Introduction
Pyroptosis, as a lytic programmed cell death, is
mediated by the gasdermin family of proteins and is common in
inflammatory cells (1). Cell
pyroptosis is mainly formed by caspase-1/3/4/5/11 cutting the
gasdermin family of proteins to form an N-terminal structure, and
is then transferred to the cell membrane for cutting and
perforation, in order that cell content can be released (2–4). It is
characterized by nuclear aggregation, formation of a large number
of 1–2-nm holes on the cell membrane, cell swelling, dissolution
and rupture of the plasma membrane, as well as release of
inflammatory factors (such as IL-1β and IL-18) to further amplify
the inflammatory response (5,6).
As aforementioned, one of the significant
morphological features of pyroptosis is excessive cell swelling,
which signifies that the capacity of cell volume regulation,
particularly regulatory volume decrease (RVD), is impaired. Several
studies, including a previous study by the authors, verified that
cells could regulate their volume to counteract osmotic swelling,
as well as during cell division, growth, migration and cell death
by RVD (7–11). Volume-regulated anion channels
(VRACs) were activated by hypotonic-stimulus and played pivotal
roles in RVD (12). VRAC inhibition
could decrease the capacity of RVD, ultimately resulting in cell
swelling (8,9,13–15).
At present, the involvement of chloride channels in cell pyroptosis
is poorly understood. Recently, Ye et al (16) proposed that lipopolysaccharides
(LPS) and nigericin, the classic pyroptosis inducers, can decrease
the hypotonicity-activated chloride currents and the capability of
RVD in bone marrow-derived macrophages (BMDMs). In addition,
leucine-rich repeat-containing 8a (LRRC8A), the constituent protein
of VRACs, was significantly downregulated in pyroptotic BMDMs
treated with LPS and nigericin. These results indicated that
VRAC/LRRC8A was involved in the pyroptosis of BMDM cells induced by
LPS and nigericin.
The mechanisms of pyroptosis in cancer and immune
cells appear to be different. Recently, the roles of pyroptosis in
cancer and the developments in inducing pyroptosis for cancer
therapy have attracted the attention of researchers (17). Paclitaxel (PTX) plus platinum were
used as first-line chemotherapy against ovarian cancer (18). To date, PTX has been proven to be
closely associated with cell pyroptosis in a number of studies. On
the one hand, PTX can induce pyroptosis in nasopharyngeal carcinoma
cells through the caspase-1/gasdermin D (GSDMD) pathway (19), and in lung cancer through
caspase-3/gasdermin E (GSDME) activation (20). On the other hand, PTX can enhance
innate immunity by promoting PYD domains-containing protein 3
(NLRP3) inflammasome activation in macrophages and inducing cell
pyroptosis by enhancing the cleavage of GSDMD (21). A previous study by the authors
reported that PTX inhibited the background chloride currents,
hypotonicity- and cisplatin-activated chloride currents in A2780
ovarian cancer cells (22). It is
well known that blocking chloride channels inhibited the RVD,
ultimately causing cell swelling (23,24).
Therefore, in the present study, whether PTX could induce cell
swelling and finally lead to ovarian cancer cell pyroptosis by
inhibiting chloride channels was investigated.
Materials and methods
Cell culture
A2780 (cat. no. CC0803; http://www.culturecollections.org.uk/collections/ecacc.aspx)
and SKOV3 (cat. no. CC0801; http://www.ATCC.org/) human ovarian cancer cell lines
were purchased from Guangzhou Cellcook Biotech Co., Ltd. A2780 and
SKOV3 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Biological Industries) and 100
µg/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. A2780 was subcultured
every 2 days, and SKOV3 was subcultured every 3 days. In subsequent
cell experiments, DMSO was used as the solvent control by default
for PTX.
Cell viability assay and half maximal
inhibitory concentration (IC50) measurement
The effect of PTX on A2780 and SKOV3 cell viability
was evaluated by MTT assay. Cells were seeded in 96-well plates
with a density of 7,000-8,500 cells/well and cultured overnight at
37°C with 5% CO2. The cells were exposed to PTX (0, 0.5,
1, 2, 4, 8, 16, 32, 64, 128 and 256 nM) in culture medium for 24
and 48 h respectively. Next, 20 µl of 5 mg/µl MTT was added to each
well and incubated for 4 h. After the supernatant was removed, 150
µl dimethyl sulfoxide was added to each well. Finally, the plates
were shaken for 10 min to fully dissolve the formation crystals.
All experiments were carried out in the dark. Subsequently, an
infinite M200PRO microplate reader (Bio-Rad Laboratories, Inc.) was
used to measure the optical density (OD) value at an absorbance of
490 nm. The IC50 values for each group of cells were
determined using the following formula: Inhibition
(%)=(ODcontrol-ODtest)/ODcontrol
×100%.
Cell apoptosis assay by flow cytometry
and Annexin V-FITC-A double staining
For cell apoptosis analysis, A2780 and SKOV3 cells
were seeded in 6-well plates at a density of 15,000 cells/well
overnight. A2780 was treated with 10 nM PTX for 24 h at 37°C and
SKOV3 was treated with 10 and 250 nM PTX for 48 h at 37°C.
Following collection, the cells were washed with ice-cold PBS
twice, and fixed with 70% ethanol at −20°C for >4 h. Next, the
collected cells were stained with 400 µl propidium iodide (PI) at
room temperature for 30 min. Finally, cell apoptosis distribution
was examined by flow cytometry (BD FACSCanto 8.0; Beckman Coulter,
Inc.). Data were analyzed using the Modfit program (version 8.0; BD
Biosciences).
Cell volume assay by flow
cytometry
As for volume analysis, cells were seeded in
six-well plates with a density of 15,000 cells/well overnight.
A2780 was treated with 10 nM PTX for 0–24 h at 37°C and SKOV3 was
treated with 250 nM PTX for 0–48 h at 37°C. Following collection,
the cells were washed with ice-cold PBS twice, and fixed with 70%
ethanol at −20°C for >4 h. Following fixation, the fixation
solution was removed and the cells were collected again and
resuspended in PBS. The forward scattered light (FSC) was
proportional to the cell volume. Finally, FSC was examined by flow
cytometry (BD FACSCanto 8.0; Beckman Coulter, Inc.). All data were
analyzed using the Modfit program (version 8.0; BD
Biosciences).
Lactate dehydrogenase (LDH) release
assay and PI staining
LDH release in cells was assessed using an LDH
Cytotoxicity Assay Kit (cat. no. C0017; Beyotime Institute of
Biotechnology) with a full-wavelength enzyme reader (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions. In
brief, 120 µl of the supernatant culture medium of A2780 and SKOV3
cells treated with PTX were collected in a 96-well plate, and the
response mixture was added and incubated in the dark for 30 min at
room temperature. The absorbance value at 490 nm was then measured,
referenced by 600 nm.
The A2780 and SKOV3 cells were cultured for 15 min
with 2.5 µg/ml PI at 20°C (cat. no. 556463; BD Biosciences). At the
end of the incubation, cells were slowly washed with PBS twice. The
cells were then exposed to 1 µg/ml Hoechst 33342 (cat. no. C1022;
Beyotime Institute of Biotechnology) for 5 min at 20°C, and
observed under a fluorescence microscope.
Scanning electron microscopy
(SEM)
Cell morphology was monitored by SEM: Following
fixing in 2.5% glutaraldehyde overnight at 20°C, cells were
dehydrated through a graded series of ethanol (30, 50, 70, 95 and
100%) and treated for 5 min at each concentration. The alcohol in
the sample was gradually replaced with isoamyl acetate, then were
critical-point dried. Dried specimens were chilled at −80°C and
transferred to a freezing dryer overnight. Each sample was coated
with gold-palladium and imaged by SEM (S-3400 N; Hitachi Science
Systems Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from A2780 cells was obtained using Total
RNA Extraction Kit (cat. no. RIJ26-01; Magen Biotechnology Co.,
Ltd.) and reversed-transcribed to cDNA with a Prime ScriptRT
reagent (cat. no. RR037A; Takara Bio, Inc.), according to the
manufacturer's instructions. The amplification of cDNA was
performed using TB Green Premix Ex Taq (cat. no. RR820A; Takara
Bio, Inc). The parameters of a real-time system (CFX Connect;
Bio-Rad Laboratories, Inc.) were set at 95°C for 30 sec, followed
by 40 cycles of 5 sec at 95°C and 30 sec at 60°C. The primers used
are listed in Table I.
Quantification of gene expression was performed by quantification
curve. The expression levels of the target genes were quantitated
using the 2−ΔΔCq method and GAPDH was used as the
internal control to normalize the qPCR data (25).
 | Table I.Sequences of the primers for reverse
transcription-quantitative PCR. |
Table I.
Sequences of the primers for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′à3′) |
|---|
| GSDMD | F:
GTGTGTCAACCTGTCTATCAAGG |
|
| R:
CATGGCATCGTAGAAGTGGAAG |
| GSDME | F:
ACATGCAGGTCGAGGAGAAGT |
|
| R:
TCAATGACACCGTAGGCAATG |
| Caspase-1 | F:
ATGTCTGTGGGCAGGAAGTG |
|
| R:
CCCTGTTTCTTCAGTGTGGGA |
| Caspase-3 | F:
CATGGAAGCGAATCAATGGACT |
|
| R:
CTGTACCAGACCGAGATGTCA |
| Caspase-4 | F:
CAAGAGAAGCAACGTATGGCA |
|
| R:
AGGCAGATGGTCAAACTCTGTA |
| Caspase-5 | F:
TTCAACACCACATAACGTGTCC |
|
| R:
GTCAAGGTTGCTCGTTCTATGG |
| GAPDH | F:
GGTGGTCTCCTCTGACTTCAACA |
|
| R:
GTTGCTGTAGCCAAATTCGTTGT |
Western blotting for protein
expression
Cells were washed with ice-cold PBS, and lysed with
cell lysis buffer for western blotting and IP (cat. no. P0013;
Beyotime Institute of Biotechnology) containing 1% PMSF. Protein
lysates were quantified using the BCA Protein Assay Kit (cat. no.
KGP903; Nanjing KeyGEN Biotech Co., Ltd.). Equal amounts of protein
(20 µg) were separated using 10% SDS-PAGE and transferred onto PVDF
membranes (cat. no. PR 05505; MilliporeSigma). Following blocking
with 5% non-fat milk in Tris-buffered saline with Tween-20 (0.05%)
at room temperature for 2 h, the membranes were incubated with
rabbit anti-LRRC8A primary antibody (cat. no. 24979S; dilution,
1:800; Cell Signaling Technology, Inc.), rabbit anti-ClC-2 primary
antibody (cat. no. ab192526; dilution, 1:800; Abcam), rabbit
anti-ClC-3 primary antibody (cat. no. ab28736; dilution, 1:1,000;
Abcam), rabbit anti-anoctamin-1 (ANO1) primary antibody (cat. no.
ab64085; dilution, 1:800; Abcam), rabbit anti-caspase-3 primary
antibody (cat. no. ab49822; dilution, 1:600; Abcam), rabbit
anti-GSDME primary antibody (cat. no. ab215191; dilution, 1:800;
Abcam) and rabbit anti-GAPDH antibody (cat. no. RIP008; dilution,
1:1,000; Beijing Dingguo Changsheng Biotechnology Co. Ltd.)
overnight at 4°C. Membranes were then incubated for 2 h at room
temperature with HRP-linked goat anti-rabbit secondary antibody
(dilution, 1:10,000; cat. no. IH-0011; Beijing Dingguo Changsheng
Biotechnology Co. Ltd.). Proteins were visualized using Ultra High
Sensitivity ECL Kit (GlpBio Technology, Inc.). Images were acquired
and quantified using the gel imaging system (Alliance 4.7; Bio-Rad
Laboratories, Inc.).
Whole-cell current recording
Whole-cell chloride currents were measured using a
patch clamp amplifier system (EPC-10; HEKA GmbH Technology &
Trade). The pipette solution contained 70 mM N-methyl-d-glucamine,
1.2 mM MgCl2, 10 mM
N-2-hydroxyethylpiperazine-N‧-2-ethanesulfonic acid (HEPES), 1 mM
ethylene glycol tetraacetic acid, 140 mM D-mannitol and 2 mM
adenosine triphosphate, with a pH of 7.25 and osmolality of 300
mOsm/kg·H2O. The isotonic bath solution contained 70 mM
NaCl, 0.5 mM MgCl2, 2 mM CaCl2, 10 mM HEPES
and 140 mM D-mannitol, pH 7.4, osmolality 300
mOsm/kg·H2O. The 47% hypotonic bath solution was similar
to the isotonic bath solution, except for D-mannitol, and its
osmolality was 160 mOsm/kg·H2O. All chemicals were the
highest grade available and purchased from Sigma-Aldrich (Merck
KGaA).
A2780 cells (20,000 cells/ml) plated on coverslips
were washed with the isotonic bath solution. The whole-cell current
was recorded with a resistance of 4–5 MΩ under the aforementioned
patch-clamp amplifier (EPC-10) using patch-clamp pipettes, which
were made from glass microtubules on a two-stage vertical pipette
puller (PC-10; Sutter Instrument Company). Cell membrane potential
was held at the Cl− equilibrium potential (0 mV) and
repeatedly stepped to 0, ±40 and ±80 mV for 200 msec pulses in 4
sec throughout the experiments. A computer recorded the whole-cell
currents using a laboratory interface (MICRO 1401-4; Cambridge
Electronic Design, Ltd.) at a sampling rate of 3 kHz. All
experiments were processed at room temperature (20–24°C). The
inhibition rate of PTX of the background Cl− current was
calculated using the following equation: Inhibition (%)=(CIso-CIso
+ PTX)/CIso ×100%. The inhibition rate of PTX of the
hypotonicity-activated Cl− current was calculated using
the following equation: Inhibition (%)=(Chypo-Chypo + PTX)/Chypo
×100%, where CIso is the background current under isotonic
condition, Chypo is the peak value of hypo-activated currents, and
CIso + PTX and Chypo + PTX are the currents recorded after adding
PTX in isotonic or hypotonic solution.
Measurement of intracellular chloride
ion concentration ([Cl−]i)
Ovarian cancer cells A2780 were washed with
Krebs-HEPES buffer (20 mM HEPES, 128 mM NaCl, 2.5 mM KCl, 2.7 mM
CaCl2, 1 mM MgCl2, 16 mM glucose, pH 7.4) and
exposed to 10 mM N-(ethoxycarbonylmethyl)-6-methoxyquinolinium
bromide (MQAE; Beyotime Institute of Biotechnology), a novel
fluorescent probe for Cl− whose fluorescence intensity
quenches when it binds to Cl−, for 60 min at 37°C and
washed again with Krebs-HEPES buffer.
4-(2-butyl-6,7-dichloro-2-cyclopentyl-indan-1-on5-yl) oxobutyric
acid (DCPIB; MedChemExpress) was configured as a 5 mM
high-concentration storage solution with DMSO. This was then
further diluted with PBS to a concentration of 1 µM. Fluorescence
emission was used to detect the fluorescence intensity of MQAE and
quantify the concentration of Cl−, including inverted
fluorescence photography and real-time fluorescence detection.
Real-time fluorescence detection used fast laser scanning confocal
microscopy (cat. no. SP8; Leica Microsystems, Inc.) to detect
cellular fluorescence. The instrument first recorded the
fluorescence intensity of the cells without drug stimulation for ~5
min, added the Krebs-Hepes buffer containing purple stripes
according to the experimental needs and recorded the changes of
MQAE fluorescence intensity following drug stimulation.
Small interfering RNA (siRNA)
transfection
A2780 ovarian cancer cells were transfected (37°C
for 8 h) using GP-transfect-mate (4 µl in 500 µl serum-free medium;
Shanghai GenePharma Co., Ltd.) transfection reagents with siRNA
(0.16 µM) targeting human LRRC8A (Shanghai GenePharma Co., Ltd.).
The time interval between transfection and subsequent experiments
was 48 h. The siRNA target sequences used are presented in Table II.
 | Table II.siRNA target sequences. |
Table II.
siRNA target sequences.
| siRNA name | Sequence
(5′à3′) |
|---|
| LRRC8A siRNA1 | F:
GCAGCAACUUCUGGUUCAATT |
|
| R:
UUGAACCAGAAGUUGCUGCTT |
| LRRC8A siRNA2 | F:
CCUCAAGAAGUACUCGUUUTT |
|
| R:
AAACGAGUACUUCUUGAGGTT |
| LRRC8A siRNA3 | F:
GCCUUAAGCUGUGGUACAATT |
|
| R:
UUGUACCACAGCUUAAGGCTT |
| Negative
control | F:
UUCUCCGAACGUGUCACGUTT |
| siRNA | R:
ACGUGACACGUUCGGAGAAT |
Statistical analysis
Data are presented as the mean ± SEM. Comparative
studies of means were performed using one-way analysis of variance
(ANOVA) followed by a post hoc test (projected least significant
difference Fisher). Differences between two groups were assessed
using an unpaired Student's t-test, and between paired variables
were assessed using a paired Student's t-test. Dunnett's t-test was
used to compare the differences between the control group and
multiple experimental groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
PTX induces pyroptosis-like cell death
in A2780 ovarian cancer cells
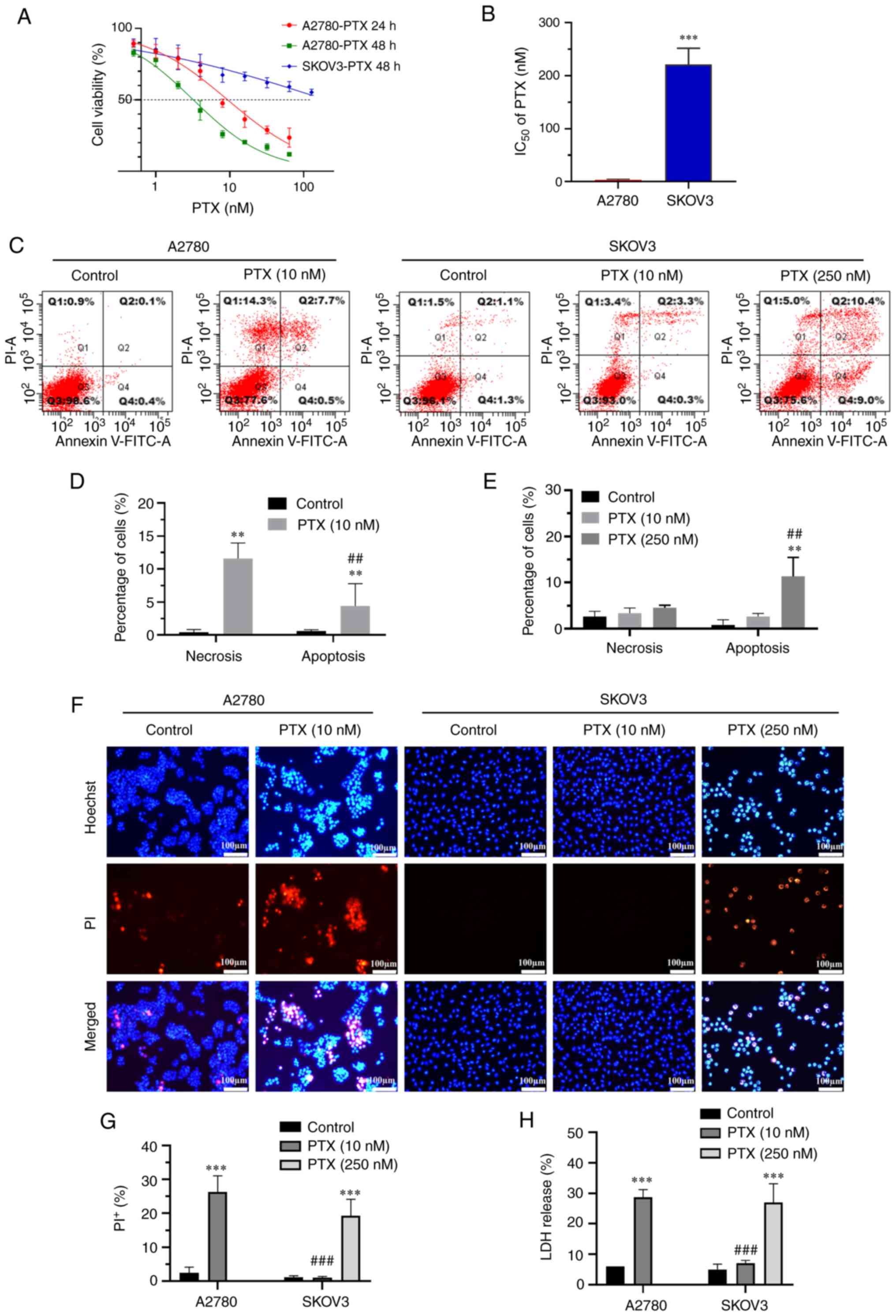
The cell viability of A2780 and SKOV3 ovarian cancer
cell lines was inhibited to various degrees following treatment
with PTX at concentrations of 0.5–256 nM for 24–48 h. As shown in
Fig. 1A and B, the IC50
in A2780 cells following treatment with PTX for 24 h was 9.67±1.46
nM, while little inhibition can be observed in SKOV3 cells.
Following treatment with PTX for 48 h, the IC50 in A2780
cells and SKOV3 cells were 3.23±0.33 and 221.9±31.72 nM (P<0.001
vs. A2780, n=4), respectively. Flow cytometry revealed a higher
level of necrosis (Annexin V+/PI+ cells) as
compared with that of apoptosis (Annexin
V+/PI− cells) in A2780 cells (Fig. 1C and D) following 10 nM PTX
treatment for 24 h. However, apoptosis was dominantly exhibited in
SKOV3 cells treated with 250 nM PTX for 48 h (Fig. 1C and E).
Similarly, Hoechst 33342/PI staining also
demonstrated that the ratio of necrosis (PI+ cells) was
markedly elevated in A2780 cells following treatment with PTX
(Fig. 1F-G; P<0.001; n=3). The
effect of PTX on plasma membrane damage was evaluated by measuring
LDH release in the culture supernatant. LDH release was
significantly increased in A2780 cells following treatment with 10
nM PTX compared with the control group (Fig. 1H; P<0.001; n=3), while it was
increased in SKOV3 cells at a PTX concentration of 250 nM. These
results indicated that PTX induced cell death by causing plasma
membrane damage in ovarian cancer cells.
PTX-induced cell swelling and cleavage
of caspase-3/GSDME in A2780 cells
Membrane rupture and cell swelling induced by
cleaved gasdermin family members are the main morphological
characteristics of pyroptosis (26). In the present study, the volume of
A2780 cells was gradually increased following treatment with PTX 10
nM for 1.5–24 h (Fig. 2A),
suggesting cell swelling, while no obvious changes were observed in
SKOV3 cells (Fig. 2B). The volume
only increased after 12 h of 250 nM PTX treatment in SKOV3 cells
(Fig. 2C). Therefore, the next
experiments focused on A2780 cells.
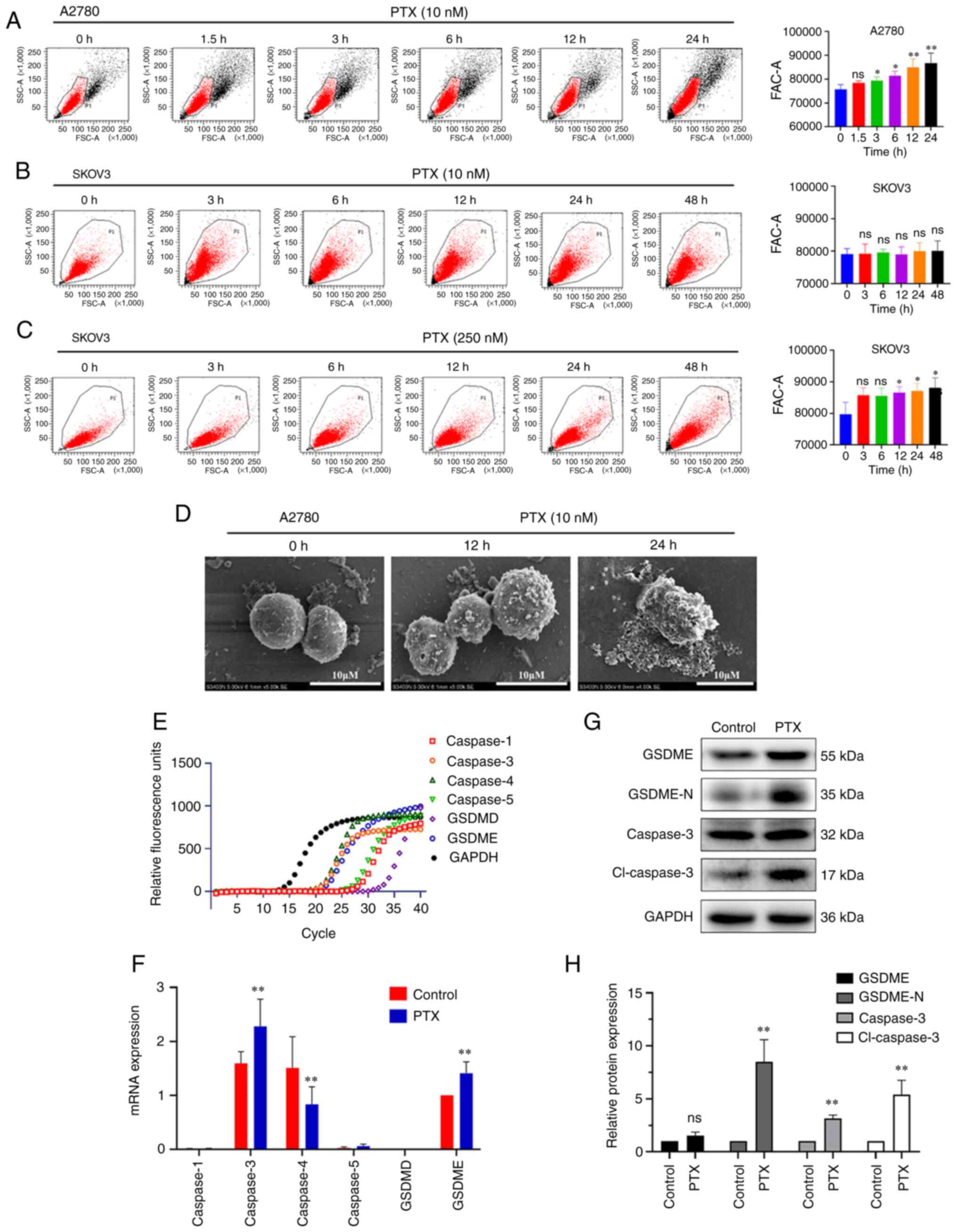 | Figure 2.PTX induces pyroptosis in ovarian
cancer cells through the caspase-3/GSDME pathway. (A-C) Flow
cytometry was used to detect the effect of PTX on the volume of
A2780 and SKOV3 ovarian cancer cells. The A2780 ovarian cancer cell
line was treated with 10 nM PTX and the SKOV3 ovarian cancer cell
line with 10 or 250 nM PTX for different time points (n=4;
*P<0.05 and **P<0.01; ns>0.05). (D) Scanning electron
micrographs of A2780 cells treated with 10 nM PTX for 12 and 24 h
(scale bar, 10 µm). (E and F) Relative fluorescence units of
caspases-1/3/4/5, GSDMD, GSDME and GAPDH mRNA in A2780 cells in the
control and PTX treatment groups, as detected by reverse
transcription-quantitative PCR (n=3; **P<0.01 vs. Control). (G
and H) Protein expression levels of full-length GSDME, GSDME-N,
caspase-3 and cl-caspase-3 were assessed using western blotting
(n=3; **P<0.01 vs. Control). PTX, paclitaxel; GSDMD, gasdermin
D; GSDME, gasdermin E; GSDME N-terminal fragment; ns, not
significant; cl-caspase-3, cleaved-caspase-3. |
The morphological changes of A2780 cells were then
examined by SEM. As revealed in Fig.
2D, the membrane surface of normal cells was intact and
relatively smooth. Following treatment with PTX, the cells were
swollen with pores and evident large bubbles extending from the
plasma membrane, suggesting the occurrence of a distinct
morphological change characteristic of pyroptosis (5).
GSDMD and GSDME are common executive proteins in
pyroptosis, and the GSDME protein is highly expressed in ovarian
tissue (27,28). However, it is unclear which member
of the gasdermin family is involved in the induction of pyroptosis
by PTX. In the present study, the genes associated with pyroptosis
were screened using RT-qPCR. The results showed that GSDME,
caspase-3 and caspase-4 genes were mainly expressed in A2780 cells,
and the expression of both caspase-3 and GSDME were increased while
that of caspase-4 was significantly decreased following treatment
with PTX (Fig. 2E and F). At the
protein level, PTX also induced the cleavage of caspase-3 and
GSDME, creating cleaved caspase-3 (cl-caspase-3) and GSDME
N-terminal fragment (GSDME-N), respectively, in A2780 cells
(Fig. 2G and H). These results
indicated that PTX induced pyroptosis through the caspase-3/GSDME
pathway in A2780 cells.
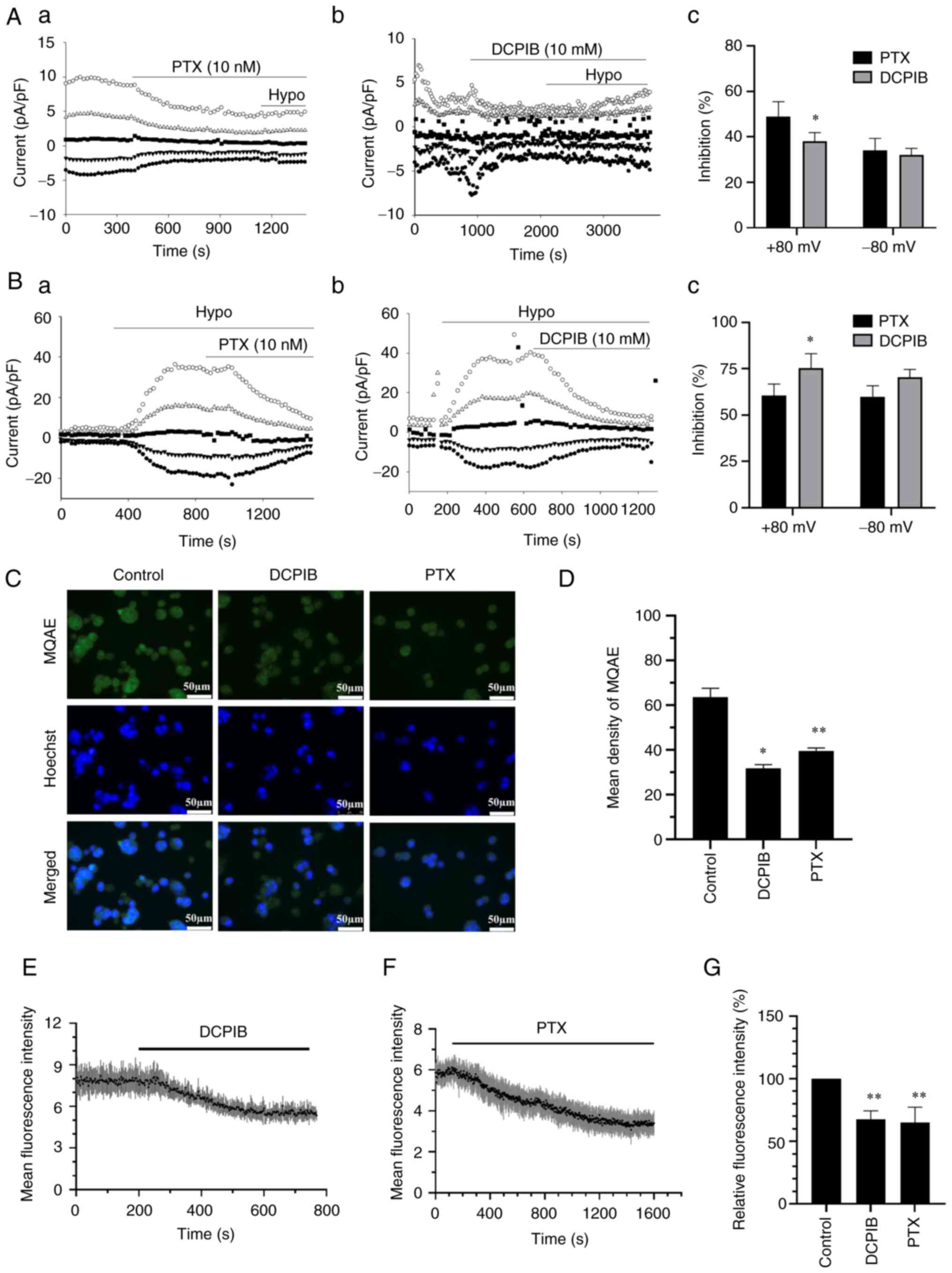
PTX inhibits chloride currents and
increases [Cl−]i in A2780 cells
The above data showed that PTX can cause cell
swelling. A previous study by the authors revealed that PTX
inhibited background- and hypotonicity-activated chloride currents
in A2780 cells (22). In addition,
it has previously been revealed that the inhibition of the chloride
channel caused an increase in the cell volume (23,29).
Herein, it was also confirmed that the inhibition of background-
and hypotonicity-activated chloride currents by PTX was similar to
the effect of classic VRAC inhibitor, DCPIB (Fig. 3A and B). It is known that the
inhibition of chloride currents leads to a decrease in
Cl− efflux. In the present study, the MQAE fluorescence
intensity was significantly decreased following treatment with
DCPIB and PTX, which indicated that the [Cl−]i increased
(Fig. 3C and D). Real-time
fluorescence assay also showed that the [Cl−]i increased
following treatment with PTX and DCPIB (Fig. 3E and F). The statistical analysis
results are shown in Fig. 3G. These
results suggested that PTX can inhibit the volume-sensitive
chloride channels in the same way as DCPIB, and cause an increase
in the [Cl−]i.
The component of volume-activated
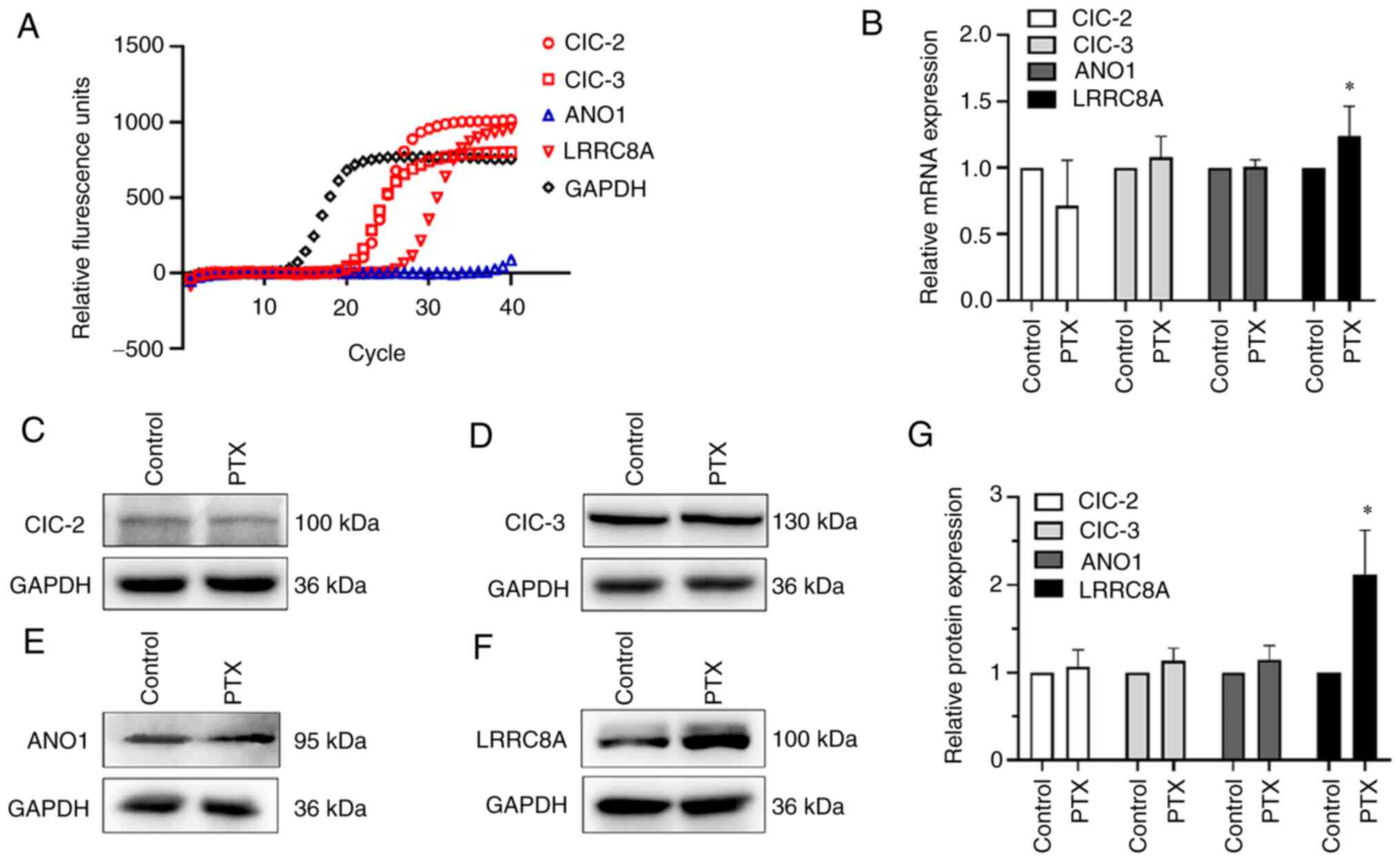
chloride channels, LRRC8A, is influenced by PTX in A2780 cells
There are several molecular categories of
volume-activated chloride channel proteins (7). In the present study, RT-qPCR was used
to detect the mRNA expression of the chloride channels of
ClC-2/ClC-3/ANO1/LRRC8A in A2780 cells. As revealed in Fig. 4A and B, volume-sensitive or
volume-related chloride channel genes expressed in A2780 cells
mainly included ClC-2, ClC-3 and LRRC8A. Only the LRRC8A mRNA
expression was increased, while that of the other genes exhibited
no significant changes following treatment with PTX. Similar to the
mRNA expression results, the protein expression of LRRC8A was
significantly increased following PTX treatment, while the
expression of the other proteins did not exhibit any significant
changes (Fig. 4C-G). It was
therefore inferred that LRRC8A may be involved in PTX-induced
pyroptosis in A2780 cells.
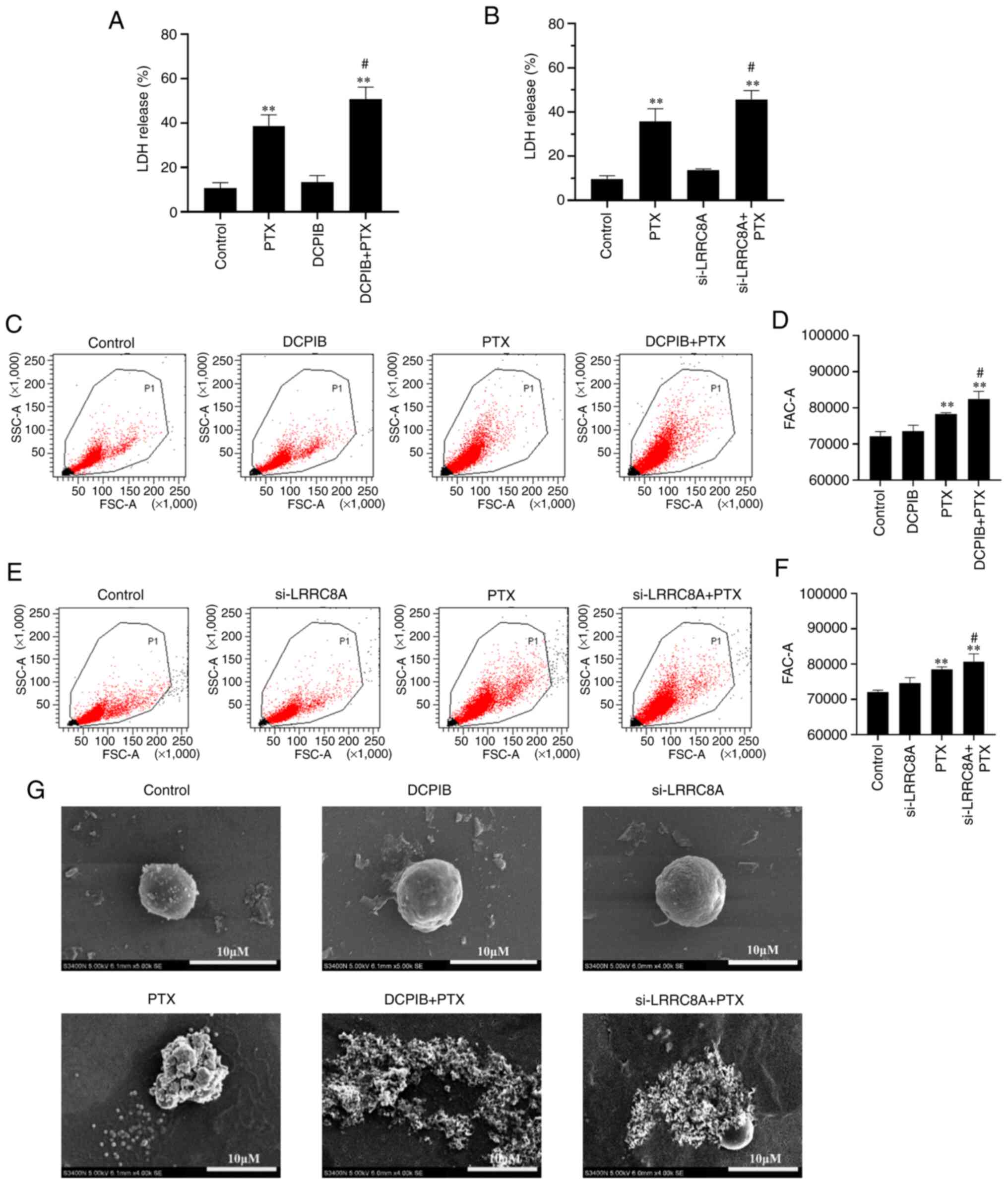
The VRAC inhibitor, DCPIB, or the
downregulation of LRRC8A protein enhance the efficacy of
PTX-induced pyroptosis in A2780 cells
VRAC plays a major role in cell volume regulation,
and its main component is LRRC8A (30). To further investigate the role of
VRAC in cell pyroptosis, PTX combined with VRAC inhibitor, DCPIB,
or LRRC8A siRNA (Fig. S1; the
si-L-3 sequence was selected by default for the subsequent
downregulation of LRRC8A) was used in the experiment. In the
experimental group, the concentration of PTX-treated cells was 10
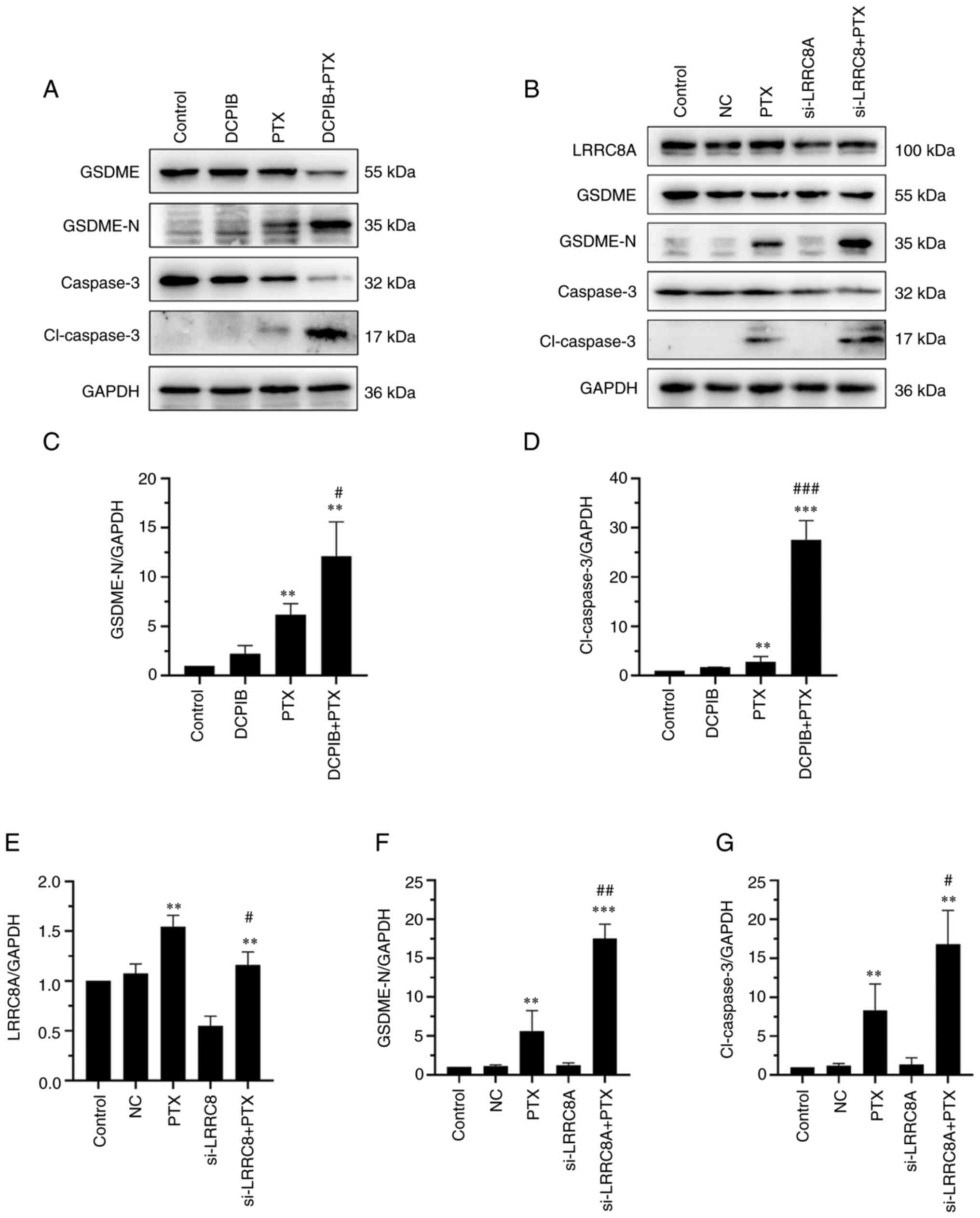
nM and the treatment time was 24 h. As shown in Fig. 5A and B, DCPIB or si-LRRC8A alone had
no effect on LDH release. However, both markedly enhanced the
PTX-induced LDH release. The effect of DCPIB and si-LRRC8A on cell
volume was investigated, and the cells in the combination group
were found to be larger, while DCPIB or siRNA alone had little
effect on cell volume (Fig. 5C-F).
Next, the morphological changes of the cells were examined using
SEM. Consistently, it was found that A2780 cells treated with DCPIB
or si-LRRC8A combined with PTX for 24 h exhibited more severe cell
membrane damage and could not maintain a normal morphology compared
with cells treated with PTX alone (Fig.
5G). However, the treatment of cells with DCPIB or si-LRRC8A
alone had little effect on cell morphology. According to these
results, the inhibition of VRAC function or the decrease in LRRC8A
protein expression could enhance the degree of PTX-induced
pyroptosis.
DCPIB or si-LRRC8A increases the
expression of pyroptosis-related proteins in A2780 cells
Given that the inhibition of VRAC combined with PTX
can increase pyroptosis in A2780 cells, the changes in
pyroptosis-related proteins, including caspase-3, cl-caspase-3,
GSDME and GSDME-N were further investigated. The levels of
pyroptosis proteins, such as cl-caspase-3 and GSDME-N, were
significantly increased by DCPIB/si-LRRC8A and PTX combination
therapy, as compared with those following treatment with PTX alone
(Fig. 6A-D and F and G). However,
there was no significant change in pyroptosis-related protein
following treatment with DCPIB or si-LRRC8A alone. In addition,
cells treated with si-LRRC8 combined with PTX showed an increase in
LRRC8 protein compared with si-LRRC8 alone (Fig. 6E). These results indicated that the
inhibition of VRAC or decrease in LRRC8A expression promoted
PTX-induced pyroptosis in A2780 cells.
Discussion
PTX was first approved for the treatment of ovarian
cancer (31), with its application
expanding into various other types of cancer some years later
(32–34). To date, it is well known that PTX
can induce multiple types of cell death, including mitotic
catastrophe, apoptosis, senescence, autophagy and pyroptosis
(35). In the present study, it was
first observed that A2780 ovarian cancer cells exhibited features
of pyroptosis, such as cell swelling, LDH release, deep PI staining
and membrane perforation. As shown by SEM, A2780 cells exhibited
evident swelling, and characteristic large bubbles appeared from
the plasma membrane following PTX treatment. These data
demonstrated that pyroptosis may be one of the underlying
mechanisms of PTX for the treatment of ovarian cancer.
The gasdermin family of proteins plays a key role in
cell pyroptosis. The family comprises six paralogous proteins in
humans: GSDMA/B/C/D/E and PJVK (36). GSDMD and GSDME are best known for
their functions in pore formation and pyroptosis (17). GSDMD activates cutting via
caspases-1/4/5/11 (36), while
GSDME activates cutting via caspase-3 (37). PTX has been shown to induce
pyroptosis in both caspase-1/GSDMD and caspase-3/GSDME pathways in
different types of cancer (19–21).
Among ovarian tissues, the gasdermin family proteins mainly express
GSDME (27,28). Previous studies have shown that
chemotherapy drug-activated caspase-3 can induce secondary
necrosis/pyroptosis in both cancer and normal cells that express
high levels of GSDME (37–39). In the present study, GSDME was
mainly expressed in A2780 cells, while GSDMD expression was very
low. The upstream proteins of pyroptosis with the highest
expression were caspases-3/4. Caspase-3 and GSDME mRNA expression
was increased while caspase-4 mRNA expression was significantly
decreased following treatment with PTX. Further western blotting
also confirmed that the protein expression of caspase-3 and GSDME-N
fragment, which was cleaved by caspase-3, were significantly
increased in A2780 cells following treatment with PTX. Therefore,
it was inferred that PTX induced pyroptosis mainly by activating
caspase-3 to cleave GSDME in A2780 ovarian cancer cells.
Following the activation of caspase proteins,
gasdermin family proteins have been revealed to cleave to separate
their N-terminal pore-forming domain from the C-terminal repressor
domain, and finally the N-terminal is inserted into cell membranes
and forms large oligomeric pores, disrupting ion homeostasis and
subsequently inducing osmotic swelling (27,36).
Several studies, including the present one, have demonstrated that
the blockage of chloride channels led to cell swelling in both
isotonic and hypotonic conditions (8,23,29,40).
It was also demonstrated that PTX could inhibit the background-,
hypotonicity- and cisplatin-induced chloride currents in A2780
ovarian cancer cells (22). It was
also confirmed herein that PTX inhibited the basic/background- and
hypotonic-induced chloride currents of A2780 cells, and increased
the [Cl−]i, which may decrease the passive water efflux
and cause osmotic swelling. The above phenomena were also induced
by DCPIB, a classic blocker of VRACs. It could therefore be deduced
that the inhibition of chloride channels by PTX may contribute to
the underlying mechanism of cell swelling in the formation of
pyroptosis. However, it has also been reported that Cl−
outflow can initiate and activate NLRP3 inflammasomes by promoting
the polymerization of ASC, and then induce caspase-1/GSDMD
pathway-mediated pyroptosis (41,42).
Therefore, the flow direction of chloride ions on both sides of the
cell membrane is likely to be different during pyroptosis and to
depend on different cell types and pyroptosis inducers.
The candidate proteins for VRACs have been debated
for years. LRRC8A was identified as an essential component of the
VRACs in 2014 (30). In the present
study, the expression of LRRC8A, ClC-2, ClC-3 and ANO1, which have
been proposed to be associated with VRACs, were measured. Only
LRRC8A was overexpressed, which was inconsistent with the findings
of a previous study by Ye et al (16), which reported that LRRC8A expression
was reduced during pyroptosis in BMDM cells induced by LPS and
nigericin. Even in the present study, the increased LRRC8A protein
expression was inconsistent with the PTX-induced inhibition of
chloride currents. DCPIB is a known specific and potent inhibitor
of VRAC (43). Unlike the
inhibition by si-RNA, the inhibition by DCPIB is completely
reversible (44). In recent years
DCPIB has been increasingly used for probing the physiologic and
pathologic roles of VRAC. Ye et al (16) reported that VRAC inhibitors, such as
DCPIB and tamoxifen, and LRRC8A downregulation by siRNA
interference can induce pyroptosis-like phenotypes similar to those
by LPS and nigericin in BMDM cells. However, the present findings
showed that DCPIB and LRRC8A siRNA alone did not cause pyroptosis
in A2780 ovarian cancer cells, but could enhance the activities of
PTX in inducing pyroptosis. A reason why VRACs exhibit these
differences during the pyroptosis process is that the mechanisms of
pyroptosis are different in cancer and immune cells with different
stimuli. LPS plus nigericin triggered caspase-1/GSDMD-dependent
pyroptosis in BMDM cells (16),
while PTX activated caspase-3/GSDME-dependent pyroptosis. It has
also been reported that different chemotherapeutic agents may
induce different secondary pyroptosis even in the same cell line
with GSDME expression (20,45). In addition, the cancer cells
exhibited more adaptive changes than normal cells. PTX induced an
increase in the expression of LRRC8A which may be associated with
its adaption in ovarian cancer cells, since ongoing experiments by
the authors found that LRRC8A was overexpressed in PTX-resistant
cell lines (data not shown). Therefore, whether individual
characteristics of differential chloride channel expression in
ovarian cancer are associated with different degrees of PTX-induced
pyroptosis will be investigated in the future.
In conclusion, caspase-3 was activated and
specifically cleaved GSDME following PTX treatment to form a large
number of membrane pores, and water molecules entered the cell
causing cell swelling and inducing pyroptosis-like phenotypes. In
addition, PTX and DCPIB increase the intracellular [Cl-]i by
blocking the VRAC channel (LRRC8), which further promotes water
entry and reduces water outflow, disrupting the osmotic balance and
ultimately leading to persistent cell swelling (Fig. S2). PTX-induced pyroptosis was more
effective when combined with the VRAC blocker or LRRC8A knockdown,
the essential component of VRACs in A2780 ovarian cancer cells.
These findings revealed a potential mechanism of PTX, and
identified a combination of PTX and VRAC blockers that can
synergistically treat ovarian cancer. However, in order to optimize
their use in cancer treatment, whether the combination of PTX and
VRAC blockers can harm non-tumorous cells needs to be
determined.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81872133 and 81372382), the Basic
Research Funds for Central Universities of Jinan University (grant
no. 21620408).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and CL contributed equally to this work. XY and
CL designed the study, performed the experiments and interpreted
the data. XY wrote the manuscript. SL and XLiao performed
statistical analyses. KW and XLi revised the manuscript. LZ, GL and
HY contributed to the conception of the work, supervised the study
design, and corrected the manuscript. XY and CL confirm the
authenticity of all the raw data. All authors have read and agreed
to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shao F: Gasdermins: Making pores for
pyroptosis. Nat Rev Immunol. 21:620–621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of Pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kepp O, Galluzzi L, Zitvogel L and Kroemer
G: Pyroptosis-a cell death modality of its kind? Eur J Immunol.
40:627–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okada Y, Okada T, Sato-Numata K, Islam MR,
Ando–Akatsuka Y, Numata T, Kubo M, Shimizu T, Kurbannazarova RS,
Marunaka Y and Sabirov RZ: Cell volume-activated and
volume-correlated anion channels in mammalian cells: Their
biophysical, molecular, and pharmacological properties. Pharmacol
Rev. 71:49–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada Y, Sato K and Numata T:
Pathophysiology and puzzles of the volume-sensitive outwardly
rectifying anion channel. J Physiol. 587:2141–2149. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Li H, Liu E, Guang Y, Yang L, Mao
J, Zhu L, Chen L and Wang L: The AQP-3 water channel and the ClC-3
chloride channel coordinate the hypotonicity-induced swelling
volume in nasopharyngeal carcinoma cells. Int J Biochem Cell Biol.
57:96–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Tang X, Xu J, Wang J, Yang Y, Chen
Y, Chen L, Wang L, Zhu L and Yang H: Opening of the CLC-3 chloride
channel induced by dihydroartemisinin contributed to early
apoptotic events in human poorly differentiated nasopharyngeal
carcinoma cells. J Cell Biochem. 119:9560–9572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J,
Jacob T, Ye W, Wang L and Chen L: ClC-3 chloride channel proteins
regulate the cell cycle by up-regulating cyclin D1-CDK4/6 through
suppressing p21/p27 expression in nasopharyngeal carcinoma cells.
Sci Rep. 6:302762016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jentsch TJ: VRACs and other ion channels
and transporters in the regulation of cell volume and beyond. Nat
Rev Mol Cell Biol. 17:293–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Ye D, Ye W, Jiao C, Zhu L, Mao J,
Jacob TJC, Wang L and Chen L: ClC-3 is a main component of
background chloride channels activated under isotonic conditions by
autocrine ATP in nasopharyngeal carcinoma cells. J Cell Physiol.
226:2516–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao G, Zuo W, Fan A, Zhang H, Yang L, Zhu
L, Ye W, Wang L and Chen L: Volume-sensitive chloride channels are
involved in maintenance of basal cell volume in human acute
lymphoblastic leukemia cells. J Membr Biol. 240:111–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Zhu L, Xu Y, Zhang H, Ye W, Mao J,
Chen L and Wang L: Uncoupling of K+ and Cl- transport across the
cell membrane in the process of regulatory volume decrease. Biochem
Pharmacol. 84:292–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye X, Liu X, Wei W, Yu H, Jin X, Yu J, Li
C, Xu B, Guo X and Mao J: Volume-activated chloride channels
contribute to lipopolysaccharide plus nigericin-induced pyroptosis
in bone marrow-derived macrophages. Biochem Pharmacol.
193:1147912021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Bettadapura SN, Smeltzer MS, Zhu H
and Wang S: Pyroptosis and pyroptosis-inducing cancer drugs. Acta
Pharmacol Sin. 43:2462–2473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gregory RE and DeLisa AF: Paclitaxel: A
new antineoplastic agent for refractory ovarian cancer. Clin Pharm.
12:401–415. 1993.PubMed/NCBI
|
|
19
|
Wang X, Li H, Li W, Xie J, Wang F, Peng X,
Song Y and Tan G: The role of Caspase-1/GSDMD-mediated pyroptosis
in Taxol-induced cell death and a Taxol-resistant phenotype in
nasopharyngeal carcinoma regulated by autophagy. Cell Biol Toxicol.
36:437–457. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang CC, Li CG, Wang YF, Xu LH, He XH,
Zeng QZ, Zeng CY, Mai FY, Hu B and Ouyang DY: Chemotherapeutic
paclitaxel and cisplatin differentially induce pyroptosis in A549
lung cancer cells via caspase-3/GSDME activation. Apoptosis.
24:312–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng QZ, Yang F, Li CG, Xu LH, He XH, Mai
FY, Zeng CY, Zhang CC, Zha QB and Ouyang DY: Paclitaxel enhances
the innate immunity by promoting NLRP3 inflammasome activation in
macrophages. Front Immunol. 10:722019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng J, Peng Z, Gao L, Yang X, Sun Z, Hou
X, Li E, Zhu L and Yang H: ClC-3 promotes paclitaxel resistance via
modulating tubulins polymerization in ovarian cancer cells. Biomed
Pharmacother. 138:1114072021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao J, Yuan J, Wang L, Zhang H, Jin X, Zhu
J, Li H, Xu B and Chen L: Tamoxifen inhibits migration of estrogen
receptor-negative hepatocellular carcinoma cells by blocking the
swelling-activated chloride current. J Cell Physiol. 228:991–1001.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu R, Wang X and Shi C: Volume-regulated
anion channel as a novel cancer therapeutic target. Int J Biol
Macromol. 159:570–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu D, Wei C, Li Y, Yang X and Zhou S:
Pyroptosis, a new breakthrough in cancer treatment. Front Oncol.
11:6988112021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Broz P, Pelegrin P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Schutter E, Croes L, Ibrahim J, Pauwels
P, de Beeck KO, Vandenabeele P and Camp GV: GSDME and its role in
cancer: From behind the scenes to the front of the stage. Int J
Cancer. 148:2872–2883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LX, Zhu LY, Jacob TJ and Wang LW:
Roles of volume-activated Cl- currents and regulatory volume
decrease in the cell cycle and proliferation in nasopharyngeal
carcinoma cells. Cell Prolif. 40:253–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Voss FK, Ullrich F, Munch J, Lazarow K,
Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T and
Jentsch TJ: Identification of LRRC8 heteromers as an essential
component of the volume-regulated anion channel VRAC. Science.
344:634–638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menzin AW, King SA, Aikins JK, Mikuta JJ
and Rubin SC: Taxol (paclitaxel) was approved by FDA for the
treatment of patients with recurrent ovarian cancer. Gynecol Oncol.
54:1031994.PubMed/NCBI
|
|
32
|
Kelly WK, Curley T, Slovin S, Heller G,
McCaffrey J, Bajorin D, Ciolino A, Regan K, Schwartz M, Kantoff P,
et al: Paclitaxel, estramustine phosphate, and carboplatin in
patients with advanced prostate cancer. J Clin Oncol. 19:44–53.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cortazar P, Justice R, Johnson J, Sridhara
R, Keegan P and Pazdur R: US food and drug administration approval
overview in metastatic breast cancer. J Clin Oncol. 30:1705–1711.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ranson M and Thatcher N: Paclitaxel: A
hope for advanced non-small cell lung cancer? Expert Opin Investig
Drugs. 8:837–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao S, Tang Y, Wang R and Najafi M:
Mechanisms of cancer cell death induction by paclitaxel: An updated
review. Apoptosis. 27:647–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Zhang P, An L, Sun N, Peng L,
Tang W, Ma D and Chen J: Miltirone induces cell death in
hepatocellular carcinoma cell through GSDME-dependent pyroptosis.
Acta Pharm Sin B. 10:1397–1413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen X, Wang H, Weng C, Jiang H and Chen
J: Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy
drug-induced nephrotoxicity. Cell Death Dis. 12:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kittl M, Winklmayr M, Preishuber-Pflugl J,
Strobl V, Gaisberger M, Ritter M and Jakab M: Low pH attenuates
apoptosis by suppressing the volume-sensitive outwardly rectifying
(VSOR) chloride current in chondrocytes. Front Cell Dev Biol.
9:8041052021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang T, Lang X, Xu C, Wang X, Gong T, Yang
Y, Cui J, Bai L, Wang J, Jiang W and Zhou R: CLICs-dependent
chloride efflux is an essential and proximal upstream event for
NLRP3 inflammasome activation. Nat Commun. 8:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Green JP, Yu S, Martin-Sanchez F, Pelegrin
P, Lopez-Castejon G, Lawrence CB and Brough D: Chloride regulates
dynamic NLRP3-dependent ASC oligomerization and inflammasome
priming. Proc Natl Acad Sci USA. 115:E9371–E9380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Decher N, Lang HJ, Nilius B, Bruggemann A,
Busch AE and Steinmeyer K: DCPIB is a novel selective blocker of
I(Cl,swell) and prevents swelling-induced shortening of guinea-pig
atrial action potential duration. Br J Pharmacol. 134:1467–1479.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pedersen SF, Okada Y and Nilius B:
Biophysics and physiology of the volume-regulated anion channel
(VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR).
Pflugers Arch. 468:371–383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zeng CY, Li CG, Shu JX, Xu LH, Ouyang DY,
Mai FY, Zeng QZ, Zhang CC, Li RM and He XH: ATP induces
caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked
murine macrophages. Apoptosis. 24:703–717. 2019. View Article : Google Scholar : PubMed/NCBI
|