Introduction
Medulloblastoma (MB) is a highly malignant and
invasive childhood brain tumor most predominantly located in the
cerebellum (1–4). Currently, the majority of patients
with MB undergo surgical resection and receive multi-agent
chemotherapy, while children ˃3 years of age are also treated with
craniospinal radiation therapy (5,6).
Although current treatment strategies have increased the average
survival of rate of patients with MB up to 70%, the major
disadvantages of such therapies include the development of drug
resistance, metastasis, disease recurrence which is universally
fatal, and long-term toxicities (7–9). There
is therefore a need for the development of novel personalized
targeted therapies focusing on molecular alterations and individual
tumor molecular profiles.
According to the renewed 2021 World Health
Organization (WHO) Classification of Tumor of the Central Nervous
System, MB is classified into four genetically-defined subgroups,
including WNT-activated (best prognosis), Sonic hedgehog
(SHH)-activated TP53 wild-type, SHH-activated TP53-mutant, and
non-WNT/non-SHH (group 3, worst prognosis; and group 4,
intermediate outcomes) (10–17).
The understanding of specific tumorigenic mutations,
molecular drivers and deregulated signaling pathways in molecular
subgroups accelerates the discovery of potential therapeutic
targets. Potential targets are poly(ADP-ribose) (PAR) polymerase
(PARP)1 and PARP2, which are known as the key proteins coordinating
the DNA damage response, specifically the damage detection and
repair of both single- and double-strand breaks (18–21).
In particular, PARP1 and PARP2 (PARP1/2) are involved in the
synthesis of PAR chains, which in turn recruit DNA repair proteins.
PARP inhibitors have therefore been actively used in the treatment
of cancers harboring defects in homologous recombination, such as
in tumors with BRCA1 and BRCA2 mutations, thereby
leading to synthetic lethality (22,23).
Among the PARP inhibitors, BMN673 (talazoparib) is the most potent
selective PARP1/2 inhibitor (24,25).
BMN673 is currently approved by the US Food and Drug Administration
(FDA) for the treatment of BRCA1 or BRCA2 mutated,
negative HER2 locally advanced or metastatic breast cancer
(26). In other clinical studies,
an ongoing phase II trial is testing the effects of BMN673 in
patients with recurrent high-grade glioma (NCT04740190). Although
BMN673 has not extensively been tested in MB, previous studies have
demonstrated that other PARP inhibitors, such as olaparib,
rucaparib and veliparib are potential chemo- and radiosensitizing
agents in MB cells and xenograft models (27–29).
Another promising molecular target is the WEE1-like
protein kinase (WEE1), which is involved in the regulation of the S
phase and G2/M checkpoint of the cell cycle (30–32).
In response to DNA damage, WEE1 mediates the inhibition of the
phosphorylation of cyclin-dependent kinase (CDK) 1 and CDK2,
resulting in cell cycle arrest and possible DNA repair (33,34).
Multiple studies have reported increased expression levels of WEE1
in various types of cancer, including pediatric high-grade gliomas,
glioblastoma, ovarian cancer, melanoma, breast cancer and MB
(33,35–38).
The inhibition of WEE1 can impair the G2/M checkpoint, allowing
cancer cells with DNA damage to divide, eventually leading to
mitotic catastrophe (39).
Currently, MK-1775 is the first selective small-molecule inhibitor
of WEE1, which has exhibited promising antitumor efficacy, when
combined with chemotherapeutics agents, against pancreatic, breast,
colon and ovarian cancer (40–43).
In addition, it has been reported that MK-1775 is highly effective
in tumor cells harboring p53 mutations (44,45).
Specifically, in MB, previous research has demonstrated that
MK-1775 single treatment can inhibit MB tumor growth in vivo
(46).
The crucial roles of PARP and WEE1 in the DNA damage
response render them potential therapeutic targets against MB
tumors. The present study focused on targeting proteins that are
critical for DNA damage repair, potentially making this treatment
strategy applicable to different molecular subgroups. More
specifically, the present study examined the effects of the
FDA-approved PARP inhibitor, BMN673, and the not yet FDA-approved
WEE1 inhibitor, MK-1775, as single agents and in combination on
four MB cell lines DAOY, UW228-3, MED8A and D425.
Materials and methods
Tumor cell lines and cell seeding
The MB cell lines DAOY, UW228-3 (both group SHH) and
D425 and MED8A (both group 3), were obtained from Professor Per
Kogner, Karolinska Institutet and cultured in minimum essential
medium (MEM), Dulbecco's modified Eagle's medium (DMEM):nutrient
mixture F-12 (DMEM/F-12) and DMEM with GlutaMAX (both Group 3 cell
lines), respectively, with the addition of 10% fetal bovine serum
(FBS) (all from Gibco; Thermo Fisher Scientific, Inc.), together
with 1% L-glutamine, 100 U/ml of penicillin as well as 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.). The DAOY, UW228-3
and D425 cells are p53 mutated and D425 and MED8A have a MYC
amplification (47) (https://www.cellosaurus.org/CVCL_1275;
http://www.cellosaurus.org/CVCL_M137). For the
viability and proliferation/cytotoxicity assays, 2.5×103
cells/well were plated for the DAOY cells, 5×103
cells/well were plated for the UW228-3 cells, and 104
cells/well were plated for the D425 and MED8A cells (group 3 were
grown in suspension) in 90 and 200 µl medium in 96-well plates,
respectively. For western blot (WB) and FACS analyses,
5×105 cells/Ti25 flask in 5 ml medium were plated for
both the DAOY and UW228-3 MB cell lines.
Inhibitors
The PARP inhibitor, BMN673 (talazoparib), the WEE1
inhibitor, MK-1775 (AZD-1775, adavosertib), the phosphoinositide
3-kinase (PI3K) inhibitor, BYL719 (alpelisib), and the CDK4/6
inhibitor, PD-0332991 (palbociclib), in DMSO stock solutions
(Selleck Chemicals GmbH), were used in various dilutions in PBS
[further details have been previously described (47,48)].
WST-1 viability assay
Following the addition of (0.1, 0.5, 1 and 10
MK-1775 and BMN673 and their combinations), for 24, 48 and 72 h,
the viability, i.e., the estimation of remaining live healthy cells
in response to therapy of the cell population was estimated using
WST-1 viability assay (Roche Diagnostics GmbH) as previously
described in more detail (47,48).
Cell confluency, cytotoxicity and
apoptosis assays
The IncuCyte S3 Live Cell Analysis System was used
to examine the cell confluency, as a measure of proliferation
following treatment and cytotoxicity, as a measure of cell damage
following treatment of the DAOY and UW228-3 cells, both grown as
monolayers (47,48). More specifically, at 24 h after
seeding, the medium was changed to a new medium containing the
Incucyte™ Cytotox Red Reagent (Essen Bioscience), that enters the
damaged plasma membrane and binds to DNA in the nuclei, the
treatments were then added and the plates were incubated at 37°C in
the machine for 72 h. Images were collected every 2 h to follow
cell confluence/proliferation with IncuCyte S3 Live Cell Analysis
System (Satorius). Cytotoxicity was quantified by counting the red
nuclei. Apoptosis was also assayed in the DAOY and UW228-3 cells
using the IncuCyte S3 Live Analysis System by the addition of the
IncuCyte Caspase-3/7 Green Apoptosis reagent, that enters live
cells, as previously described in further detail (47,48).
FACS analysis
For cell cycle analysis, the cells were collected
following 48 h of treatment and fixed with 70% ethanol. A total of
5×105 cells were counted and stained with FxCycleR/RNAse
solution (Invitrogen™, ThermoFisher Scientific, Inc.). All samples
were analyzed with the FACS NovoCyte 3000, while the analysis of
the data was conducted using FlowJo_v10.8.1 software (BD
Biosciences).
Statistical analysis
All the results were subjected to statistical
analysis. To estimate the efficacy of the single or combination
treatments compared to the negative control, a multiple t-test
accompanied by the correction for multiple comparisons of the means
using the Holm Sidak method were used as previously described
(49). To investigate the efficacy
of the drug combinations the Synergy FinderPlus computational tool
(https://synergyfinderplus.org/#!/)
with the highest single agent (HSA) was used. HSA values >10
indicated synergistic effects of the drugs, HSA values from −10 to
10 indicated additive effects, and HSA values <-10 indicated
antagonism (50).
Results
Effects of single and combination drug
treatment with WEE1 and PARP inhibitors on MB cell lines measured
using WST-1 assays
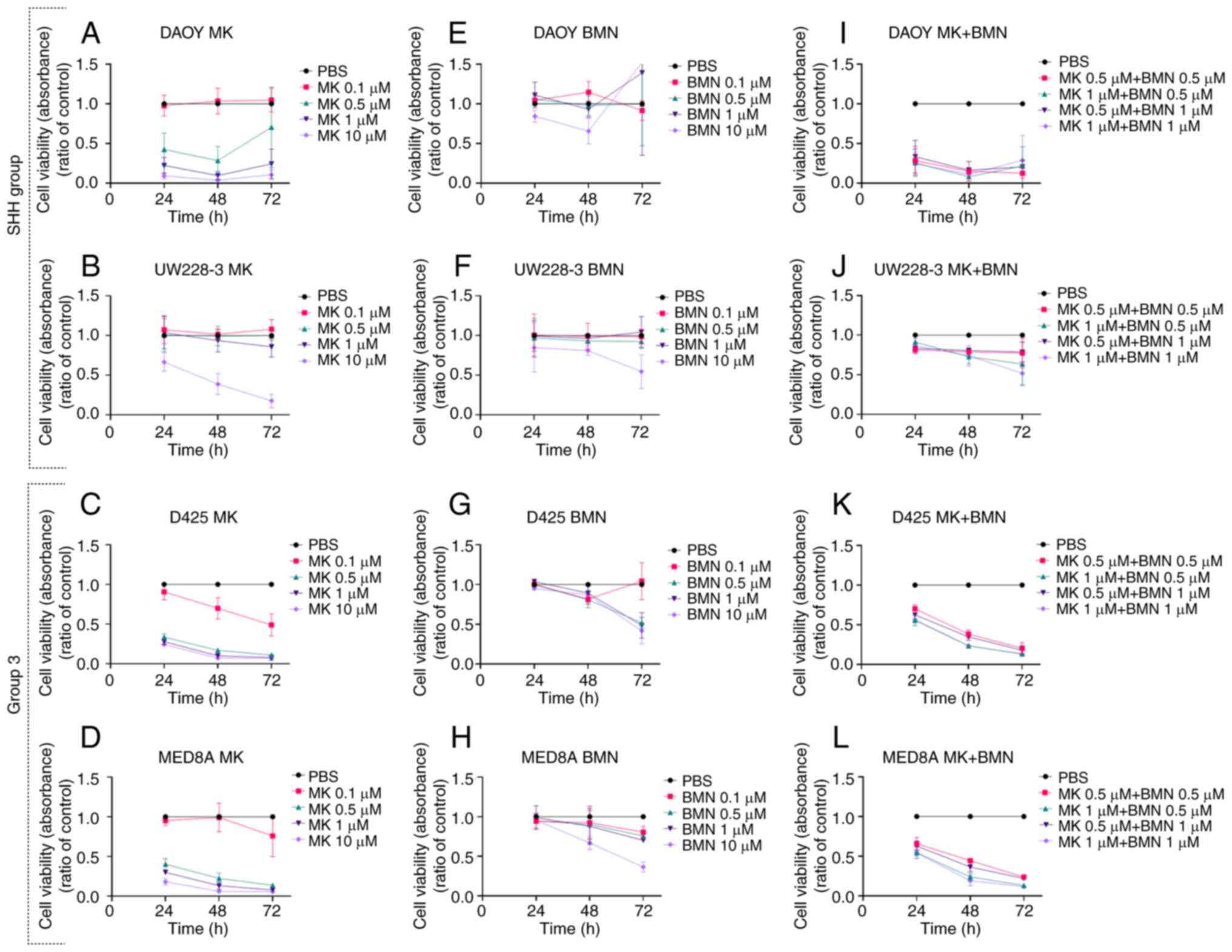
All MB cell lines (DAOY, UW228-3, D425 and MED8A)
exhibited a concentration-dependent inhibition of viability at
24–72 h following treatment (determined using WST-1 assays) with
the WEE1 inhibitor, MK-1775 (0.1–10 µM), and likewise, with the
exception of DAOY cells, with the PARP inhibitor, BMN673 (0.1–10
µM).
MK-1775
All MB cell lines exhibited a >50% significant
decrease in viability in comparison to PBS control with the highest
concentration (10 µΜ) of the WEE1 inhibitor, MK-1775, at almost all
time points following treatment (for all at least P<0.005)
(Fig. 1A-D). In addition, all MB
cell lines, apart from the UW228-3 cells, exhibited a >50%
decrease in viability compared to the PBS control following
treatment with 0.5 and 1 µΜ MK-1775 at most time points (for all
those at least P<0.05) (Fig.
1A-D).
BMN673
Only the highest concentration (10 µΜ) of the PARP
inhibitor, BMN673, led to a >50% decrease in viability in
comparison to the PBS control at 72 h following treatment in all MB
cell lines, apart from the DAOY cells (for all those at least
P<0.001) (Fig. 1E-H).
MK-1775 and BMN673 in combination
All MB cell lines, apart from the UW228-3 cells,
exhibited a >50% decrease in viability compared to the PBS
control at both 48 and 72 h following treatment with all drug
combinations, while the UW228-3 cells only exhibited a significant
decrease with the highest combination concentrations at 72 h
following treatment (for all at least P<0.05) (Fig. 1I-L).
To summarize, all four MB cell lines exhibited
concentration-dependent responses to both inhibitors with the
exception of the DAOY cells to BMN673, and with MK-1775 being more
efficient than BMN673 at the drug concentrations used. Moreover,
upon combining the inhibitors, the UW228-3 cells were generally
more resistant as compared to the other three MB cell lines.
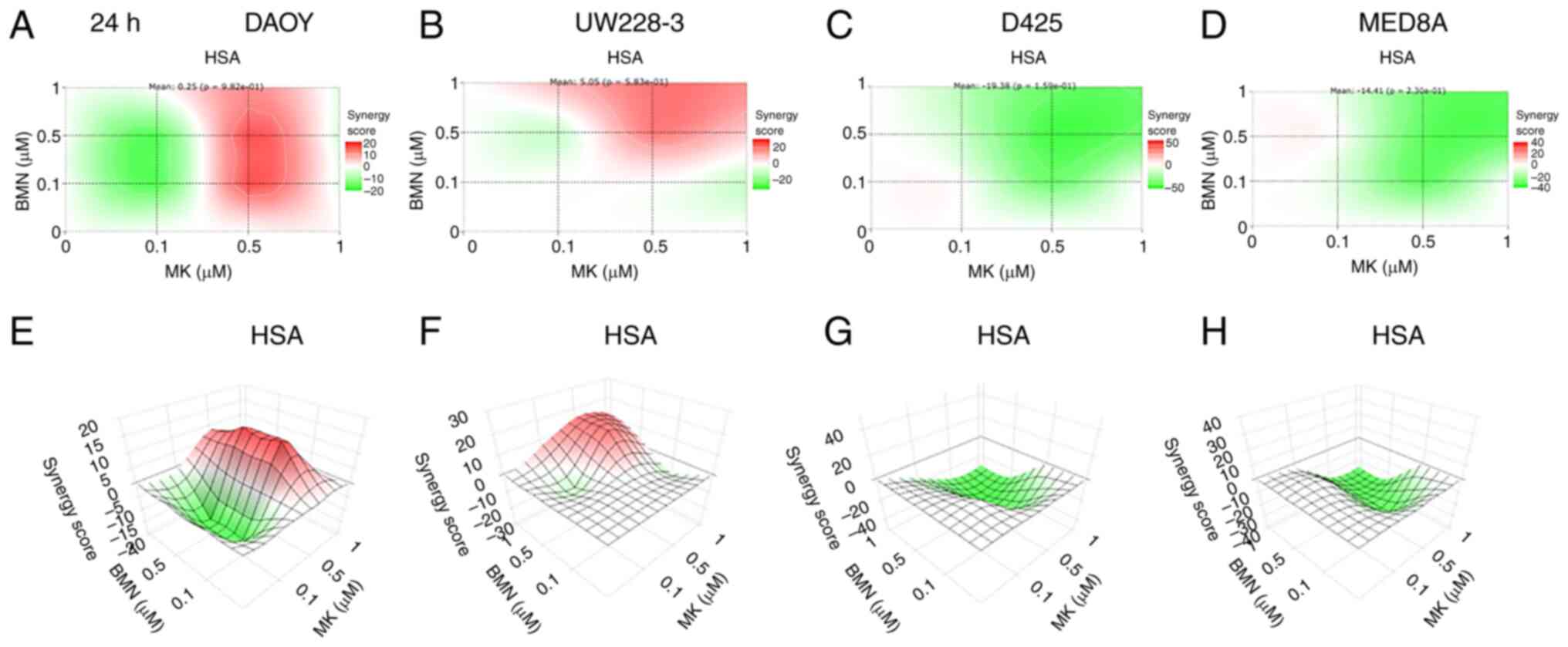
Synergistic effects of combined drug
treatment with WEE1 and PARP inhibitors on MB cell lines
Based on the cell viability data described above,
the synergy between WEE1 and PARP inhibitors was examined using the
Synergy FinderPlus program. To examine the synergy, the synergy
scores for the four MB cell lines treated with a range of
combinations of WEE1 and PARP inhibitors were calculated (Fig. 2). HSA values >10 indicated
synergistic effects of the drugs, HSA values from −10 to 10
indicated additive effects, and HSA values <-10 indicated
antagonism (50).
The calculations obtained revealed that synergy was
most clearly found when combining MK-1775 and BMN673 in the DAOY
and UW228-3 cells, while the same drug combinations exerted mainly
antagonistic effects in the D425 and MED8A cells (Fig. 2). Since synergy was optimal in the
UW228-3 and DAOY cells, these two cell lines were selected for use
in further experiments.
Other combinations
MK-1775 was also combined with BYL719 and
PD-0332991, two other regulators of the cell cycle, in order to
examine the joint effects of MK-1775 with BYL719 or PD-0332991 on
DAOY and UW228-3 cells. However, at the concentrations used (5 and
10 µΜ of BYL719, and 5 and 10 µΜ of PD-0332991), no major
enhancements were observed as compared to the effect of MK-1775
alone, with possibly one exception of a slight enhancement using
MK-1775 and PD-0332991 in combination on the UW228-3 cells (data
not shown). These data were therefore not pursued further
herein.
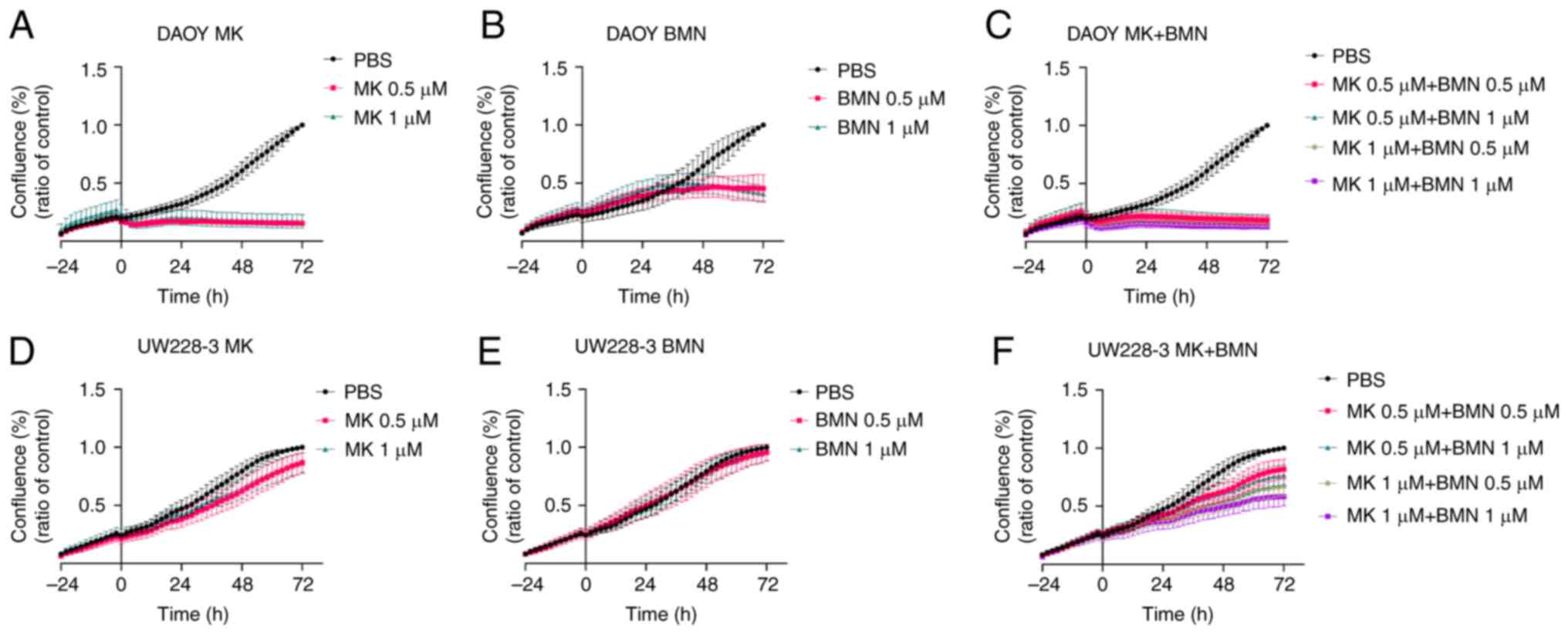
Cell confluency and cytotoxicity
following treatment of the DAOY and UW228-3 cells with WEE1 and
PARP inhibitors either alone or in combination
The effects of treatment with the MK-1775 and BMN673
inhibitors either alone or in combination on the DAOY and UW228-3
cells were further analyzed up to 72 h following treatment using
cell confluency and cytotoxicity assays utilizing the IncuCyte S3
Live-Cell Analysis System (Figs. 3
and 4).
Cell confluency
MK-1775
For the DAOY cells, both the MK-1775 concentrations
(0.5 and 1 µΜ) used induced an almost complete reduction in cell
confluency as compared to the PBS control, while for the UW228-3
cells, only a marginal reduction in cell confluency was noted
(Fig. 3A and D).
BMN673
For the DAOY cells, both concentrations (0.5 and 1
µΜ) of BMN673 used resulted in a reduced cell confluency compared
to the PBS control, while for the UW228-3 cells, no effect on cell
confluency was observed (Fig. 3B and
E).
MK-1775 and BMN673
For the DAOY cells, all drug combinations resulted
in an almost complete reduction of cell confluency compared to the
PBS control, while for the UW228-3 cells, the effects were
concentration-dependent and less pronounced (Fig. 3C and F).
To conclude, MK-1775 alone considerably reduced the
confluency of the DAOY cells and this was also the case, to a
certain extent, for cell confluency upon treatment with BMN673; an
enhanced was not detected for the DAOY cells following combination
treatment. By contrast, while almost no effect on UW228-3 cell
confluency was observed upon treatment with the inhibitors alone,
when used in combination, the inhibitors led to a
concentration-dependent inhibition of cell confluency.
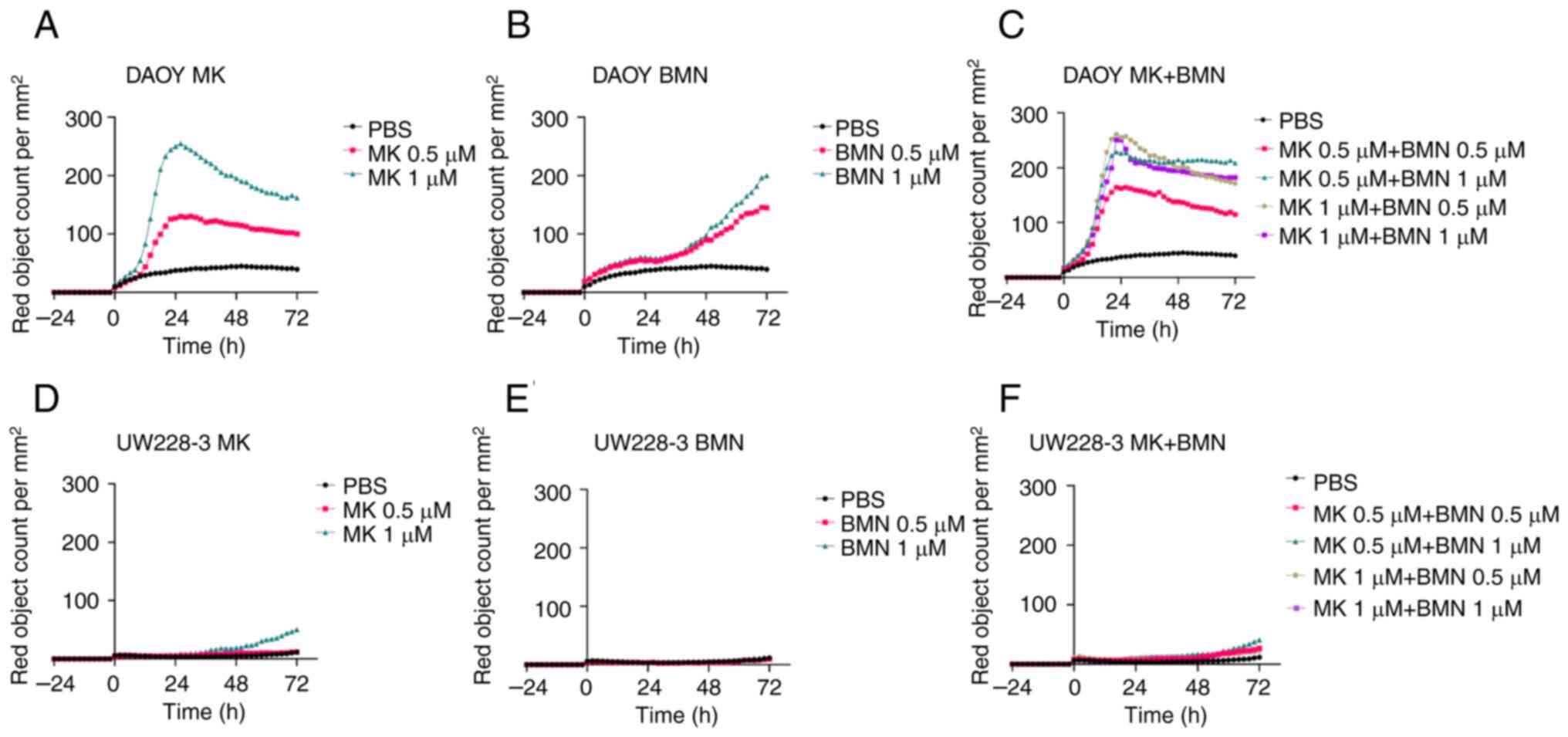
Cytotoxicity
MK-1775
Both the MK-1775 concentrations (0.5 and 1 µΜ)
exerted marked cytotoxic effects on the DAOY cells, whereas no
marked cytotoxic effects were observed on the UW228-3 cells
(Fig. 4A and D).
BMN673
Both the BMN673 concentrations (0.5 and 1 µΜ)
exerted some cytotoxic effects on the DAOY cells, whereas no
cytotoxic effects were observed on the UW228-3 cells (Fig. 4B and E).
MK-1775 and BMN673
The combined use of MK-1775 with BMN673 did not
exert any exerted enhanced cytotoxic effects on either the DAOY or
UW228-3 cells as compared to using the most efficient single
inhibitor, MK-1775 (Fig. 4C and
F).
To investigate this further, and to determine
whether part of the cytotoxic response was due to apoptosis, an
apoptosis assay was also performed. However, no major effects on
apoptosis were observed with any of the single or combined drug
administrations used above (Fig.
S1).
To conclude, although treatment with MK-1775, but
also BMN673 alone exerted cytotoxic effects on the DAOY cells,
their combined use did not exert any enhanced effects. In the
UW228-3 cells, none of the single or combined treatments exerted
pronounced cytotoxic effects.
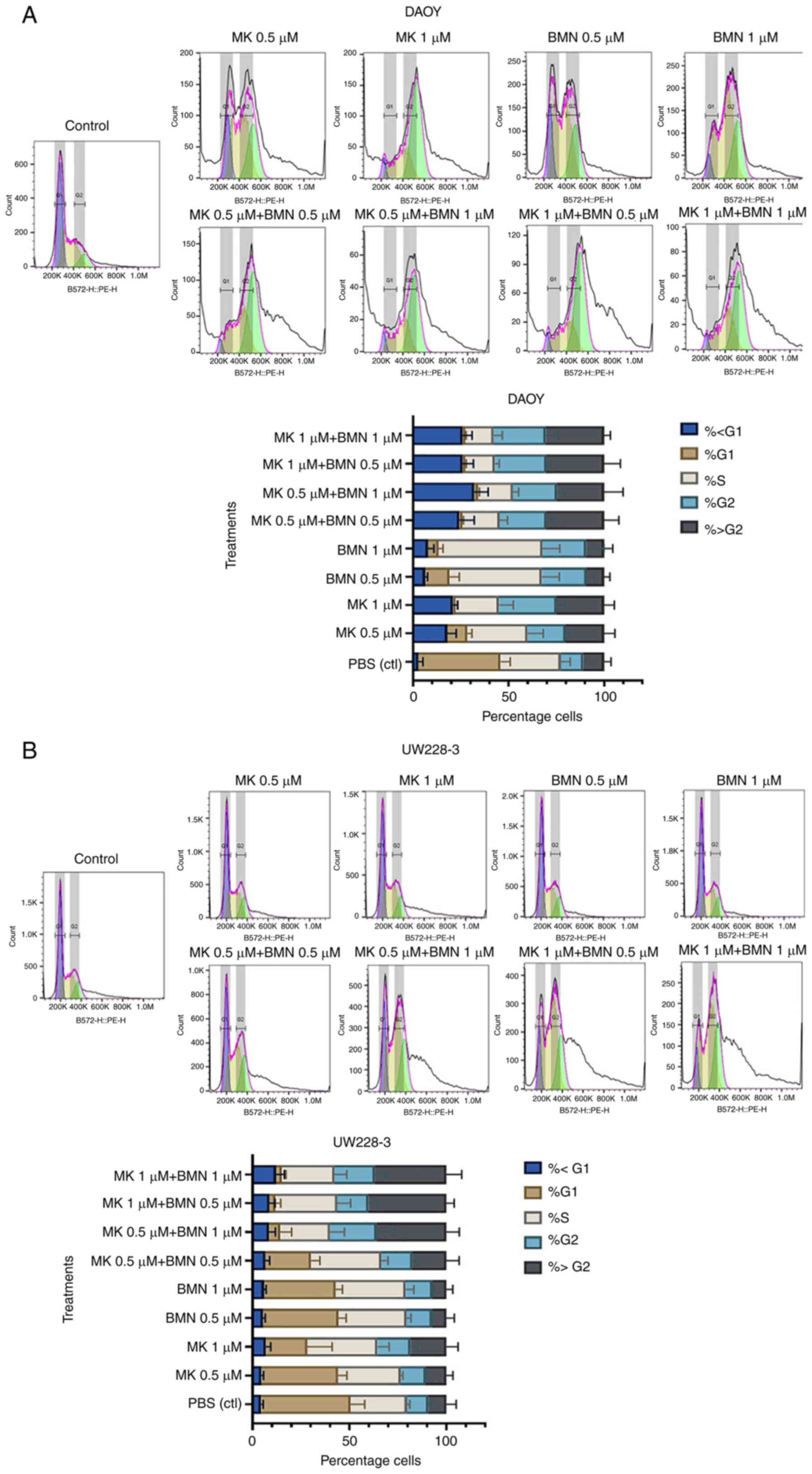
Effects of PARP and WEE1 inhibitors
alone or in combination on the cell cycle progression of MB
cells
The effects on cell cycle progression were examined
using a FACS NovoCyte 3000 machine and FlowJo_v10.8.1 software.
More specifically, the effects of WEE1 and PARP inhibitors alone or
in combination (MK-1775 and BMN673) were examined on the SHH cell
lines, DAOY and UW228-3 (Figs. 5
and S2).
MK-1775
Both single MK-1775 concentrations (0.5 and 1 µΜ)
induced an increase in the proportion of cells in the S and G2
phases (compared to the PBS control), with a higher percentage of
cells arrested in the G2 phase using the 1 µΜ concentration in both
cell lines, although with a higher amount of UW228-3 cells
remaining in the G1 phase in comparison to the DAOY cells (Fig. 5).
BMN673
Both single BMN673 concentrations (0.5 and 1 µΜ)
induced an increase in the proportion of cells in the S phase and
to a lower extent in the G2 phase (compared to the PBS control) in
both cell lines, although with a higher number of UW228-3 cells
remaining in the G1 phase in comparison to the DAOY cells (Fig. 5).
MK-1775 and BMN673
All MK-1775 and BMN673 combinations, irrespective of
the concentrations used, led to a decrease in the percentage of
cells in the G1 phase, with a clear shift of the cells from the S
to the G2 and >G2 phases in both the DAOY and UW228-3 (Fig. 5).
To conclude, the single MK-1775 and BMN673
administrations increased the proportion of cells in the S and G2
phases in both cell lines, and their combination exerted additive
effects on both cell lines; however, the shifts in cell cycle
progression were generally delayed in the UW228-3 cells when
compared to the DAOY cells.
Discussion
In the present study, WEE1 and PARP inhibitors
(MK-1775 and BMN673, respectively) were evaluated alone or in
combination in four MB cell lines, namely DAOY, UW228-3 (both group
SHH), and D425 and MED8A (both group 3). All MB cell lines
exhibited, at the concentrations used, concentration-dependent
responses, with a decrease in viability upon single MK-1775
treatment and with the exception of the DAOY cells, which to
certain to a certain extent, also exhibited a response to BMN673
treatment. Furthermore, upon combining the two drugs, synergy was
noted in the DAOY and UW228-3 cells (both group SHH), but not in
the group 3 cell lines; thus, only the former two cell lines were
examined in further detail for the effects of single and combined
inhibitor treatments on cell confluency, cytotoxicity and the cell
cycle.
Presently, the authors have no explanation as to why
synergy with regard to viability upon combination treatments with
MK-1775 and BMN673 was not obtained in group 3 MB cell lines. One
could have argued that this could have been due to the fact that
the two SHH cell lines, DAOY and UW228-3, have p53
mutations; however, this was also the case for the D425 cells;
thus, obviously, this is an issue that warrants further
investigation in future studies.
Notably, at the concentrations used, MK-1775 alone
was superior to BMN673 upon single treatments as regards its
effects on viability in all cell lines. A similar outcome was also
observed in cell confluency and cytotoxicity, where the DAOY cells
were more sensitive than the UW228-3 cells, which in turn exhibited
marginal effects with regard to both cell confluency and
cytotoxicity. The data obtained herein for BMN673 are thereby in
line with previous data on BMN673 in other childhood cancers; a
previous study demonstrated limited clinical activity and suggested
that single treatment with BMN673 possibly would be more efficient
in patients whose tumors had defects in homologous recombination
repair (51).
When MK-1775 and BMN673 were used in combination,
synergy was disclosed, particularly as mentioned above, in
viability, in which a reduction was observed in both the DAOY and
UW228-3 cells, while the synergistic effects on cell confluency
mainly applied to the UW228-3 cells, where the single drugs had
limited effects. On the other hand, a synergistic effect on
cytotoxicity was not detected in any cell line, although the DAOY
cells were generally more sensitive than the UW228-3 cells,
corresponding to similar findings from previous research by the
authors using other inhibitors (47).
The concentration-dependent effects which were
observed on the MB cell lines with single MK-1775 treatments were
expected, since this has been previously demonstrated with
corresponding inhibitors on MB and other tumor cell lines, such as
head and neck cancer (46,52–55).
Previous analysis with other PARP inhibitors, such
as olaparib, rucaparib and veliparib has shown that they are
potential chemo- and radiosensitizing agents in MBs, even though
there are, to the best of our knowledge, no other publications
available using BMN673 on MB (27–29).
Furthermore, to the best of our knowledge, there are no other
studies available examining the MK-1775 and BMN673 combinations in
MB cell lines. Nevertheless, it is known that WEE1 inhibition is
efficient in cell lines exhibiting p53 mutations, although
the response is not always only related to a p53 mutation
alone (56).
In addition, in the present study, as mentioned
above in the two SHH MB cell lines, the effects on cell cycle
progression were examined, since both MK-1775 and BMN673 induce
G2/M cell cycle arrest (30–32,57).
Following single MK-1775 and BMN673 treatments, there was a
decrease in the proportion of cells in the G1 phase in both the
DAOY and UW228-3 cells, and an increase in the proportion of cells
shifting towards the S and G2/M phase, which is in accordance with
recent study, particularly for MK-1775, since it exerts cytotoxic
effects in both the S and G2/M phase (56).
Combined treatment with MK-1775 and BMN673 exerted
synergistic effects on both cell lines and further decreased the
proportion of cells in the G1 and S phases, and increased the
number of cells in the G2/M phase. Of note, combined treatment in
both cell lines resulted in a clear increase in the proportion of
cells in the >G2 phase; this is not surprising, as previous
studies on MK-1775 have suggested that WEE1 inhibition causes an
impairment in cytokinesis, leading to tetraploid cells (55). This needs to be examined further in
order to validate the obtained results. When comparing DAOY and
UW228-3 with regard to shifting towards the G2/M phase, the
responses of the UW228-3 cells were generally more delayed as
compared to those of the DAOY cells. The reason for this currently
remains unknown, apart from the fact that the UW228-3 cells are
generally more resistant than the DAOY cells (47,48).
Notably, since it has been previously demonstrated
that targeted therapy may cause problems in G1 control and can lead
to cancer cells becoming dependent on the G2 control to repair DNA
damage (58), targeting the G2
checkpoint could be proposed as a possible additional anticancer
strategy. Based on the aforementioned hypothesis, the present study
combined the WEE1 inhibitor, MK-1775, with the FDA-approved PI3K
and CDK4/6 inhibitors, BYL719 and PD-0332991; however, at the
concentrations used, only slight synergistic effects were observed
with MK-1775 and PD-0332991 on the UW228-3 cells (data not shown);
thus, this was not pursued further herein.
There were some limitations to the present study,
since only a small number of inhibitors and cell lines were used.
Nevertheless, of note, the obtained data demonstrate that drug-drug
interactions using WEE1 and PARP inhibitors are complex and can
result in either synergistic or antagonistic interactions,
depending on the MB subgroup profile and the mutation profile of
the different cell lines. While broader concentration ranges and
modified incubated periods may shed further light on the drug
interactions with respect to their antitumor efficacy, the
concentrations used herein adhere to commonly used standard
conditions, and therefore allow for more direct comparisons
(47,55).
Further and more detailed studies are warranted in
order to disclose the possible mechanisms underlying the tested
drug combinations exerting synergistic or antagonistic effects and
to provide a pre-clinical rationale of how to apply the
corresponding combinations clinically. Nevertheless, combining the
WEE1 inhibitor, MK-1775, and the PARP inhibitor, BMN673, in two SHH
MB cell lines, exerted synergistic effects and allowed for the use
of lower inhibitor concentrations compared to those of single
treatments, and this could possibly reduce some side-effects.
Moreover, targeting MB with two different mechanisms may decrease
the risk of resistance.
In conclusion, the present study suggests that
treatment with WEE1 alone can have effects on SHH and group 3 MB,
and combining WEE1 and PARP inhibitors may be of potential interest
for the treatment of the SHH MB group.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Swedish Childhood Cancer
Foundation (grant no. TJ2022-0067), the Swedish Cancer Foundation
(grant no. 20 0704), the Stockholm Cancer Society (grant no.
201092), the Stockholm City Council (grant no. 20180037), AnnaBrita
o Bo Casters Minne Foundation (Lindhés Advokatbyrå) (grant no.
LA2022-0070), Svenska Läkaresällskapet (grant no. SLS-934161), Åke
Wiberg Foundation (grant no. M21-0012) Karolinska Institutet Sweden
(grant no. 2022-01587), and Tornspiran Foundation (grant no.
839).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML, AT and SH performed the majority of the
experiments, interpreted the data, performed the statistical
analyses and contributed to the writing of the manuscript. ML
contributed together with ONK to the graphs of the manuscript. ML
and SH initiated the experiments and the interpretation of the
initial experiments, and contributed to the writing of the material
and methods section, all under the supervision of ONK. TD and ONK
made substantial contributions to the conception and design of the
study, as well as to the acquisition of data, and analysis and
interpretation of data, and were also involved in the drafting of
the manuscript and revising it critically for important
intellectual content. TD also provided the resources for the
performance of the experiments such as laboratory space and
consumables. TD and ONK were responsible for obtaining financial
support for conducting the research Project. All authors critically
read and approved the manuscript. ML and ONK confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Northcott PA, Robinson GW, Kratz CP,
Mabbott DJ, Pomeroy SL, Clifford SC, Rutkowski S, Ellison DW,
Malkin D, Taylor MD, et al: Medulloblastoma. Nat Rev Dis Primers.
5:112019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahapatra S and Amsbaugh MJ:
Medulloblastoma. StatPearls Treasure Island (FL): pp. pp12021
|
|
3
|
Northcott PA, Jones DT, Kool M, Robinson
GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P,
Taylor MD and Pfister SM: Medulloblastomics: The end of the
beginning. Nat Rev Cancer. 12:818–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar LP, Deepa SF, Moinca I, Suresh P and
Naidu KV: Medulloblastoma: A common pediatric tumor: Prognostic
factors and predictors of outcome. Asian J Neurosurg. 10:502015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin AM, Raabe E, Eberhart C and Cohen
KJ: Management of pediatric and adult patients with
medulloblastoma. Curr Treat Options Oncol. 15:581–594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Ding C, Tan W and Zhang A:
Medulloblastoma: Molecular understanding, treatment evolution, and
new developments. Pharmacol Ther. 210:1075162020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leary SE and Olson JM: The molecular
classification of medulloblastoma: Driving the next generation
clinical trials. Curr Opin Pediatr. 24:33–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulhern RK, Palmer SL, Merchant TE,
Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt
R, Ashley DM, et al: Neurocognitive consequences of risk-adapted
therapy for childhood medulloblastoma. J Clin Oncol. 23:5511–5519.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salloum R, Chen Y, Yasui Y, Packer R,
Leisenring W, Wells E, King A, Howell R, Gibson TM, Krull KR, et
al: Late morbidity and mortality among medulloblastoma survivors
diagnosed across three decades: A report from the childhood cancer
survivor study. J Clin Oncol. 37:731–740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cotter JA and Hawkins C: Medulloblastoma:
WHO 2021 and beyond. Pediatr Dev Pathol. 25:23–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Remke M, Ramaswamy V and Taylor M:
Medulloblastoma molecular dissection: The way toward targeted
therapy. Curr Opin Oncol. 25:674–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kijima N and Kanemura Y: Molecular
classification of medulloblastoma. Neurol Med Chir (Tokyo).
56:687–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lospinoso Severini L, Ghirga F, Bufalieri
F, Quaglio D, Infante P and Di Marcotullio L: The SHH/GLI signaling
pathway: A therapeutic target for medulloblastoma. Expert Opin Ther
Targets. 24:1159–1181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gajjar AJ and Robinson GW:
Medulloblastoma-translating discoveries from the bench to the
bedside. Nat Rev Clin Oncol. 11:714–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Northcott PA, Korshunov A, Pfister SM and
Taylor MD: The clinical implications of medulloblastoma subgroups.
Nat Rev Neurol. 8:340–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kool M, Korshunov A, Remke M, Jones DT,
Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van
Meeteren A, van Vuurden D, et al: Molecular subgroups of
medulloblastoma: An international meta-analysis of transcriptome,
genetic aberrations, and clinical data of WNT, SHH, group 3, and
group 4 medulloblastomas. Acta Neuropathol. 123:473–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rouleau M, Patel A, Hendzel MJ, Kaufmann
SH and Poirier GG: PARP inhibition: PARP1 and beyond. Nat Rev
Cancer. 10:293–301. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beck C, Robert I, Reina-San-Martin B,
Schreiber V and Dantzer F: Poly(ADP-ribose) polymerases in
double-strand break repair: Focus on PARP1, PARP2 and PARP3. Exp
Cell Res. 329:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mladenov E and Iliakis G: Induction and
repair of DNA double strand breaks: The increasing spectrum of
non-homologous end joining pathways. Mutat Res. 711:61–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benafif S and Hall M: An update on PARP
inhibitors for the treatment of cancer. Onco Targets Ther.
8:519–528. 2015.PubMed/NCBI
|
|
22
|
Ray Chaudhuri A and Nussenzweig A: The
multifaceted roles of PARP1 in DNA repair and chromatin
remodelling. Nat Rev Mol Cell Biol. 18:610–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Wang F, Tang T and Guo C: The role
of PARP1 in the DNA damage response and its application in tumor
therapy. Front Med. 6:156–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Res.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murai J, Huang SY, Renaud A, Zhang Y, Ji
J, Takeda S, Morris J, Teicher B, Doroshow JH and Pommier Y:
Stereospecific PARP trapping by BMN 673 and comparison with
olaparib and rucaparib. Mol Cancer Ther. 13:433–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoy SM: Talazoparib: First global
approval. Drugs. 78:1939–1946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buck J, Dyer PJC, Hii H, Carline B,
Kuchibhotla M, Byrne J, Howlett M, Whitehouse J, Ebert MA, McDonald
KL, et al: Veliparib is an effective radiosensitizing agent in a
preclinical model of medulloblastoma. Front Mol Biosci.
8:6333442021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Vuurden DG, Hulleman E, Meijer OL,
Wedekind LE, Kool M, Witt H, Vandertop PW, Würdinger T, Noske DP,
Kaspers GJ and Cloos J: PARP inhibition sensitizes childhood high
grade glioma, medulloblastoma and ependymoma to radiation.
Oncotarget. 2:984–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daniel RA, Rozanska AL, Mulligan EA, Drew
Y, Thomas HD, Castelbuono DJ, Hostomsky Z, Plummer ER, Tweddle DA,
Boddy AV, et al: Central nervous system penetration and enhancement
of temozolomide activity in childhood medulloblastoma models by
poly(ADP-ribose) polymerase inhibitor AG-014699. Br J Cancer.
103:1588–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garcia TB, Snedeker JC, Baturin D, Gardner
L, Fosmire SP, Zhou C, Jordan CT, Venkataraman S, Vibhakar R and
Porter CC: A small-molecule inhibitor of WEE1, AZD1775, synergizes
with olaparib by impairing homologous recombination and enhancing
DNA damage and apoptosis in acute leukemia. Mol Cancer Ther.
16:2058–2068. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McGowan CH and Russell P: Cell cycle
regulation of human WEE1. EMBO J. 14:2166–2175. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghelli Luserna di Rorà A, Cerchione C,
Martinelli G and Simonetti G: A WEE1 family business: Regulation of
mitosis, cancer progression, and therapeutic target. J Hematol
Oncol. 13:1262020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Do K, Doroshow JH and Kummar S: WEE1
kinase as a target for cancer therapy. Cell Cycle. 12:3159–3164.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin P, Gu Y and Morgan DO: Role of
inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in
human cells. J Cell Biol. 134:963–970. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mueller S, Hashizume R, Yang X, Kolkowitz
I, Olow AK, Phillips J, Smirnov I, Tom MW, Prados MD, James CD, et
al: Targeting Wee1 for the treatment of pediatric high-grade
gliomas. Neuro Oncol. 16:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caretti V, Hiddingh L, Lagerweij T,
Schellen P, Koken PW, Hulleman E, van Vuurden DG, Vandertop WP,
Kaspers GJ, Noske DP and Wurdinger T: WEE1 kinase inhibition
enhances the radiation response of diffuse intrinsic pontine
gliomas. Mol Cancer Ther. 12:141–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Magnussen GI, Holm R, Emilsen E, Rosnes
AK, Slipicevic A and Flørenes VA: High expression of Wee1 is
associated with poor disease-free survival in malignant melanoma:
Potential for targeted therapy. PLoS One. 7:e382542012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iorns E, Lord CJ, Grigoriadis A, McDonald
S, Fenwick K, Mackay A, Mein CA, Natrajan R, Savage K, Tamber N, et
al: Integrated functional, gene expression and genomic analysis for
the identification of cancer targets. PLoS One. 4:e51202009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mir SE, De Witt Hamer PC, Krawczyk PM,
Balaj L, Claes A, Niers JM, Van Tilborg AA, Zwinderman AH, Geerts
D, Kaspers GJ, et al: In silico analysis of kinase expression
identifies WEE1 as a gatekeeper against mitotic catastrophe in
glioblastoma. Cancer Cell. 18:244–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirai H, Arai T, Okada M, Nishibata T,
Kobayashi M, Sakai N, Imagaki K, Ohtani J, Sakai T, Yoshizumi T, et
al: MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor
efficacy of various DNA-damaging agents, including 5-fluorouracil.
Cancer Biol Ther. 9:514–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang G, Niu X, Zhang W, Caldwell JT,
Edwards H, Chen W, Taub JW, Zhao L and Ge Y: Synergistic antitumor
interactions between MK-1775 and panobinostat in preclinical models
of pancreatic cancer. Cancer Lett. 356:656–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghiasi N, Habibagahi M, Rosli R, Ghaderi
A, Yusoff K, Hosseini A, Abdullah S and Jaberipour M: Tumour
suppressive effects of WEE1 gene silencing in breast cancer cells.
Asian Pac J Cancer Prev. 14:6605–6611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leijen S, Beijnen JH and Schellens JH:
Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase
results in sensitization of p53-deficient tumor cells to
DNA-damaging agents. Curr Clin Pharmacol. 5:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirai H, Iwasawa Y, Okada M, Arai T,
Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K,
et al: Small-molecule inhibition of Wee1 kinase by MK-1775
selectively sensitizes p53-deficient tumor cells to DNA-damaging
agents. Mol Cancer Ther. 8:2992–3000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rajeshkumar NV, De Oliveira E, Ottenhof N,
Watters J, Brooks D, Demuth T, Shumway SD, Mizuarai S, Hirai H,
Maitra A and Hidalgo M: MK-1775, a potent Wee1 inhibitor,
synergizes with gemcitabine to achieve tumor regressions,
selectively in p53-deficient pancreatic cancer xenografts. Clin
Cancer Res. 17:2799–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harris PS, Venkataraman S, Alimova I,
Birks DK, Balakrishnan I, Cristiano B, Donson AM, Dubuc AM, Taylor
MD, Foreman NK, et al: Integrated genomic analysis identifies the
mitotic checkpoint kinase WEE1 as a novel therapeutic target in
medulloblastoma. Mol Cancer. 13:722014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Holzhauser S, Lukoseviciute M, Andonova T,
Ursu RG, Dalianis T, Wickström M and Kostopoulou ON: Targeting
fibroblast growth factor receptor (FGFR) and phosphoinositide
3-kinase (PI3K) signaling pathways in medulloblastoma cell lines.
Anticancer Res. 40:53–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lukoseviciute M, Maier H,
Poulou-Sidiropoulou E, Rosendahl E, Holzhauser S, Dalianis T and
Kostopoulou ON: Targeting PI3K, FGFR, CDK4/6 signaling pathways
together with cytostatics and radiotherapy in two medulloblastoma
cell lines. Front Oncol. 11:7486572021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kostopoulou ON, Holzhauser S, Lange BKA,
Ohmayer A, Andonova T, Bersani C, Wickström M and Dalianis T:
Analyses of FGFR3 and PIK3CA mutations in neuroblastomas and the
effects of the corresponding inhibitors on neuroblastoma cell
lines. Int J Oncol. 55:1372–1384. 2019.PubMed/NCBI
|
|
50
|
Zheng S, Wang W, Aldahdooh J, Malyutina A,
Shadbahr T, Tanoli Z, Pessia A and Jing T: SynergyFinder Plus:
Toward better interpretation and annotation of drug combination
screening datasets. Genomics Proteomics Bioinformatics. 20:587–596.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Smith MA, Hampton OA, Reynolds CP, Kang
MH, Maris JM, Gorlick R, Kolb EA, Lock R, Carol H, Keir ST, et al:
Initial testing (stage 1) of the PARP inhibitor BMN 673 by the
pediatric preclinical testing program: PALB2 mutation predicts
exceptional in vivo response to BMN 673. Pediatr Blood Cancer.
62:91–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moreira DC, Venkataraman S, Subramanian A,
Desisto J, Balakrishnan I, Prince E, Pierce A, Griesinger A, Green
A, Eberhardt CG, et al: Targeting MYC-driven replication stress in
medulloblastoma with AZD1775 and gemcitabine. J Neurooncol.
147:531–545. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kreahling JM, Gemmer JY, Reed D, Letson D,
Bui M and Altiok S: MK1775, a selective Wee1 inhibitor, shows
single-agent antitumor activity against sarcoma cells. Mol Cancer
Ther. 11:174–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guertin AD, Li J, Liu Y, Hurd MS, Schuller
AG, Long B, Hirsch HA, Feldman I, Benita Y, Toniatti C, et al:
Preclinical evaluation of the WEE1 inhibitor MK-1775 as
single-agent anticancer therapy. Mol Cancer Ther. 12:1442–1452.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Byskata K, Lukoseviciute M, Tuti F,
Zupancic M, Kostopoulou ON, Holzhauser S and Dalianis T: Targeted
therapy with PI3K, PARP, and WEE1 inhibitors and radiotherapy in
HPV positive and negative tonsillar squamous cell carcinoma cell
lines reveals synergy while effects with APR-246 are limited.
Cancers (Basel). 15:932022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Heijink AM, Blomen VA, Bisteau X, Degener
F, Matsushita FY, Kaldis P, Foijer F and van Vugt MA: A haploid
genetic screen identifies the G1/S regulatory machinery as a
determinant of Wee1 inhibitor sensitivity. Proc Natl Acad Sci USA.
112:15160–15165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guney Eskiler G, Cecener G, Egeli U and
Tunca B: Synthetically lethal BMN 673 (talazoparib) loaded solid
lipid nanoparticles for BRCA1 mutant triple negative breast cancer.
Pharm Res. 35:2182018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bucher N and Britten CD: G2 checkpoint
abrogation and checkpoint kinase-1 targeting in the treatment of
cancer. Br J Cancer. 98:523–528. 2008. View Article : Google Scholar : PubMed/NCBI
|