/AKT signaling pathway to promote tumor
metastasis (10). The results of
the present study revealed that ITGB4 may be the main downstream
mediator of SPC25-induced metastatic activity that is involved in
ECM-integrin interactions, which subsequently activate the
FAK/PI3K/AKT signaling pathway to promote metastasis in HCC.
Materials and methods
Tissue microarrays (TMAs) and
immunohistochemistry (IHC) assay
The present study was approved by the Institutional
Review Board of the First Affiliated Hospital of Zhengzhou
University. All patients signed informed consent forms, and the
study was performed in accordance with the principles dictated in
the Declaration of Helsinki. TMAs were constructed with 141 pairs
of HCC tumor and normal liver tissues, which were collected at our
center between January 2012 and December 2015. The mean age of the
patients was 53.4 years (25–77 years).
As described previously (21), immunohistochemistry (IHC) assay was
performed using an UltraVision Quanto Detection System HRP (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions, and the SPC25 antibody (1:100; cat. no. ab121395;
Abcam) was used, and incubation was performed at 4°C for 12 h.
Subsequently, the integrated optical density (IOD) value was
detected using Image-Pro Plus 6.0 software (Image-Pro Plus,
http://scicrunch.org/resolver/SCR_007369; Media
Cybernetics, Inc.).
Bioinformatics analysis based on
public databases
The expression profile of liver cancer was obtained
from The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga) and The International
Cancer Genome Consortium (ICGC) database (https://dcc.icgc.org/) and the gene expression
profiles of GSE102079 (22) and
GSE112790 (23) were downloaded
from the Gene Expression Omnibus (GEO) database. Then the gene
expression data for HCC and adjacent non-cancerous tissues were
obtained. The Student's t-test was used to detect the differential
expression of SPC25. Kaplan-Meier method was used to compare
survival analysis for the SPC25 high and low expression patients
based on the TCGA and ICGC database.
Cell culture
All HCC cell lines used (MHCC97H, MHCC97L, HCCLM3
and Huh7) and the immortalized human hepatocyte MIHA cells were
obtained from the Liver Cancer Institute at Fudan University, and
cultured in HyClone Dulbecco's modified Eagle's medium (DMEM) or
RPMI-1640 medium (for MIHA) with high glucose (Cytiva),
supplemented with 10% Gibco® fetal bovine serum (FBS)
and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in an atmosphere of 5% CO2 at 37°C.
The cell lines were authenticated via STR profiling.
Reverse transcription-quantitative
(RT-q)PCR
As previously described (21), total RNA was extracted from the HCC
cells or tissues with RNAiso Plus (Takara Bio, Inc.). cDNA was
synthesized using PrimeScript™ RT reagent Kit with gDNA Eraser
(Takara Bio, Inc.). qPCR was performed using SYBR®
Premix Ex Taq™ (Takara Bio, Inc.). The following thermocycling
conditions were used: initial denaturation at 95°C for 30 sec
followed by 40 cycles at 95°C for 5 sec and 60°C for 20 sec.
Subsequently, the levels of gene expression were quantified using
the 2−ΔΔCq method (24), and the values were normalized
against GAPDH. The primer sequences are listed in Table SI.
Small interfering RNA (siRNA)
synthesis, vector construction, and transfection
SPC25 shRNA, integrin subunit β4
(ITGB4) small interfering (si)RNA and ITGB4 cDNAs were
synthesized by Bio-link-Gene Co., Ltd. The target sequences are
listed in Table SII. Briefly,
3×105 cells (Huh7 and HCCLM3) per well were seeded in
6-well plates the day before transfection. After 24 h, the
lentiviruses were added to respective HCC cells with 1 ml of DMEM
containing no FBS and 5 µg/ml Polybrene (Sigma-Adrich; Merck KGaA).
Twelve hours later, the medium was removed and replaced with fresh
culture medium containing 10% FBS. Three days later, the cells were
collected for subsequent culture. The efficiency of the
transfections was confirmed using western blotting and RT-qPCR.
Microarray analysis
Total RNA was extracted from HCCLM3-shSPC25 and
HCCLM3-shNC cells with RNAiso Plus (Takara Bio, Inc.). The Agilent
SurePrint G3 Human Gene Expression v3 8×60K Microarray (Design ID:
072363) was used in this experiment, and data analysis of the 6
samples was conducted by OE Biotechnology Co., Ltd. (Shanghai,
China). Total RNA was quantified by the NanoDrop ND-2000 (Thermo
Fisher Scientific, Inc.) and the RNA integrity was assessed using
Agilent Bioanalyzer 2100 (Agilent Technologies). The sample
labeling, microarray hybridization and washing were performed based
on the manufacturer's standard protocols. Briefly, total RNA was
transcribed to double-strand cDNA, and then synthesized into cRNA
and labeled with Cyanine-3-CTP. The labeled cRNAs were hybridized
onto the microarray. After washing, the arrays were scanned by the
Agilent Scanner G2505C (Agilent Technologies). Feature Extraction
software (version 10.7.1.1; Agilent Technologies) was used to
analyze array images to obtain raw data. Genespring (version 14.8,
Agilent Technologies) was employed to finish the basic analysis
with the raw data. Initially, the raw data were normalized with the
quantile algorithm. The probes with at least 1 condition out of 2
conditions that had flags in ‘Detected’ were chosen for further
data analysis. Differentially expressed genes were then identified
through fold change as well as the P-value calculated with t-test.
The threshold set for upregulated and downregulated genes was a
fold change ≥1.0 and a P-value ≤0.05. Afterwards, Gene Ontology
(GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG)
analysis were applied to determine the roles of these
differentially expressed mRNAs. Finally, hierarchical clustering
was performed to display the distinguishable gene expression
patterns among the samples. We uploaded the raw data to the GEO
database (GSE188881).
Wound healing assay
Cells (HCCLM3 and Huh7) were seeded at 80%
confluency and cultured overnight. After the cells had been
scratched using a 200-µl pipette tip, they were cultured in
FBS-free DMEM and the movement of the cells was measured every 12 h
(up to 48 h) after scratching using a microscope (Olympus IX-71;
Olympus Corp.), original magnification, ×200. The wound distances
were measured and calculated as a percentage of the distance at 0
h.
Transwell assay
Transwell assays were performed using Transwell
chambers (Corning, Inc.) and Matrigel™ (Corning Life Sciences). For
Transwell assays, the upper chamber coated with Matrigel was used
for the invasion assays, whereas the migration assays were
performed without Matrigel. The cells (HCCLM3 and Huh7) were seeded
into the upper chamber and incubated for 36 h, and subsequently the
chambers were stained with 0.1% crystal violet (Beyotime Institute
of Biotechnology) for 10 min at 25°C. and counted in three
different fields with a binocular optical microscope (Olympus
Corp.) at original magnification, ×200.
In vivo assay
As described in our previous studies (25,26),
an in vivo assay was performed using nude mice (4-week-old
male mice weighing approximately 20 g purchased from Charles River
Laboratories, Inc.), which were randomly assigned to control and
experimental groups (n=5). A total of 6×106 cells
(HCCLM3 and Huh7) were injected subcutaneously in the axilla of the
nude mice. When the diameter of the subcutaneous tumor was 1-1.5 cm
4 weeks later, the subcutaneous tumors were obtained, which were
used in an orthotopic model. The orthotopic model was established
by orthotopic inoculation of tumor tissue (2×2×2 mm) into the
livers of nude mice (n=5). When some mice developed ascites or
cachexia after a subsequent 4-week period, the mice were
sacrificed, and the tumor weights and volumes (largest diameter ×
perpendicular height2/2) were measured and analyzed. In
this study, the largest diameter measured was 18 mm in for the
HCCLM3 group, and 20 mm for the Huh7 group. The nude mice were
euthanized via barbiturate overdose (sodium pentobarbital, 150
mg/kg), followed by exsanguination. General mouse health and
well-being were monitored daily and no animals were withdrawn from
the study. This research was conducted in strict accordance with
the Care and Use of Laboratory Animals of the National Institutes
of Health.
Western blot assay
As previously described (21), total protein was extracted using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration was determined using a BCA assay (Beyotime Institute
of Biotechnology). Total protein (30 µg/lane) was separated using
SDS-PAGE on a 10% gel (Beyotime Institute of Biotechnology) and
transferred onto a PVDF membrane (MilliporeSigma). The membranes
were blocked with 5% skimmed milk at room temperature for 1 h and
incubated overnight at 4°C with diluted primary antibodies.
Subsequently, the membranes were washed using TBS with 0.1%
Tween-20 (TBST) three times and incubated with HRP-conjugated
secondary antibodies for 1 h at room temperature. After being
washed with TBST, the membranes were visualized using
electrochemiluminescence (ECL) kit (Beyotime Institute of
Biotechnology). Β-actin was used as the loading control. The
following antibodies were used: mouse monoclonal anti-β-actin
(1:1,000; cat. no. AF0003; Beyotime Institute of Biotechnology),
rabbit polyclonal anti-SPC25 (1:500; cat. no. ab121395; Abcam),
rabbit monoclonal anti-ITGB4 [1:1,000; cat. no. 14803; Cell
Signaling Technology, Inc. (CST)], rabbit monoclonal anti-PI3K
(1:1,000; cat. no. 4257; CST), rabbit monoclonal anti-phospho-PI3K
(1:1,000; cat. no. 17366; CST), rabbit monoclonal anti-AKT
(1:1,000; cat. no. 4658; CST), rabbit monoclonal anti-phospho-AKT
(1:1,000; cat. no. 4060; CST), rabbit monoclonal anti-FAK (1:1,000;
cat. no. 13009; CST), rabbit monoclonal anti-phospho-FAK (1:1,000;
cat. no. 8556; CST), rabbit monoclonal anti-MMP9 (1:1,000; cat. no.
13667; CST) and rabbit monoclonal anti-MMP13 (1:1,000; cat. no.
69926; CST), HRP-linked anti-rabbit secondary antibody (1:2,000;
cat. no. 7074; CST), and HRP-linked anti-mouse secondary antibody
(1:2,000; cat. no. 7076; CST). Three independent experiments were
performed for each of the western blots.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was performed using the CCK-8 assay
kit of Dojindo Molecular Technologies, Inc. After 3,000 cells
(HCCLM3 and Huh7) were seeded for 24, 48, and 72 h, 100 µl reaction
mixture (90 µl DMEM and 10 µl CCK-8 solution) was added and
incubation was carried out for 2 h at 37°C. Finally, the absorbance
was measured at 450 nm.
Statistical analysis
Statistical analyses were performed using SPSS
Statistics 20.0 (IBM Corp.). Experimental data are presented as the
mean ± SD from three independent experiments performed in
triplicate. The significant differences between groups were
calculated by Student's t-test, Chi-square test or one-way ANOVA
and Tukey post hoc test, as appropriate. The correlation analysis
was performed using Spearman correlation analysis. Survival
analysis was conducted using the Kaplan-Meier method, and
comparisons were made using the log-rank test. Cox proportional
hazards regression models were assessed using the relative
prognostic significance of the variables for predicting overall
survival (OS) and disease-free survival (DFS). P-values were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of SPC25 tissues is
associated with poor prognosis in HCC
To study the role of SPC25 in HCC, the expression of
SPC25 was first analyzed based on publicly available databases [The
Cancer Genome Atlas (TCGA), International Cancer Genome Consortium
(ICGC) and Gene Expression Omnibus (GEO)]. These analyses revealed
that SPC25 is expressed at higher levels in HCC compared
with that in normal liver tissues in the databases, TCGA [HCC
(n=374) cf. normal (n=50) tissues, P<0.001] (Fig. 1A), ICGC [HCC (n=240) cf. normal
(n=202) tissues, P<0.001] (Fig.
1B), GSE102079 [HCC (n=243) cf. normal (n=14) tissues,
P<0.001] (Fig. 1C) and
GSE112790 [HCC (n=183) cf. normal (n=15) tissues, P<0.001]
(Fig. 1D). To validate these
results, SPC25 expression was detected in 30 pairs of HCC
and normal liver tissues using RT-qPCR, which confirmed that the
expression of SPC25 in HCC tissues was indeed higher
compared with the normal tissues (n=30, P<0.001) (Fig. 1E).
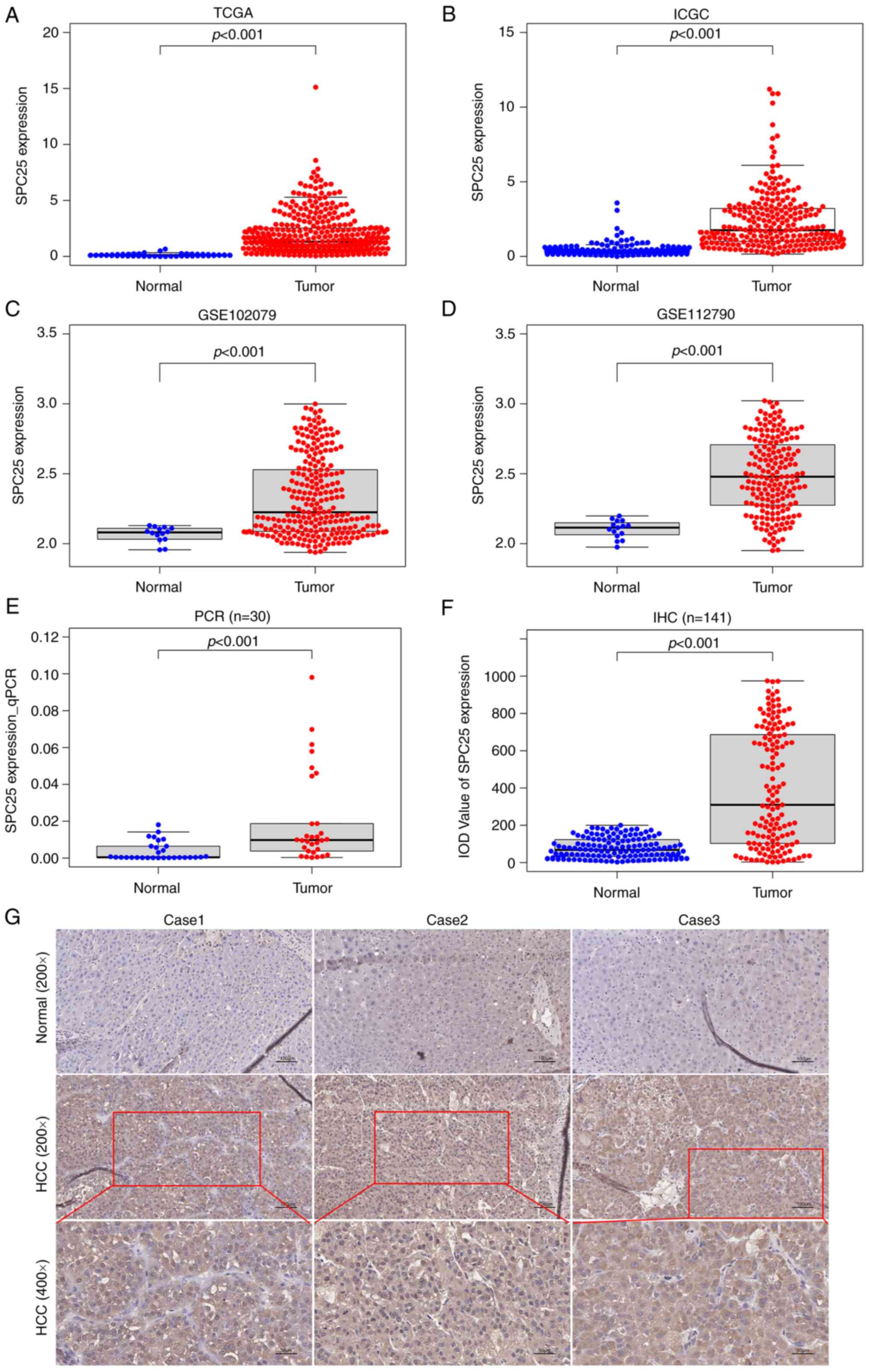 | Figure 1.Differential expression analysis of
SPC25 in HCC and normal liver tissues is shown. (A) TCGA [HCC
(n=374) cf. normal (n=50) tissues, P<0.001 (independent-samples
t-test)]. (B) ICGC [HCC (n=240) cf. normal (n=202) tissues,
P<0.001 (independent-samples t-test)]. (C) GSE102079 [HCC
(n=243) cf. normal (n=14) tissues, P<0.001 (independent-samples
t-test)]. (D) GSE112790 [HCC (n=183) cf. normal (n=15) tissues,
P<0.001 (independent-samples t-test)]. (E) The results from the
RT-qPCR analysis is shown [n=30, P<0.001 (matched samples
t-test)]. (F) The results from the IHC analysis are shown [(n=141,
P<0.001) (matched samples t-test)]. (G) IHC staining indicated
that SPC25 was expressed more highly in HCC tumor tissues compared
with the expression in normal liver tissues. Original
magnification, ×200 (scale bars, 100 µm) and ×400 (scale bars, 50
µm). SPC25, spindle pole body component 25 homolog; TCGA, The
Cancer Genome Atlas; ICGC, International Cancer Genome Consortium;
GEO, Gene Expression Omnibus; HCC, hepatocellular carcinoma. |
To explore the association between SPC25 and HCC
metastasis, the expression levels of SPC25 were detected in 141
pairs of HCC and normal liver tissues by IHC. This analysis
revealed that SPC25 was expressed at a higher level in HCC tissues
(n=141, P<0.001) (Fig. 1F and
G). Subsequently, the patients were divided into high and low
expression groups, based on the median IOD of SPC25 expression.
Further analysis was conducted with the factors and
the basic information related to HCC, which could objectively
reflect the tumor burden and metastatic risk. The results revealed
that thrombus, microvascular invasion (MVI), tumor number and
encapsulation were markedly different when comparing the high and
low expression groups (Table I),
which strongly suggested that SPC25 may be involved in HCC
malignancy, especially metastasis.
 | Table I.Association between intratumor SPC25
and clinicopathologic features (N=141) of the HCC patients. |
Table I.
Association between intratumor SPC25
and clinicopathologic features (N=141) of the HCC patients.
|
| SPC25 |
|---|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Age (years) | 52.61±11.4 | 54.12±10.3 | 0.316 |
| Sex |
|
| 0.379 |
|
Female | 9 | 14 |
|
|
Male | 58 | 60 |
|
| Cirrhosis |
|
| 0.180 |
| No | 11 | 19 |
|
|
Yes | 56 | 55 |
|
| Thrombus |
|
| 0.002 |
| No | 65 | 59 |
|
|
Yes | 2 | 15 |
|
| Tumor size
(cm) | 6.34±3.36 | 8.57±4.12 | 0.091 |
| MVI |
|
| 0.042 |
| No | 49 | 42 |
|
|
Yes | 18 | 32 |
|
| Tumor number |
|
| 0.039 |
|
Single | 50 | 43 |
|
|
Multiple | 17 | 31 |
|
| Encapsulation |
|
| 0.042 |
|
Yes | 44 | 36 |
|
| No | 23 | 38 |
|
Kaplan-Meier analysis showed that SPC25 was
associated with poor OS and DFS in HCC (Fig. 2A and B). The univariate and
multivariate analyses revealed that SPC25 expression was an
independent risk factor for OS [hazard ratio (HR), 1.864; 95%
confidence interval (CI), 1.125-3.089; P<0.001] and DFS (HR,
1.712; 95% CI, 1.097-2.672; P=0.018) rates of patients with HCC
(Table II). In order to validate
these results, survival analysis was performed based on TCGA and
ICGC data, which demonstrated that SPC25 was associated with poor
OS rates (Fig. 2C and D).
Therefore, it was possible to speculate that SPC25 overexpression
was associated with the aggressiveness and poor prognosis of
HCC.
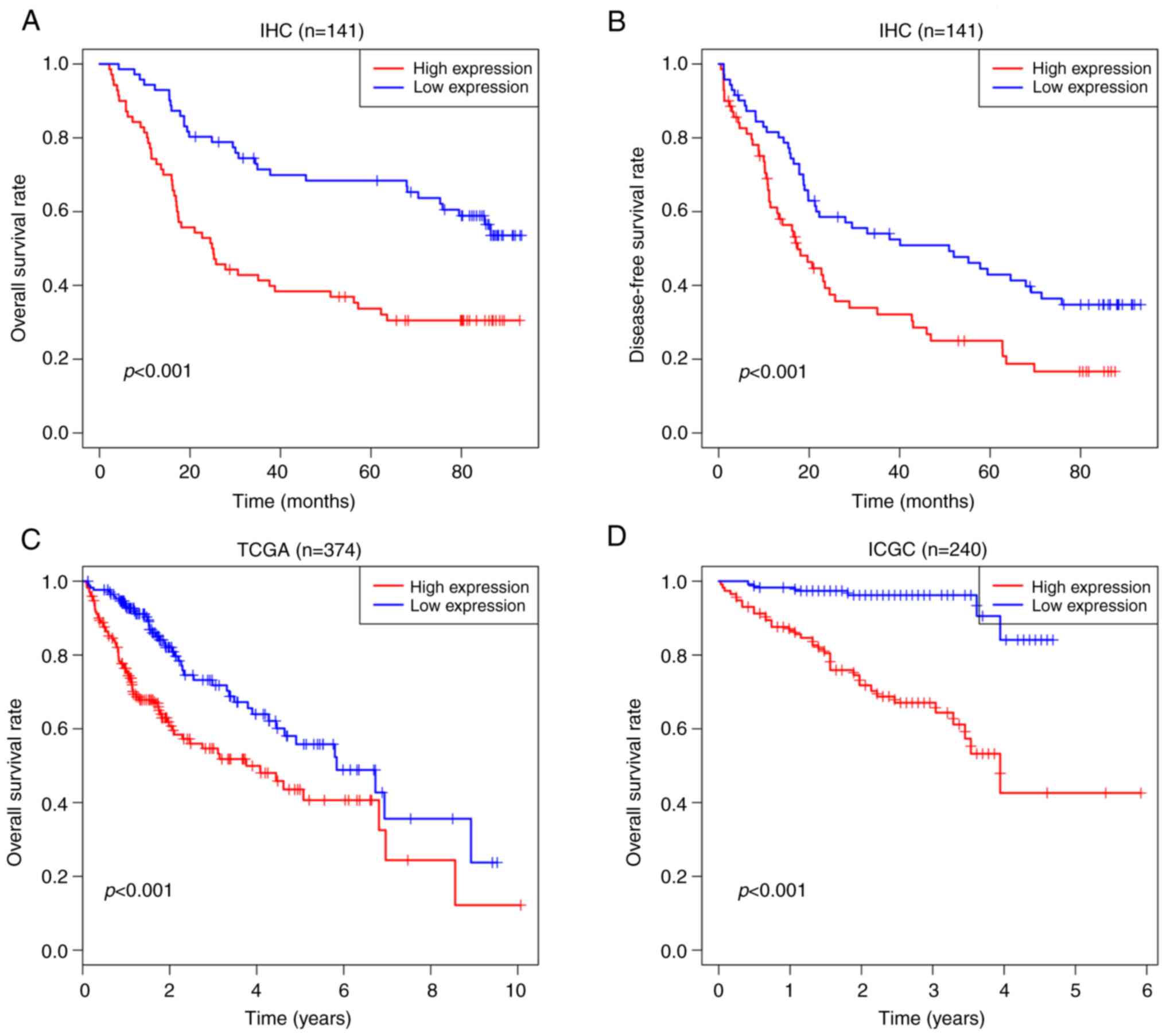 | Figure 2.Kaplan-Meier analysis, showing that
high expression of SPC25 is associated poor prognosis in HCC. (A)
OS of patients at our center (n=141, P<0.001). (B) DFS of
patients at our center (n=141, P<0.001). (C) OS of TCGA (n=374,
P<0.001). (D) OS of ICGC (n=240, P<0.001). SPC25, spindle
pole body component 25 homolog; HCC, hepatocellular carcinoma; OS,
overall survival; DFS, disease-free survival; TCGA, The Cancer
Genome Atlas; ICGC, International Cancer Genome Consortium. |
 | Table II.Univariate and multivariate analysis
of factors associated with overall survival and disease-free
survival of the HCC patients. |
Table II.
Univariate and multivariate analysis
of factors associated with overall survival and disease-free
survival of the HCC patients.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
|
|
| Multivariate |
| Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | Univariate
P-value | HR | 95% CI | P-value | Univariate
P-value | HR | 95% CI | P-value |
|---|
| Age (years) (≤50
vs. >50) | 0.707 |
|
| NA | 0.495 |
|
| NA |
| Sex (female vs.
male) | 0.676 |
|
| NA | 0.877 |
|
| NA |
| Cirrhosis (no vs.
yes) | 0.489 |
|
| NA | 0.447 |
|
| NA |
| Tumor size (cm) (≤5
vs. >5) | 0.002 | 1.541 | 0.882-2.691 | NS | 0.002 | 1.743 | 1.089-2.791 | 0.021 |
| Tumor number
(single vs. multiple) | 0.918 |
|
| NA | 0.675 |
|
| NA |
| MVI (no vs.
yes) | 0.002 | 2.296 | 1.432-3.680 | 0.001 | 0.002 | 1.347 | 0.857-2.119 | NS |
| Thrombus (no vs.
yes) | <0.001 | 2.283 | 1.244-4.187 | 0.008 | <0.001 | 2.922 | 1.527-5.558 | 0.001 |
| Encapsulation (no
vs. yes) | 0.377 |
|
| NA | 0.300 |
|
| NA |
| SPC25 (high vs.
low) | <0.001 | 1.864 | 1.125-3.089 | 0.016 | <0.001 | 1.712 | 1.097-2.672 | 0.018 |
SPC25 promotes invasion and migration
of HCC cells in vitro and in vivo
Subsequently, western blotting was performed to
assess the expression levels of SPC25 in the different liver cancer
cell lines, and this analysis revealed that SPC25 was more highly
expressed in HCCLM3 and Huh7 cells (Fig. 3A). SPC25 was then silenced
in HCCLM3 and Huh7 cells using shRNAs, and its silencing was
confirmed by western blotting and RT-qPCR (n=3, P<0.05)
(Fig. 3B and C). CCK-8 assay
showed that SPC25 silencing led to a decrease in the proliferation
of HCC cells (n=3, P<0.01) (Fig.
3D). Wound healing assays were subsequently performed, and
these experiments revealed that SPC25 silencing inhibited cell
motility (n=3, P<0.05) (Fig.
3E). Finally, Transwell assays consistently confirmed that
SPC25 silencing inhibited the invasion and migration abilities of
HCCLM3 and Huh7 cells (n=3, P<0.05) (Fig. 3F). Taken together, the results
obtained from these in vitro experiments suggested that
SPC25 could facilitate HCC migration and invasion.
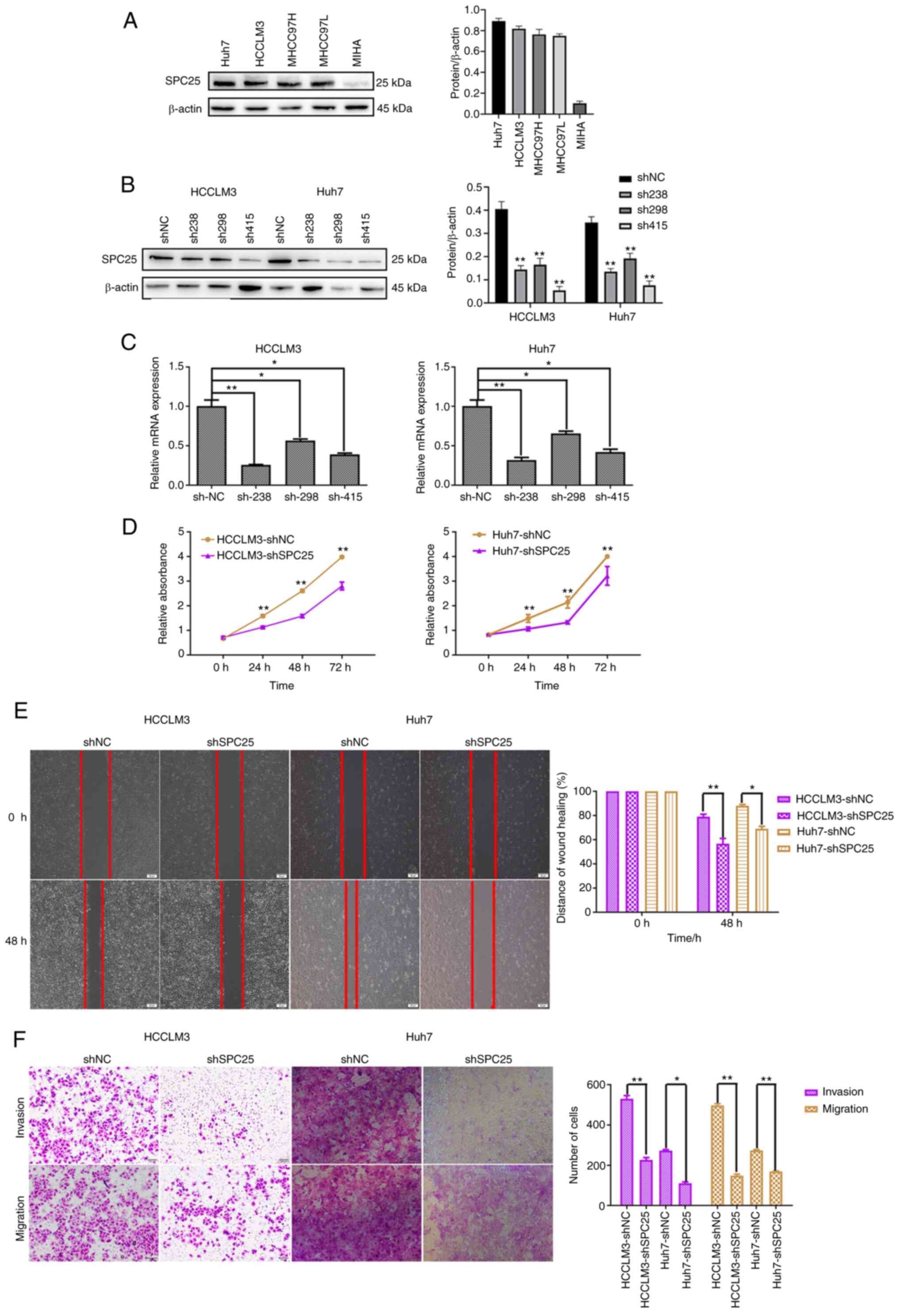 | Figure 3.SPC25 promotes HCC migration in
vitro. (A) Western blot analysis revealed the levels of SPC25
protein expression in liver cancer cell lines. (B and C) Western
blotting and RT-qPCR were used to confirm the silencing of SPC25 in
the HCC cell lines (*P<0.05 and **P<0.01, one-way ANOVA,
compared with the shNC). (D) CCK-8 assay indicated that SPC25
silencing reduced the proliferation of Huh7 and HCCLM3 cells
(**P<0.001, independent-samples t-test, compared with shNC
group). (E) Wound-healing assay was used to show that SPC25
silencing reduced the migration of Huh7 and HCCLM3 cells. Original
magnification, ×200. Scale bars, 50 µm (*P<0.05 and **P<0.01,
independent-samples t-test, compared to the shNC group). (F)
Transwell assay, indicating that SPC25 silencing reduced the
invasion and migration of Huh7 and HCCLM3 cells (*P<0.05,
**P<0.01, independent-samples t-test, compared to the shNC
group). Original magnification, ×200. Scale bars, 100 µm. SPC25,
spindle pole body component 25 homolog; HCC, hepatocellular
carcinoma. |
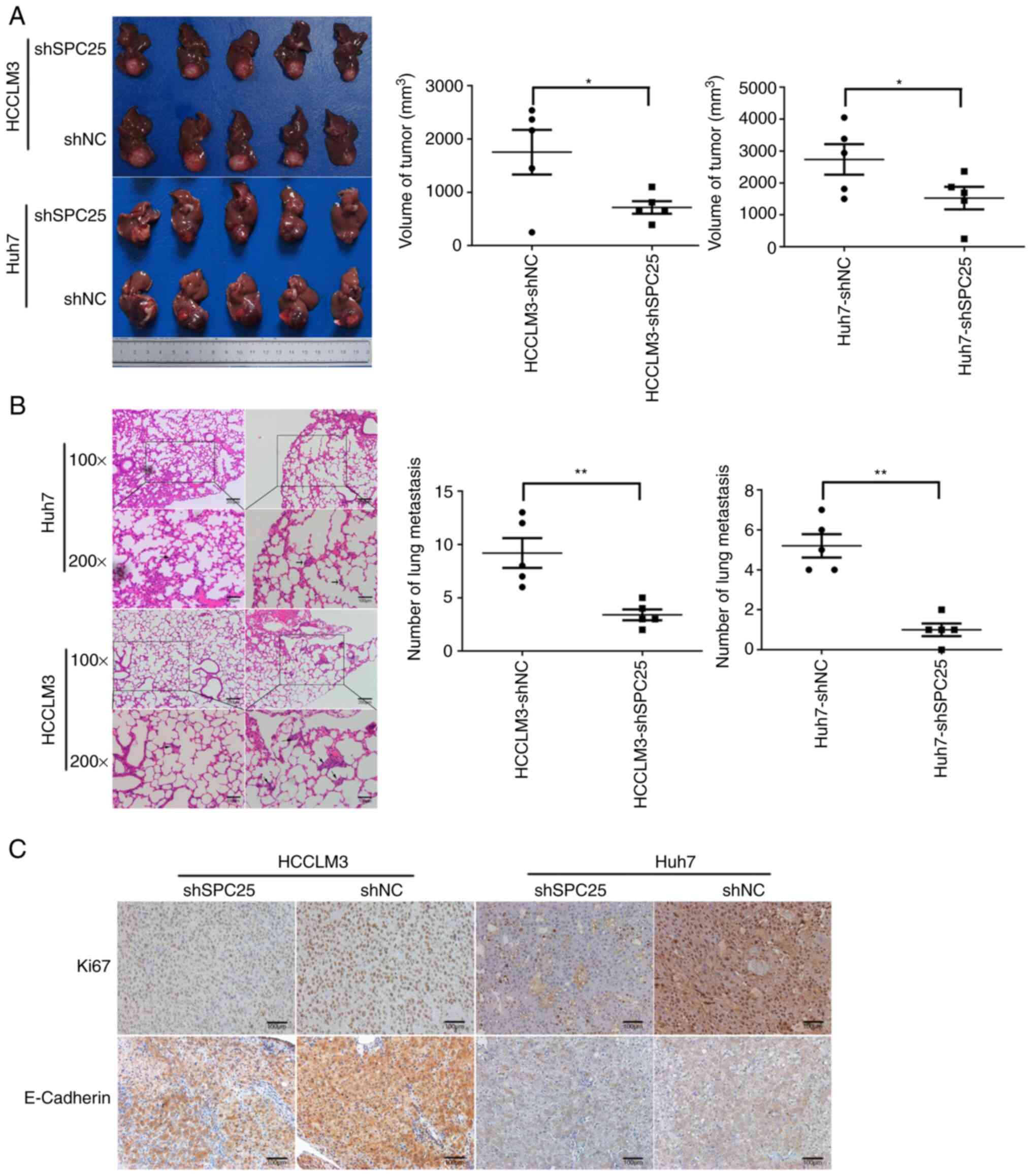
To explore the promotion of metastasis by SPC25
in vivo, transfected HCCLM3 and Huh7 cells were transplanted
in situ into nude mice (n=5 each group). SPC25 silencing
resulted in markedly reduced tumor sizes (n=5, P<0.05) (Fig. 4A) and metastasis to the lungs (n=5,
P<0.01) (Fig. 4B). IHC staining
with Ki-67 and E-cadherin antibodies suggested that SPC25 could
promote tumor proliferation and metastasis (Fig. 4C). Therefore, these data support a
role of SPC25 in promoting HCC metastasis in vitro and in
vivo.
SPC25 upregulates the expression of
genes associated with ECM-receptor interactions and focal adhesion
pathways
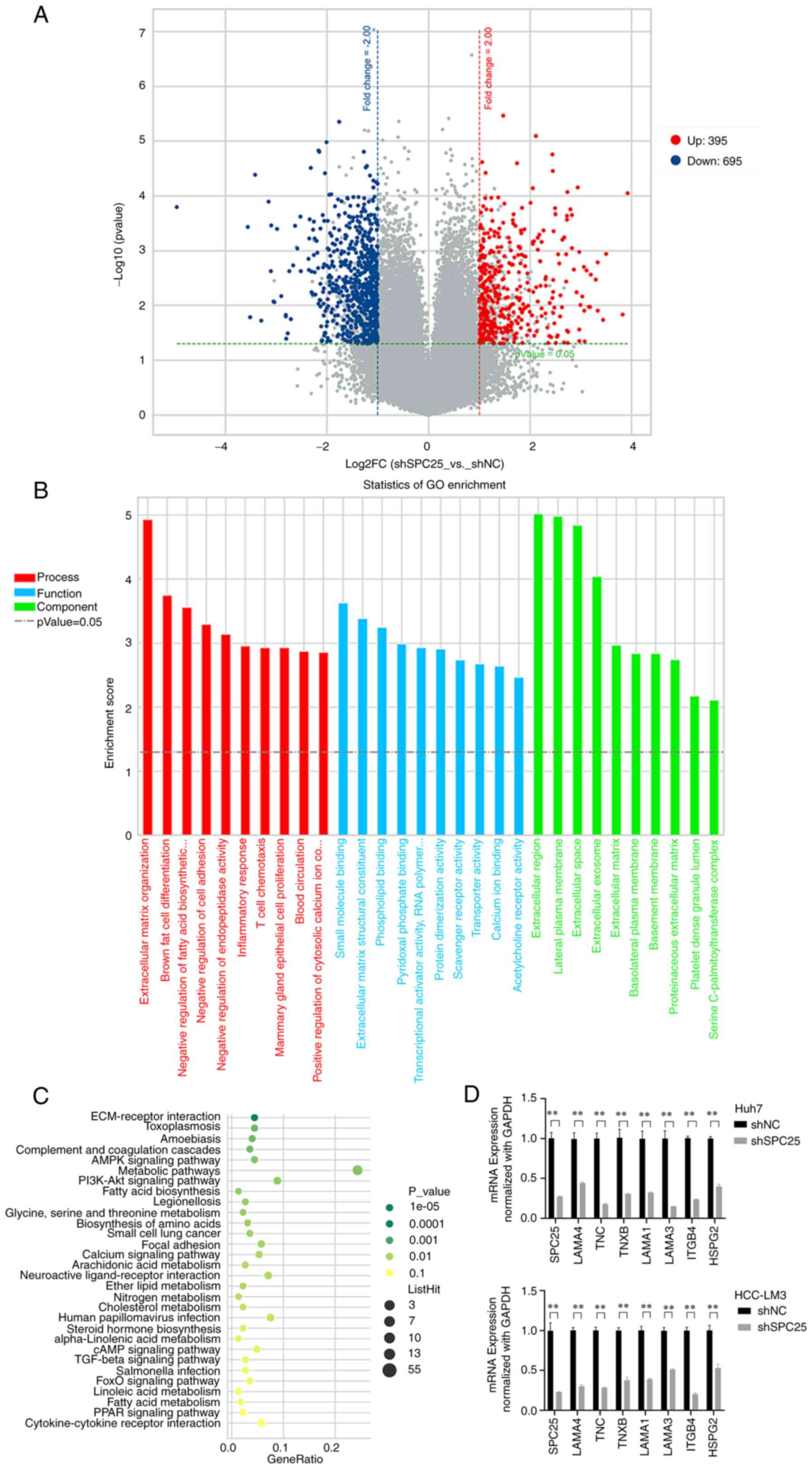
To gain an improved understanding of the mechanism
of SPC25 in promoting metastasis, Agilent cDNA microarray analysis
was performed, and the gene expression levels of shSPC25 and shNC
(negative control) in HCCLM3 cells were compared (GSE188881). A
total of 1,091 differentially expressed genes (DEGs) were screened
out, including 695 downregulated and 396 upregulated genes
(P<0.05, |log2FoldChange|≥1) (Fig. 5A). Subsequently, Gene Ontology (GO)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment
analysis was performed. The results obtained showed that
SPC25 silencing mainly influenced the functions
‘extracellular region cellular component’, ‘small molecule binding
molecular function’, ‘extracellular matrix organization biological
process’ (Fig. 5B) and
‘ECM-receptor interactions’ (Fig.
5C). The ECM-receptor interactions included numerous genes that
promote metastasis and cell motility, a finding that was in keeping
with the metastasis-promoting role of SPC25. Subsequently, RT-qPCR
was used to confirm the genes identified by microarray analysis in
SPC25-silenced cells (n=3, P<0.05) (Fig. 5D). The results obtained showed that
SPC25 could affect the expression of metastasis-associated
genes.
SPC25-mediated promotion of metastasis
is mediated by ITGB4
Among the DEGs, ITGB4 is an integrin-encoding gene,
and laminin subunit α1 (LAMA1), laminin subunit α3 (LAMA3), laminin
subunit α4 (LAMA4) and laminin subunit γ3 (LAMC3) are
laminin-coding genes that lie upstream of the integrin pathway.
Therefore, our conjecture was that SPC25 may control ECM-integrin
interactions to regulate the integrin pathway. The associations
among SPC25 and ITGB4, LAMA1, LAMA3, LAMA4 and LAMC3 were analyzed
based on the HCC data of TCGA, which revealed that ITGB4 and SPC25
had the strongest positive correlation (n=369;
ρspearman=0.34; 95% CI: 0.25-0.43; P<0.001) (Fig. 6A and Fig. S1). Subsequently, the correlation
between ITGB4 and SPC25 was analyzed based on the
data of HCC tissues by RT-qPCR, which revealed that ITGB4 and SPC25
were positively correlated (n=30; ρspearman=0.451; 95%
CI: 0.16-0.64; P<0.01) (Fig.
6B). Finally, the decrease in ITGB4 in response to SPC25
knockdown was confirmed via western blot analysis (n=3, P<0.01)
(Fig. 6C). Taken together, these
results indicated that ITGB4 may be a downstream target of
SPC25.
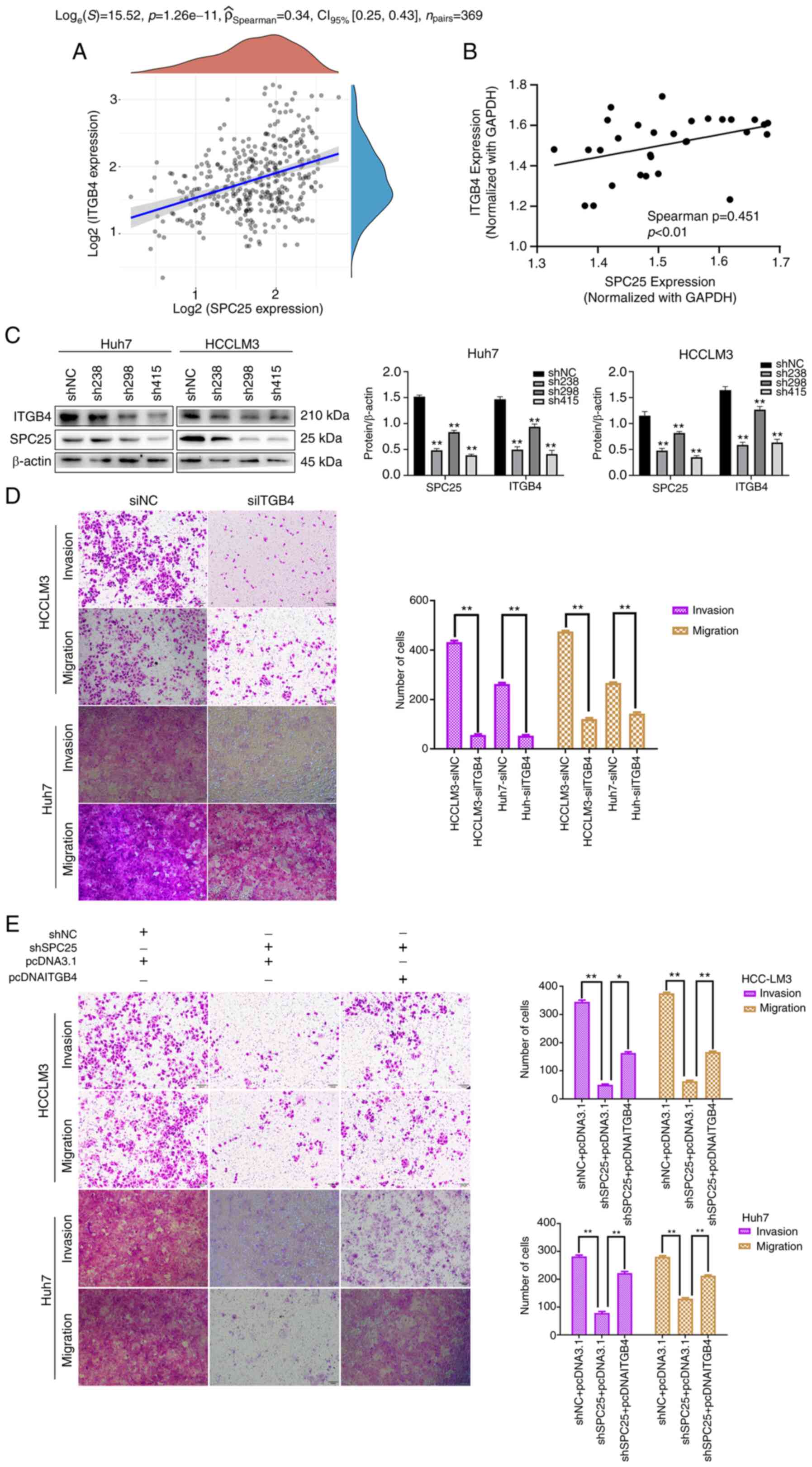 | Figure 6.Promotion of metastasis by SPC25 is
mediated by ITGB4. (A) Correlation analysis of SPC25 and ITGB4
based on TCGA data is shown. (B) Correlation analysis of SPC25 and
ITGB4 by RT-qPCR is shown. (C) Western blot analysis showed that
alterations in the level of ITGB4 were accompanied by changes in
the level of SPC25 (**P<0.01, one-way ANOVA, compared with the
shNC group). (D) Transwell assay, showing that ITGB4 silencing
reduced the invasion and migration of Huh7 and HCCLM3 cells
(**P<0.01, independent-samples t-test). (E) A rescue experiment
with Transwell assays was performed in Huh7 and HCCLM3 cells
co-transfected with shNC or shSPC25 and pcDNA3.1 or pcITGB4
(*P<0.05 and **P<0.01, one-way ANOVA). Original
magnification, ×200. Scale bars, 100 µm. 001. SPC25, spindle pole
body component 25 homolog; ITGB4, integrin subunit β4; TCGA, The
Cancer Genome Atlas. |
Since the results obtained suggest a role for SPC25
in promoting metastasis, the role of ITGB4 in the transmission of
HCC metastatic potential was subsequently explored. Silencing of
ITGB4 caused a significant reduction in the invasion and migration
abilities of HCCLM3 and Huh7 cells in vitro (n=3, P<0.01)
(Fig. 6D). The findings revealed
that ITGB4 and SPC25 have a similar role in promoting HCC
metastasis. Subsequently, rescue experiments were performed to
explore whether SPC25 could promote HCC metastasis via ITGB4. The
results obtained showed that ectopic overexpression of ITGB4 with
simultaneous silencing of SPC25 partially mitigated the reduction
in cell invasion and migration capability caused by SPC25 silencing
(n=3, P<0.05) (Fig. 6E). Hence,
these results suggest that ITGB4 is the main downstream mediator of
SPC25-induced metastatic activity.
SPC25 activates the FAK/PI3K/AKT
signaling pathway through ITGB4
A previous study has shown that integrin-laminin
binding increases FAK phosphorylation, which induces activation of
the PI3K/AKT signaling pathway to promote tumor metastasis
(10). In the present study, the
results obtained showed that neither SPC25 nor ITGB4 silencing
exerted any effect on the total protein levels of FAK, PI3K and
AKT, whereas their silencing did markedly reduce the levels of
phosphorylated (p-)FAK, p-PI3K and p-AKT (n=3, P<0.05) (Fig. 7A) and the ratios of phosphorylated
vs. total protein (PI3K, FAK and AKT) (Fig. S2A). Subsequently, rescue
experiments were performed, and ITGB4 overexpression was observed
to reverse the decrease in expression of p-FAK, p-PI3K and p-AKT
levels induced by SPC25 silencing (n=3, P<0.05) (Fig. 7B) and the ratios of phosphorylated
vs. total protein (PI3K, FAK and AKT) (Fig. S2B). Furthermore, SPC25 silencing
was also found to decrease the expression of MMP9 and MMP13 (n=3,
P<0.05) (Fig. 7C and D), which
are proteins involved in the FAK/PI3K/AKT signaling pathway. Taken
together, these findings suggest that SPC25 could promote HCC
metastasis, mainly through regulating ITGB4 to activate the
FAK/PI3K/AKT signaling pathway.
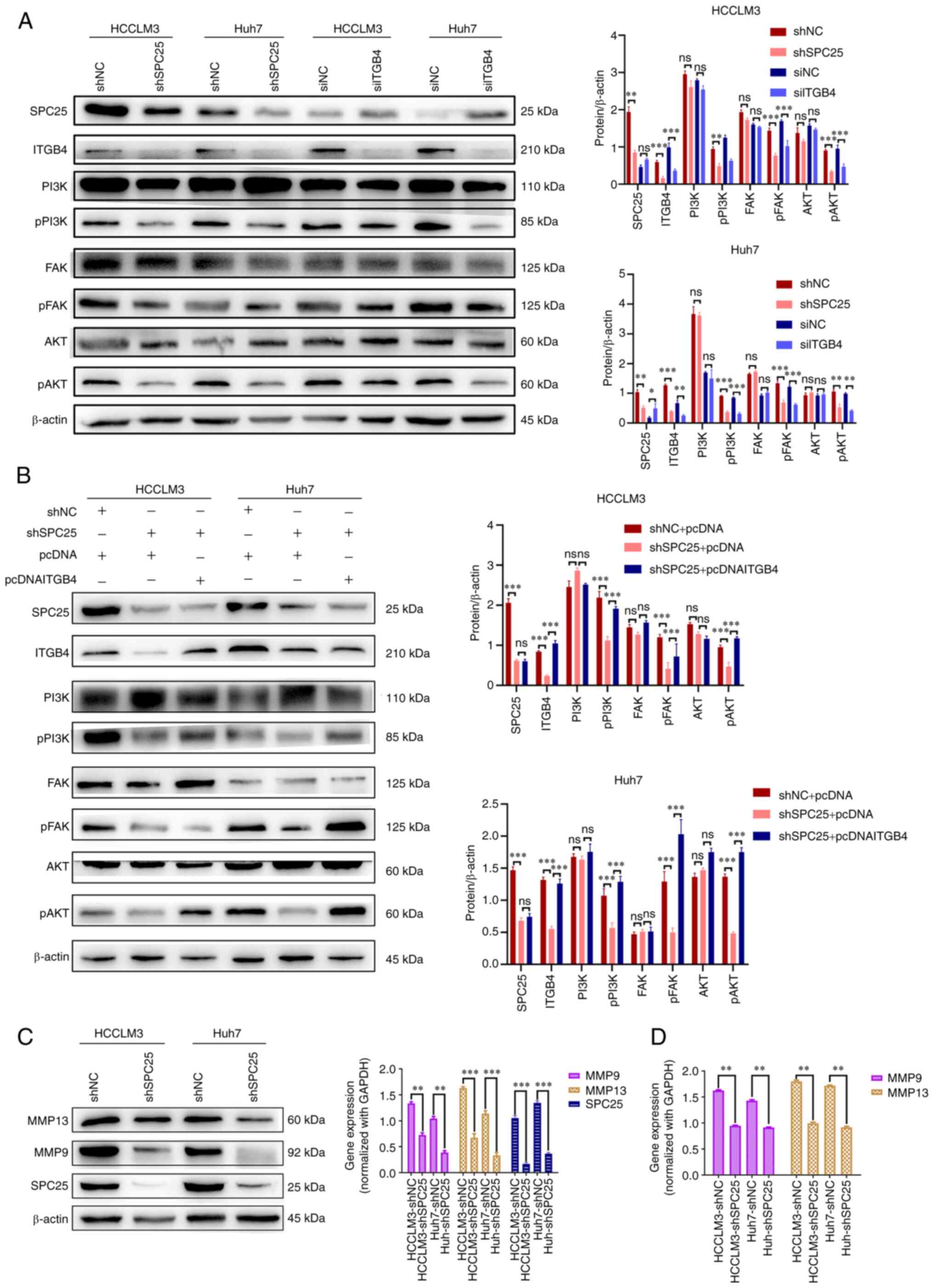 | Figure 7.SPC25 activates the FAK/PI3K/AKT
signaling pathway through ITGB4. (A) Western blot analysis revealed
the changed levels in phosphorylated (p)FAK, pPI3K and pAKT after
SPC25 or ITGB4 silencing in Huh7 and HCCLM3 cells (*P<0.05,
**P<0.01, ***P<0.001; ns, not significant,
independent-samples t-test). (B) Western blot analysis, revealing
the results of the rescue experiment on proteins associated with
the FAK/PI3K/AKT signaling pathway (***P<0.001; ns, not
significant, one-way ANOVA). (C and D) The altered levels of MMP9
and MMP13 in Huh7 and HCCLM3 after SPC25 silencing are shown [(C)
Results of western blot, (D) Results of RT-qPCR (**P<0.01,
***P<0.001, independent-samples t-test]. SPC25, spindle pole
body component 25 homolog; FAK, focal adhesion kinase; ITGB4,
integrin subunit β4; p, phosphorylated; MMP, matrix
metalloproteinase; PI3K, phosphoinositide 3-kinase. |
Discussion
The present study has mainly focused on the role of
spindle pole body component 25 homolog (SPC25) in promoting
metastasis in hepatocellular carcinoma (HCC), and in the underlying
mechanism. Previous studies have shown that SPC25 is able to
promote HCC proliferation, and that it was found to be a prognostic
indicator of poor survival in patients with HCC (18–20).
However, these studies were mainly based on bioinformatics
analysis, and relevant experimental studies have found only an
in vitro phenomenon. There are no in-depth and reliable
experimental studies on the specific roles and mechanisms of SPC25
in HCC. In the present study, the expression of SPC25 was first
examined in specimens from 141 patients with HCC and survival
analyses were performed to verify the results from the public
database. We firstly detected the changes of genes and signaling
pathways in HCC cells (HCCLM3) induced by SPC25 knockdown by
Agilent cDNA microarray analysis and uploaded the relevant data to
the GEO database (GSE188881). Based on the results, we identified
the main pathway of SPC25 regulating the invasion and metastasis of
HCC cells and was verified by rescue experiments. In conclusion, we
firstly examined the role and mechanism of SPC25 in regulating the
invasion and metastasis of HCC cells in a systematic and in-depth
experimental study.
In the present study, SPC25 expression was examined
in HCC tissues, and microarray analysis was performed to clarify
the mechanism. SPC25 was found to be expressed highly in HCC
tissues, and this high level of expression was associated with
thrombus, microvascular invasion (MVI), tumor number and
encapsulation, suggesting that SPC25 may be a predictor for HCC
prognosis and metastasis. Subsequently, further experiments
revealed that SPC25 could accelerate HCC metastasis both in
vivo and in vitro.
The present study also demonstrated that SPC25
accelerated HCC metastasis by regulating a number of genes that are
associated with extracellular matrix (ECM)-integrin interactions. A
previously published study revealed that integrin imbalances
resulting from genomic variation or expression disorder are
associated with tumorigenesis (27). For example, ITGB4 was shown to be
associated with the progression of NSCLC, pancreatic cancer, colon
cancer, prostate cancer and other types of cancer (28–31).
In particular, ITGB4 expression is often found at the forefront of
cancer cell invasion (32,33). In the present study, it was found
that ITGB4 expression decreased with SPC25 silencing. Moreover,
ITGB4 exerted the same role as SPC25 in terms of promoting HCC
metastasis. In addition, it was found that SPC25 is closely
associated with the expression of ITGB4 in HCC tissues, and that
upregulation of ITGB4 partly alleviated the reduced cell migration
ability caused by downregulated expression of the SPC25 gene.
Collectively, these results provided sufficient evidence for the
hypothesis that ITGB4 may be a target gene of SPC25.
In the present study, it has also been shown that
SPC25 promotes HCC metastasis mainly through activation of the
FAK/PI3K/AKT signaling pathway. ECM proteins locally adhere to
integrin elements on the cell membrane, forming the basic adhesive
molecule known as a hemidesmosome (34,35).
Upon ligation, FAK becomes phosphorylated to activate the PI3K/AKT
signaling pathway, a phenomenon that has also been reported in HCC
and gastric cancer (12,36). In the present study, the results
showed that silencing both SPC25 and ITGB4 reduced the activation
of FAK, PI3K and AKT. Furthermore, the upregulation of ITGB4
inhibited the inactivation induced by SPC25 silencing. Activated
signaling pathways often occur in many different tumor types, and
the regulatory networks among them are particularly complex. The
present study demonstrated that ITGB4 could induce the
phosphorylation of FAK, and the PI3K/AKT signaling pathway was
identified as being affected by SPC25. It has also been shown that
the activated FAK/PI3K/AKT signaling pathway induced by SPC25 was
regulated via ECM-integrin interactions.
Genomic instability fulfills an important role in
tumor metastasis (37,38), and metastasis is the leading cause
of cancer-associated deaths (5).
SPC25, as a gene associated with genomic instability, has an
important role in promoting HCC metastasis. The results based on
clinical samples in the present study showed that SPC25 is
associated with thrombus, MVI, tumor number and encapsulation,
which are indicators either of tumor metastasis or a high
propensity for metastasis. In analyzing the underlying mechanism,
it was found that SPC25 could activate the FAK/PI3K/AKT signaling
pathway and regulate ECM-integrin interactions to promote
metastasis in HCC. Based on these findings, it is possible to
conclude that SPC25 is able to regulate the invasion and metastasis
of HCC cells, and promote metastasis in patients with HCC.
Considering all the results obtained and this discussion thus far,
we consider that targeting SPC25 as a means of therapeutic
intervention may be a viable strategy for reducing the invasive
ability of HCC cells, thereby improving the survival time of the
patients.
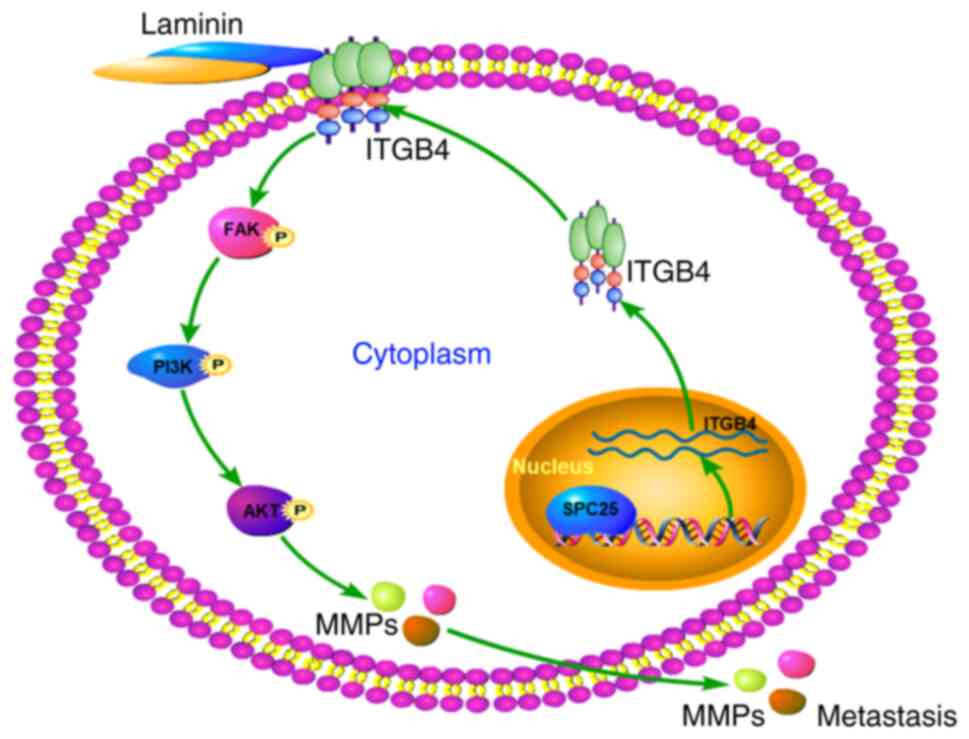
In conclusion, the present study suggests a role for
SPC25 as a prognostic indicator, as SPC25 was shown to be able to
promote metastasis in HCC. The results obtained have demonstrated
that SPC25 is able to promote metastasis through the ITGB4-mediated
FAK/PI3K/AKT signaling pathway (Fig.
8). This study has enabled us to recognize the importance of
SPC25 and its role in the invasion and migration of HCC cells and,
consequently, its potential prognostic and therapeutic value.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Joint Construction Project of
Medical Science and Technology of Henan Province (grant no.
LHGJ20190041).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WKS and YFZ conceived and designed the study. WKS
and QLS analyzed the data and wrote the paper. WKS, QLS and YFZ
were all involved in preparing the manuscript of the study. All
authors read and approved the manuscript, and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work in particular the
data are appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of the First Affiliated Hospital of Zhengzhou University
before specimen collection and animal tests (approval no.
2019-KY-21). All patients provided signed informed consent, and the
collection of clinical samples was conducted in accordance with the
Declaration of Helsinki. The animal tests in this study complied
with the ethical guidelines of the Laboratory Animal Care
International Council for Science (ICLAS) and the NC3Rs ARRIVE
Guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hindson J: Lenvatinib plus EGFR inhibition
for liver cancer. Nat Rev Gastroenterol Hepatol. 18:6752021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP investigators study group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J, da Fonseca LG and Reig M:
Insights into the success and failure of systemic therapy for
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
16:617–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holle AW, Young JL and Spatz JP: In vitro
cancer cell-ECM interactions inform in vivo cancer treatment. Adv
Drug Deliv Rev. 97:270–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra SK and Schlaepfer DD: Integrin
regulated FAK-Src signaling in normal and cancer cells. Curr Opin
Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: The dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang
K, Guan X, Yang K and Bai Y: LncRNA CASC9 promotes esophageal
squamous cell carcinoma metastasis through upregulating LAMC2
expression by interacting with the CREB-binding protein. Cell Death
Differ. 25:1980–1995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tai YL, Chu PY, Lai IR, Wang MY, Tseng HY,
Guan JL, Liou JY and Shen TL: An EGFR/Src-dependent β4 integrin/FAK
complex contributes to malignancy of breast cancer. Sci Rep.
5:164082015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leng C, Zhang ZG, Chen WX, Luo HP, Song J,
Dong W, Zhu XR, Chen XP, Liang HF and Zhang BX: An integrin
beta4-EGFR unit promotes hepatocellular carcinoma lung metastases
by enhancing anchorage independence through activation of FAK-AKT
pathway. Cancer Lett. 376:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun SC, Lee SE, Xu YN and Kim NH:
Perturbation of Spc25 expression affects meiotic spindle
organization, chromosome alignment and spindle assembly checkpoint
in mouse oocytes. Cell Cycle. 9:4552–4559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aguilera A and García-Muse T: Causes of
genome instability. Annu Rev Genet. 47:1–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Chen H, Yang H and Dai H: SPC25
upregulation increases cancer stem cell properties in non-small
cell lung adenocarcinoma cells and independently predicts poor
survival. Biomed Pharmacother. 100:233–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Zhu Y, Li Z, Bu Q, Sun T, Wang H,
Sun H and Cao X: Up-regulation of SPC25 promotes breast cancer.
Aging (Albany NY). 11:5689–5704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui F, Tang H, Tan J and Hu J: Spindle
pole body component 25 regulates stemness of prostate cancer cells.
Aging (Albany NY). 10:3273–3282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Sun H, Song Y, Yang L and Liu H:
Diagnostic and prognostic values of upregulated SPC25 in patients
with hepatocellular carcinoma. PeerJ. 8:e95352020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Zhou Q, Xie Q, Lin X, Miao W, Wei
Z, Zheng T, Pang Z, Liu H and Chen X: SPC25 overexpression promotes
tumor proliferation and is prognostic of poor survival in
hepatocellular carcinoma. Aging (Albany NY). 13:2803–2821. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Zhang K, Huang Y, Luo F, Hu K and
Cai Q: SPC25 may promote proliferation and metastasis of
hepatocellular carcinoma via p53. FEBS Open Bio. 10:1261–1275.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi WK, Zhu XD, Wang CH, Zhang YY, Cai H,
Li XL, Cao MQ, Zhang SZ, Li KS and Sun HC: PFKFB3 blockade inhibits
hepatocellular carcinoma growth by impairing DNA repair through
AKT. Cell Death Dis. 9:4282018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiyonobu N, Shimada S, Akiyama Y, Mogushi
K, Itoh K, Akahoshi K, Matsumura S, Ogawa K, Ono H, Mitsunori Y, et
al: Fatty Acid Binding Protein 4 (FABP4) overexpression in
intratumoral hepatic stellate cells within hepatocellular carcinoma
with metabolic risk factors. Am J Pathol. 188:1213–1224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimada S, Mogushi K, Akiyama Y, Furuyama
T, Watanabe S, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, et al:
Comprehensive molecular and immunological characterization of
hepatocellular carcinoma. EBioMedicine. 40:457–470. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao
DM and Ma ZC: Establishment of a metastatic model of human
hepatocellular carcinoma in nude mice via orthotopic implantation
of histologically intact tissues. Int J Cancer. 66:239–243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM,
Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al: Flot2 promotes
tumor growth and metastasis through modulating cell cycle and
inducing epithelial-mesenchymal transition of hepatocellular
carcinoma. Am J Cancer Res. 7:1068–1083. 2017.PubMed/NCBI
|
|
27
|
Lee JS and Thorgeirsson SS: Functional and
genomic implications of global gene expression profiles in cell
lines from human hepatocellular cancer. Hepatology. 35:1134–1143.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Jiang X, Wang G, Zhai C, Liu Y, Li
H, Zhang Y, Yu W and Zhao Z: ITGB4 is a novel prognostic factor in
colon cancer. J Cancer. 10:5223–5233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu P, Wang Y, Wu Y, Jia Z, Song Y and
Liang N: Expression and prognostic analyses of ITGA11, ITGB4
and ITGB8 in human non-small cell lung cancer. PeerJ.
7:e82992019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilkinson EJ, Woodworth AM, Parker M,
Phillips JL, Malley RC, Dickinson JL and Holloway AF: Epigenetic
regulation of the ITGB4 gene in prostate cancer. Exp Cell Res.
392:1120552020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang H, Zhou Z, Ma Z, Li Z, Liu C, Huang
S, Zhang C and Hou B: Characterization of the prognostic and
oncologic values of ITGB superfamily members in pancreatic cancer.
J Cell Mol Med. 24:13481–13493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung JS, Kang CW, Kang S, Jang Y, Chae YC,
Kim BG and Cho NH: ITGB4-mediated metabolic reprogramming of
cancer-associated fibroblasts. Oncogene. 39:664–676. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li XL, Liu L, Li DD, He YP, Guo LH, Sun
LP, Liu LN, Xu HX and Zhang XP: Integrin β4 promotes cell invasion
and epithelial-mesenchymal transition through the modulation of
Slug expression in hepatocellular carcinoma. Sci Rep. 7:404642017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Zuidema A, Te Molder L, Nahidiazar
L, Hoekman L, Schmidt T, Coppola S and Sonnenberg A: Hemidesmosomes
modulate force generation via focal adhesions. J Cell Biol.
219:e2019041372020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walko G, Castañón MJ and Wiche G:
Molecular architecture and function of the hemidesmosome. Cell
Tissue Res. 360:363–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu L, Hou Y, Tu G, Chen Y, Du YE, Zhang H,
Wen S, Tang X, Yin J, Lang L, et al: Nuclear Drosha enhances cell
invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by
dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in
gastric cancer. Cell Death Dis. 8:e26422017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radisky DC, Levy DD, Littlepage LE, Liu H,
Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et
al: Rac1b and reactive oxygen species mediate MMP-3-induced EMT and
genomic instability. Nature. 436:123–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walen KH: Genomic Instability in cancer I:
DNA-Repair triggering primitive hereditary 4n-Skewed, amitotic
division-system, the culprit in EMT/MET/Metaplasia cancer-concepts.
J Cancer Ther. 9:974–997. 2018. View Article : Google Scholar
|