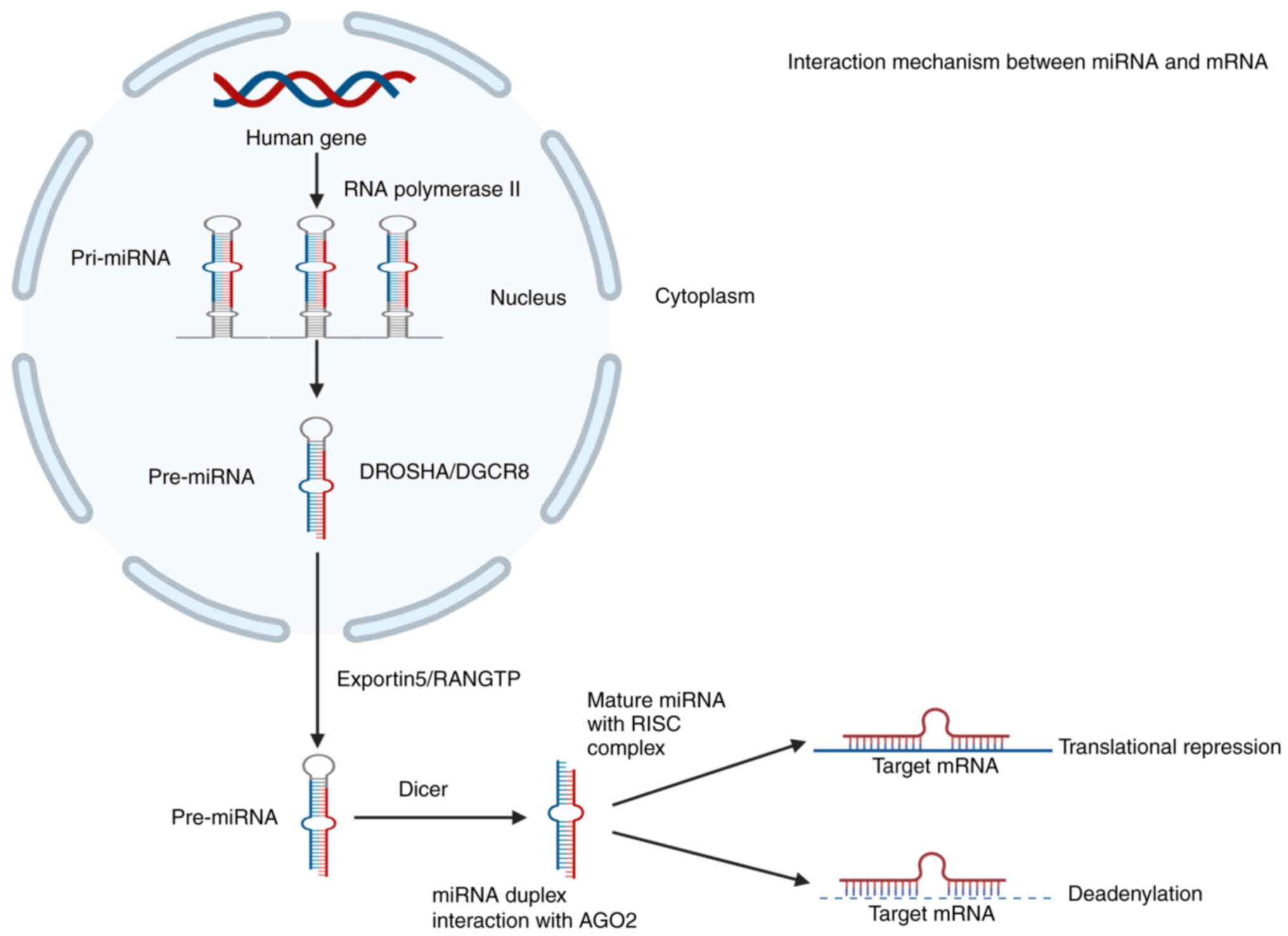
miRNAs are a class of non-coding single-stranded RNA
molecules with lengths of ~22–24 nucleotides. They are widely
present in animals, plants and viruses (12). Pri-miRNA is produced by RNA
polymerase II via a clear miRNA-processing mechanism (13). Subsequently, pri-miRNA is
transformed into pre-miRNA through the processing of RNase III,
Drosha and DGCR8 (14). DGCR8
identifies double-stranded structures and recruits substrates
(15). Drosha is responsible for
cleaving pri-miRNAs. This process occurrs as the first shear in the
nucleus. The newly generated pre-miRNA is transferred to the
cytoplasm through RANGTP/exportin-5 (16).
The ribonuclease Dicer then combines with the TRBP
protein to synthesize a mature double-stranded miRNA from pre-miRNA
(17). In the process of assembling
miRNA particles, the RNA helicase separates the two strands of
duplex miRNA (18). The 5′ end of
the single strand forms an active double strand with its partner,
which enters a complex containing miRNA and ribonucleoprotein
particles (19). The other strand
breaks down (20). After a series
of reactions, single-stranded miRNAs combine with Argonaute
(2) in RNA-induced silencing
complexes and then bind to the 3′ untranslated region of the target
mRNA, leading to translation suppression or de-adenylation
(Fig. 1) (21). In recent years, numerous studies
have confirmed that miRNAs are associated with numerous diseases,
such as diabetic kidney disease (22) and neurodegenerative disorders
(23). In addition, miRNAs are
known to participate in various malignant biological behaviors of
tumors, such as proliferation and epithelial-mesenchymal transition
(EMT) (24,25) (Table
I).
RNA microarrays and sequencing have been widely used
to screen differentially expressed miRNAs in LUAD. The results were
validated using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) (102).
Bioinformatics was employed to identify downstream target genes and
enriched pathways (103). Petkova
et al (104) used 12 pairs
of tissues to screen 107 significantly dysregulated miRNAs through
microarrays and performed RT-qPCR validation on the obtained
results using 50 pairs of samples. A total of eight significantly
differentially expressed miRNAs were successfully validated. Gene
Ontology and Kyoto Encyclopedia of Genes and Genomes analyses
revealed enrichment in the cell cycle, gene expression and EGFR
pathways. The present study highlighted the potential of exploring
differential miRNA expression profiles to understand their impact
on tumor diagnosis and prognosis (104). Beyond human 365, it can also be
detected in plasma. Jin et al (105) performed next-generation sequencing
on samples from 16 patients with LUAD and 12 healthy individuals.
Subsequently, a validation set including 10 LUAD patients and 30
healthy individuals was used to confirm significant differential
expression of four miRNAs, including miR-181-5p. These miRNAs were
further evaluated for diagnostic accuracy in an additional 60
patients initially diagnosed with non-small cell lung cancer,
resulting in an area under curve (AUC) value of 0.936. These
results revealed that these miRNAs may be promising biomarkers for
diagnosing LUAD (105).
Various studies have shown that miRNAs play an
important role in regulating tumor biological behavior and
influencing the tumor microenvironment (106,107). Numerous miRNAs have been
recognized as tumor markers and therapeutic targets that play
prominent roles in tumor prevention, diagnosis and treatment
(108). Next, the roles of miRNAs
in LUAD were investigated.
Over the past 20 years, studies have confirmed that
miRNAs can serve as biomarkers of malignant tumors, including LUAD
(109,110). Tong et al (111) found that miRNA-365 is
significantly downregulated in LUAD, and its expression is
associated with tumor invasion and migration as well as patient
survival. Meanwhile, miR-365 upregulates ETS1 expression and
inhibits EMT by inactivating the AKT/mTOR pathway (111). Kim et al (112) also reported that high
miRNA-130b expression is significantly associated with
unfavorable clinicopathological parameters and poor survival
outcomes in LUAD. Another study revealed a significant decrease in
miR-339-5p expression in LUAD tissues and plasma, whereas
miR-21 expression was significantly elevated. Receiver
operating curve analysis demonstrated that they could be
distinguished from normal control individuals through the AUC. This
result confirmed the role of miRNAs in the early screening of LUAD
(113). These miRNAs may serve as
targeted tools for the diagnosis and evaluation of LUAD prognosis.
Several studies have demonstrated that miRNAs are involved in the
biological processes of LUAD in addition to acting as biomarkers.
Subsequently, a series of specific miRNA functions were presented
to demonstrate their significant roles in LUAD.
Cell proliferation and apoptosis are common in
tumors. Together, they constitute the ‘minimum platform’ for the
further development of tumors (114). To date, research on miRNAs in the
field of tumor cell proliferation and apoptosis has been the most
extensive. MiR-144-5p is considered a tumor suppressor gene
in ovarian and lung cancers. It is involved in almost all stages of
tumor development (115,116). Luo et al (28) found a negative regulatory
relationship between miR-144-5p and CDCA3;
miR-144-5p inhibited cell proliferation and promoted
apoptosis through the interaction between CDCA3 and p53 signaling
pathways. This result indicated that the downregulation of
miR-144-5p had an antitumor effect by affecting the
activation of p53.
Correspondingly, various tumor promoters, such as
miR-516a-3p, have been reported to promote cancer cell
proliferation and inhibit apoptosis by regulating PTPRD
expression. Researchers also found a significant relationship
between the expression of miR-516a-3p and the clinical staging of
LUAD (61). Thus, these molecules
are potential targets for the diagnosis and treatment of LUAD.
EMT is a cellular process in which cells lose their
epithelial and interstitial properties. During tumor evolution, EMT
is closely related to tumor occurrence, metastasis and treatment
resistance (119). Long et
al (26) showed that
miR-214 is overexpressed in LUAD and promotes metastasis and
EMT by regulating Sufu. During this process, epithelial and
interstitial marker genes showed significant changes in opposite
directions. Simultaneously, knockdown of miR-214 was shown
to suppress EMT activity (26).
Drug resistance and reduced sensitivity to
radiotherapy can lead to treatment failure and tumor recurrence
(120,121). miRNAs are considered to induce the
corresponding mechanisms in LUAD to improve drug resistance or
radiation sensitivity. Cao et al (36) found that miR-192 was
significantly upregulated in A549 cells and that LUAD mice carrying
miR192 inhibitors were more sensitive to cisplatin and
gemcitabine treatment. Moreover, in the process of improving
chemotherapy resistance, Bcl-2 is upregulated as a key
regulatory factor following miR-192 knockdown (36). Thus, miR-192 may be a
potential target for LUAD chemotherapy. Another miRNA,
miRNA-134, has been shown to be associated with
multiple-drug resistance in LUAD. MiR-134 has been reported
to be significantly downregulated in cisplatin-resistant LUAD
cells. Further studies have shown that miR-134
overexpression enhances the sensitivity of LUAD cells to
vincristine and 5-fluorouracil (51). Yuan et al (40) confirmed that the upregulation of
miR-195-5p promotes the expression of Bax and reduces the
expression of cyclin D1 and Bcl-2 in A549 and PC9 cells exposed to
ionizing radiation. This result indicated that miR-195-5p
enhanced the radiosensitivity of LUAD cells by promoting apoptosis
(40). In summary, different miRNAs
participate in LUAD progression by influencing the downstream
target genes. They play an important role in the different
phenotypes of LUAD.
miRNAs play an undeniable role in LUAD, yet, the
specific molecular mechanism remains controversial. Generally,
these molecules regulate tumor development by targeting downstream
genes in multiple signaling pathways (122) (Table
II).
Akt, also known as protein kinase B, is a key medium
for GF-induced cell survival (164). Upregulation of Akt activity has
been observed in numerous cancers. The interaction between tumor
suppressors and tumor-promoting factors in the Akt pathway leads to
proliferation, differentiation and inhibition of tumor cell
apoptosis (165). The Akt pathway
mediates by transporting signals from upstream regulatory proteins
(such as PTEN and PI3K) to downstream effector proteins (MDM2 and
FOXO). Subsequently, these effectors intersect with numerous other
compensatory signaling pathways (166). Furthermore, miRNAs impact tumor
progression by interfering with the expression of related genes in
the Akt pathway (167). The roles
of miRNAs in LUAD progression via the Akt pathway were
summarized.
Signal transducer and activator of transcription
(STAT) proteins are a family of cytoplasmic transcription factors
that include STAT5a, STAT4, and STAT3 that regulate numerous
signaling pathways. STAT3 is associated with diverse biological
processes, including cell proliferation, apoptosis and
differentiation (168). Lv et
al (143) found that
miR-320a not only regulates STAT3 but also affects its
related signals, such as Bcl-2, Bax and Caspase8 to suppress the
proliferation and metastasis of LUAD in vivo and in
vitro. It is well known that certain cytokines, such as
interleukin-6 (IL-6), bind to corresponding receptors on the cell
membrane to activate the JAK2-STAT3 signaling pathway (169). MiR-204 and miR-425
were based on this mechanism to suppress the malignant biological
behavior of LUAD (141,151). In addition, Xu et al
(162) confirmed from another
perspective that miR-30e-5p targets the upregulation of
USP22 and mediates the Sirt1/JAK2/STAT3 pathway, which also
inhibits LUAD.
The Wnt pathway is a critical signaling cascade in
cancer. Abnormal Wnt signaling is observed in numerous cancers,
including LUAD. The Wnt signaling pathway mainly affects the
stability, migration and immune escape of cancer stem cells
(170). Additionally, signaling
pathways, such as the Wnt and Notch pathways, typically form a
network within cells to jointly regulate tumor progression
(171). MiR-1275 has been
reported to be significantly upregulated in LUAD. This trend
increased the expression of β-catenin in the Wnt pathway and NICD
in the Notch pathway. This miRNA also directly targets and inhibits
negative regulatory factors, such as GSK3, RUNX3 and NUMB, in two
signaling pathways. This enhances the stem cell phenotype of LUAD
cells (123).
The mammalian target of rapamycin (mTOR), a
serine/threonine kinase, combines hormones, cytokines, nutrients
and other factors to regulate biological behaviors including
proliferation, differentiation and metabolism of cancer cells
(172). It has two different
complex forms in cells, mTORC1 and mTORC2, and its C-terminus is
homologous to the catalytic domain of phosphatidylinositol kinase
(PI3K). mTOR itself does not possess esterase kinase activity but
rather has Ser/Thr protein kinase activity (173).
MiR-125 has been shown to inhibit LUAD. It also
reduced the p-AKT/AKT ratio, the p-mTOR/mTOR ratio and the
expression of RhoA by downregulating TNS1 (154). Additionally, miR-363-3p
inhibited the proliferation and metastasis of LUAD cells through
the mTOR/4EBP-1 and ERK signaling pathways (145). Evidently, the effect of miRNA on
cancer often occurs in a multi-pathway and multi-target manner.
LUAD treatment with cisplatin can lead to multiple
tolerances in malignant cells. This can cause the cancer cells to
lose their sensitivity to drugs, leading to treatment failure.
Cisplatin resistance is a major bottleneck in the treatment of LUAD
(174). However, some studies have
confirmed that miRNAs affect cisplatin resistance in LUAD through
the mTOR signaling pathway. Liu et al (138) reported that the overexpression of
miR-181 in A549/DDP cells (a LUAD drug-resistant cell line)
promoted autophagy and upregulated the expression of LC3 and AGT5
proteins through the PTEN/PI3K/AKT/mTOR signaling pathway.
Additionally, downregulation of miR-21 in A549/DDP cells
slowed the loss of glucose and the production of pyruvic acid and
lactic acid, which promoted the expression of apoptosis-related
proteins. This process inhibits glucose metabolism and promotes
cell death via the PI3K/AKT/mTOR/HIF-1a pathway (130).
The NF-kB is not a single gene but a family of
transcription factors involved in multiple biological processes
(175). This signaling pathway not
only participates in inflammation and immune response but also
plays an important role in the occurrence and development of tumors
(176).
The mitogen-activated protein kinase (MAPK)
signaling pathway plays an important role in proliferation,
differentiation and inflammation-related signaling pathways. It
contains four branches, of which the main substrates are
extracellular signal-related kinase (ERK) and Jun amino terminal
kinase (JNK) (177). Among these,
the MAPK/ERK signaling pathway has been associated with
tumor-related malignant phenotypes such as cell proliferation and
apoptosis (178).
Numerous signaling pathways are involved in LUAD
tumor regulation, with numerous miRNAs associated with these
pathways. Ma et al (126)
found that miR-6077 targeted GLUT1 (glucose transporter 1)
and inhibited glucose absorption and lactate production after its
upregulation. By mediating the glucose transport pathway,
miR-6077 increased the sensitivity of LUAD cells to alotinib
(126). Other miRNAs, such as
miR-106a-5p, were upregulated in LUAD, and it has been shown
to suppress the phosphorylation of AMPK and TSC2 proteins, while
upregulating the phosphorylation of mTOR. This change promotes the
proliferation and autophagy of tumor cells (133). Ghoshal-Gupta et al
(135) showed that
miR-125a-5p regulates apoptosis in LUAD cells by
upregulating the p53 protein and altering the expression of other
related apoptotic proteins, such as Bcl-2 and BAX. There are
several additional examples. MiR-140-3p enhanced the
sensitivity of LUAD cells to antitumor drugs by suppressing the
Notch signaling pathway, and miR-182-5p plays a similar role
through the Hedgehog pathway (136,139). Additionally, TGF β, Hippo, and YAP
signaling pathways participated in the regulation of LUAD (142,158,160).
Recently, competing endogenous RNAs (ceRNAs) have
garnered significant research interest as they represent a novel
regulatory mechanism between RNAs, rather than representing a
distinct type RNA (179). This
theory reveals the presence of miRNA response elements (MREs) not
only on mRNA but also on lncRNAs and circRNAs (180). Therefore, mRNA, lncRNAs and
circRNAs compete with miRNAs to form complex regulatory networks
that affect gene expression. Some lncRNAs and circRNAs interact
with miRNAs and subsequently affect LUAD progression (Tables III and IV).
Furthermore, multiple studies have confirmed that
circRNAs regulate gene expression by suppressing miRNA activity
(206). Circle_ 0006427 was
significantly localized in the cytoplasm and was positively
regulated by DKK1 through competitive sponging of
miR-6783-3p in LUAD cells (195). Huang et al (199) reported that the overexpression of
circ_000881 slowed the malignant phenotypes of LUAD cells.
Furthermore, circRNA_000881 acts as a sponge for
miR-665 and indirectly regulates the downstream target gene
PRICKLE2 (199). Similarly,
circ_0129047 and circ-MTO1 play similar roles as
tumor suppressors in LUAD (201,202). Numerous circRNAs act as cancer
promoters in LUAD. For example, circ-CAMK2A was not only
significantly upregulated in LUAD but was also positively
correlated with an unfavorable prognosis. It upregulates the
expression of fibronectin 1 by competitively binding to
miR-615-5p, thereby enhancing the expression of MMP9
and MMP2 and promoting LUAD progression (196). In summary, the circRNA-miRNA-mRNA
axis plays a crucial role in LUAD (197,198,200,203–205).
However, these experiments also have certain
limitations. Firstly, in the article, the approach to revealing the
mechanism is relatively singular. It is nothing more than
verification at the tissue, cell and animal levels, and further
verification through functional gene experiments and phenotype
rescue experiments is required. Secondly, during experimental
verification, the number of cell line types and tissue samples is
relatively small. Thirdly, the current research on miRNAs remians
in the basic experimental stage, and how to transition to clinical
practice is an urgent issue that needs to be solved.
At present, although numerous miRNAs have been
proven to have promoting or inhibiting effects on LUAD, the
manipulation of miRNAs has not been translated into practical
clinical treatment strategies. The reasons for this are
multifaceted. Firstly, numerous miRNAs regulate tumor progression
through different target genes and signaling pathways. Therefore,
interfering with a single miRNA cannot fundamentally treat LUAD.
Correspondingly, a method or drug that can alter the regulatory
network targeting miRNAs should be developped. Secondly, the
reagents required for overexpression or low expression of miRNAs in
basic experiments are cytotoxic. In actual clinical treatment, this
is clearly unacceptable. Thirdly, even if drugs that can interfere
with miRNAs while being non-toxic are obtained, how to efficiently
and safely enter the human organism remains a challenging
issue.
Emerging evidence suggests that miRNAs are involved
in the regulation of LUAD by degrading or silencing downstream
target genes at the post-transcriptional level. miRNAs have been
shown to regulate multiple malignant biological phenotypes of LUAD
through multiple signaling pathways. The present review
systematically summarized the roles of abnormally expressed miRNAs
in LUAD and their related signaling pathways.
Research findings suggest that miRNAs hold promise
as potential biomarkers of LUAD, and the signaling pathways that
they influence could offer innovative targets for LUAD treatment.
The interactions between ceRNAs and miRNAs present a novel
mechanism for LUAD development. The lncRNA or circRNA/miRNA/mRNA
axis has emerged as a major focus in cancer research. Continued
investigation is likely to unveil additional miRNA-mediated
signaling pathways and therapeutic targets for LUAD, enhancing
diagnosis and treatment approaches for this disease.
However, basic research is not equivalent to
clinical application. There are still numerous urgent problems to
be solved in the treatment of LUAD using miRNAs. For example, there
is a lack of effective means for overall intervention in
miRNAs-regulatory networks. Meanwhile, drugs that interfere with
miRNAs need to be proven to be effective and safe. These practical
problems not only pose challenges, but also point in the direction
of progress.
Not applicable.
The present study was supported by the 345 Talent Project of the
Shengjing Hospital.
Not applicable.
JL and FZ wrote the manuscript. YW and JW reviewed
the article. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Seguin L, Durandy M and Feral CC: Lung
adenocarcinoma tumor origin: A guide for personalized medicine.
Cancers (Basel). 14:17592022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson MR, Gazdar AF and Clarke BE: The
pivotal role of pathology in the management of lung cancer. J
Thorac Dis. 5 (Suppl 5):S463–S478. 2013.PubMed/NCBI
|
|
4
|
Zito Marino F, Bianco R, Accardo M, Ronchi
A, Cozzolino I, Morgillo F, Rossi G and Franco R: Molecular
heterogeneity in lung cancer: From mechanisms of origin to clinical
implications. Int J Med Sci. 16:981–989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langer CJ, Besse B, Gualberto A, Brambilla
E and Soria JC: The evolving role of histology in the management of
advanced non-small-cell lung cancer. J Clin Oncol. 28:5311–5320.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iqbal MA, Arora S, Prakasam G, Calin GA
and Syed MA: MicroRNA in lung cancer: Role, mechanisms, pathways
and therapeutic relevance. Mol Aspects Med. 70:3–20. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishola AA, La'ah AS, Le HD, Nguyen VQ,
Yang YP, Chou SJ, Tai HY, Chien CS and Wang ML: Non-coding RNA and
lung cancer progression. J Chin Med. 83:8–14. 2020.
|
|
8
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landi MT, Zhao Y, Rotunno M, Koshiol J,
Liu H, Bergen AW, Rubagotti M, Goldstein AM, Linnoila I, Marincola
FM, et al: MicroRNA expression differentiates histology and
predicts survival of lung cancer. Clin Cancer Res. 16:430–441.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y,
Liu Z, Xu Q, Liu S, Xiao D and Tao Y: Role of non-coding RNAs and
RNA modifiers in cancer therapy resistance. Mol Cancer. 19:472020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanna J, Hossain GS and Kocerha J: The
potential for microRNA therapeutics and clinical research. Front
Genet. 10:4782019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Fang J, Wang Y, Wang H and Sun CC:
MiRNA-based therapeutic strategy in lung cancer. Curr Pharm Des.
23:6011–6018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agrawal N, Dasaradhi PVN, Mohmmed A,
Malhotra P, Bhatnagar RK and Mukherjee SK: RNA interference:
Biology, mechanism, and applications. Microbiol Mol Biol Rev.
67:657–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michlewski G and Cáceres JF:
Post-transcriptional control of miRNA biogenesis. RNA. 25:1–16.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo WT and Wang Y: Dgcr8 knockout
approaches to understand microRNA functions in vitro and in vivo.
Cell Mol Life Sci. 76:1697–1711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heyam A, Lagos D and Plevin M: Dissecting
the roles of TRBP and PACT in double-stranded RNA recognition and
processing of noncoding RNAs. Wiley Interdiscip Rev RNA. 6:271–289.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linder P and Jankowsky E: From unwinding
to clamping-the DEAD box RNA helicase family. Nat Rev Mol Cell
Biol. 12:505–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams AE: Functional aspects of animal
microRNAs. Cell Mol Life Sci. 65:545–562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du T and Zamore PD: microPrimer: The
biogenesis and function of microRNA. Development. 132:4645–4652.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and function. Thromb Haemost.
107:605–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren H and Wang Q: Non-coding RNA and
diabetic kidney disease. DNA Cell Biol. 40:553–567. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gizak A, Duda P, Pielka E, McCubrey JA and
Rakus D: GSK3 and miRNA in neural tissue: From brain development to
neurodegenerative diseases. Biochim Biophys Acta Mol Cell Res.
1867:1186962020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Long H, Wang Z, Chen J, Xiang T, Li Q,
Diao X and Zhu B: microRNA-214 promotes epithelial-mesenchymal
transition and metastasis in lung adenocarcinoma by targeting the
suppressor-of-fused protein (Sufu). Oncotarget. 6:38705–38718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han L, Huang Z, Liu Y, Ye L, Li D, Yao Z,
Wang C, Zhang Y, Yang H, Tan Z, et al: MicroRNA-106a regulates
autophagy-related cell death and EMT by targeting TP53INP1 in lung
cancer with bone metastasis. Cell Death Dis. 12:10372021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo J, Xia L, Zhang L, Zhao K and Li C:
MiRNA-144-5p down-modulates CDCA3 to regulate proliferation and
apoptosis of lung adenocarcinoma cells. Mutat Res. 825:1117982022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mo D, Yang D, Xiao X, Sun R, Huang L and
Xu J: MiRNA-145 suppresses lung adenocarcinoma cell invasion and
migration by targeting N-cadherin. Biotechnol Lett. 39:701–710.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin R, Zhang S, Wu Y, Fan X, Jiang F,
Zhang Z, Feng D, Guo X and Xu L: microRNA-145 suppresses lung
adenocarcinoma-initiating cell proliferation by targeting OCT4.
Oncol Rep. 25:1747–1754. 2011.PubMed/NCBI
|
|
31
|
Zhang JX, Yang W, Wu JZ, Zhou C, Liu S,
Shi HB and Zhou WZ: MicroRNA-32-5p inhibits epithelial-mesenchymal
transition and metastasis in lung adenocarcinoma by targeting SMAD
family 3. J Cancer. 12:2258–2267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Si L and Tian H: MicroRNA-148a
inhibits cell proliferation and cell cycle progression in lung
adenocarcinoma via directly targeting transcription factor E2F3.
Exp Ther Med. 16:5400–5409. 2018.PubMed/NCBI
|
|
33
|
Lu Y, Zheng W, Rao X, Du Y and Xue J:
MicroRNA-9-5p facilitates lung adenocarcinoma cell malignant
progression via targeting STARD13. Biochem Genet. 60:1865–1880.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu K, Lin J, Chen S and Xu Q: miR-9-5p
promotes lung adenocarcinoma cell proliferation, migration and
invasion by targeting ID4. Technol Cancer Res Treat.
20:153303382110485922021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han HS, Son SM, Yun J, Jo YN and Lee OJ:
MicroRNA-29a suppresses the growth, migration, and invasion of lung
adenocarcinoma cells by targeting carcinoembryonic antigen-related
cell adhesion molecule 6. FEBS Lett. 588:3744–3750. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao J, He Y, Liu HQ, Wang SB, Zhao BC and
Cheng YS: MicroRNA 192 regulates chemo-resistance of lung
adenocarcinoma for gemcitabine and cisplatin combined therapy by
targeting Bcl-2. Int J Clin Exp Med. 8:12397–12403. 2015.PubMed/NCBI
|
|
37
|
Ma Z, Chen G, Chen Y, Guo Z, Chai H, Tang
Y, Zheng L, Wei K, Pan C, Ma Z, et al: MiR-937-3p promotes
metastasis and angiogenesis and is activated by MYC in lung
adenocarcinoma. Cancer Cell Int. 22:312022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duan L, Wang J, Zhang D, Yuan Y, Tang L,
Zhou Y and Jiang X: Immune-related miRNA-195-5p inhibits the
progression of lung adenocarcinoma by targeting polypyrimidine
tract-binding protein 1. Front Oncol. 12:8625642022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bu L, Tian Y, Wen H, Jia W and Yang S:
miR-195-5p exerts tumor-suppressive functions in human lung cancer
cells through targeting TrxR2. Acta Biochim Biophys Sin (Shanghai).
53:189–200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan C, Bai R, Gao Y, Jiang X, Li S, Sun
W, Li Y, Huang Z, Gong Y and Xie C: Effects of MicroRNA-195-5p on
biological behaviors and radiosensitivity of lung adenocarcinoma
cells via targeting HOXA10. Oxid Med Cell Longev. 2021:45222102021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao X, Xue F, Chen H, Shen L, Yuan X, Yu
Y, Zong Y, Zhong L and Huang F: MiR-202-3p inhibits the
proliferation and metastasis of lung adenocarcinoma cells by
targeting RRM2. Ann Transl Med. 10:13742022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhuang L, Shou T, Li K, Gao CL, Duan LC,
Fang LZ, Zhang QY, Chen ZN, Zhang C, Yang ST and Li GF:
MicroRNA-30e-5p promotes cell growth by targeting PTPN13 and
indicates poor survival and recurrence in lung adenocarcinoma. J
Cell Mol Med. 21:2852–2862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao S, Gao X, Zang S, Li Y, Feng X and
Yuan X: MicroRNA-383-5p acts as a prognostic marker and inhibitor
of cell proliferation in lung adenocarcinoma by cancerous inhibitor
of protein phosphatase 2A. Oncol Lett. 14:3573–3579. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sato T, Shiba-Ishii A, Kim Y, Dai T, Husni
RE, Hong J, Kano J, Sakashita S, Iijima T and Noguchi M: miR-3941:
A novel microRNA that controls IGBP1 expression and is associated
with malignant progression of lung adenocarcinoma. Cancer Sci.
108:536–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pu X, Jiang H, Li W, Xu L, Wang L and Shu
Y: Upregulation of the coatomer protein complex subunit beta 2
(COPB2) gene targets microRNA-335-3p in NCI-H1975 lung
adenocarcinoma cells to promote cell proliferation and migration.
Med Sci Monit. 26:e9183822020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu WB, Wang L, Huang XR and Li F:
MicroRNA-204 targets SOX4 to inhibit metastasis of lung
adenocarcinoma. Eur Rev Med Pharmacol Sci. 23:1553–1562.
2019.PubMed/NCBI
|
|
47
|
Zhou Y, Zhao M, Du Y, Liu Y, Zhao G, Ye L,
Li Q, Li H, Wang X, Liu X, et al: MicroRNA-195 suppresses the
progression of lung adenocarcinoma by directly targeting apelin.
Thorac Cancer. 10:1419–1430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu X, Wu G, Zhang H, Peng X, Huang B,
Huang M, Ding J, Mao C and Peng C: MiR-196b promotes the invasion
and migration of lung adenocarcinoma cells by targeting AQP4.
Technol Cancer Res Treat. 20:15330338209858682021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan L, Tang Z, Pan L and Tang R:
MicroRNA-3666 inhibits lung cancer cell proliferation, migration,
and invasiveness by targeting BPTF. Biochem Cell Biol. 97:415–422.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mou X and Liu S: MiR-485 inhibits
metastasis and EMT of lung adenocarcinoma by targeting Flot2.
Biochem Biophys Res Commun. 477:521–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Chen Y, Jin M, Wang J, Li S, Chen Z
and Yu W: MicroRNA-134 reverses multidrug resistance in human lung
adenocarcinoma cells by targeting FOXM1. Oncol Lett. 13:1451–1455.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gao Y, Xue Q, Wang D, Du M, Zhang Y and
Gao S: miR-873 induces lung adenocarcinoma cell proliferation and
migration by targeting SRCIN1. Am J Transl Res. 7:2519–2526.
2015.PubMed/NCBI
|
|
53
|
Liu L, Bi N, Wu L, Ding X, Men Y, Zhou W,
Li L, Zhang W, Shi S, Song Y and Wang L: MicroRNA-29c functions as
a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol
Cancer. 16:502017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chai Y, Xue H, Wu Y, Du X, Zhang Z, Zhang
Y, Zhang L, Zhang S, Zhang Z and Xue Z: MicroRNA-216b-3p inhibits
lung adenocarcinoma cell growth via regulating PDZ binding
kinase/T-LAK-cell-originated protein kinase. Exp Ther Med.
15:4822–4828. 2018.PubMed/NCBI
|
|
55
|
Zhu D, Gu L, Li Z, Jin W, Lu Q and Ren T:
MiR-138-5p suppresses lung adenocarcinoma cell
epithelial-mesenchymal transition, proliferation and metastasis by
targeting ZEB2. Pathol Res Pract. 215:861–872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Wang F and Xu P: miR-590
accelerates lung adenocarcinoma migration and invasion through
directly suppressing functional target OLFM4. Biomed Pharmacother.
86:466–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang M, Wang Y, Zang W, Wang H, Chu H, Li
P, Li M, Zhang G and Zhao G: Downregulation of microRNA-182
inhibits cell growth and invasion by targeting programmed cell
death 4 in human lung adenocarcinoma cells. Tumour Biol. 35:39–46.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Greenawalt EJ, Edmonds MD, Jain N, Adams
CM, Mitra R and Eischen CM: Targeting of SGK1 by miR-576-3p
inhibits lung adenocarcinoma migration and invasion. Mol Cancer
Res. 17:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li X, Fu Q, Li H, Zhu L, Chen W, Ruan T,
Xu W and Yu X: MicroRNA-520c-3p functions as a novel tumor
suppressor in lung adenocarcinoma. FEBS J. 286:2737–2752. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fan G, Xu P and Tu P: MiR-1827 functions
as a tumor suppressor in lung adenocarcinoma by targeting MYC and
FAM83F. J Cell Biochem. 121:1675–1689. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu A, Yang X, Zhang B, Wang S and Li G:
miR-516a-3p promotes proliferation, migration, and invasion and
inhibits apoptosis in lung adenocarcinoma by targeting PTPRD. Int J
Clin Exp Pathol. 12:4222–4231. 2019.PubMed/NCBI
|
|
62
|
Qin E, Gu S, Guo Y, Wang L and Pu G:
MiRNA-30a-5p/VCAN arrests tumor metastasis via modulating the
adhesion of lung adenocarcinoma cells. Appl Biochem Biotechnol. Apr
10–2023.(Epub ahead of print). View Article : Google Scholar
|
|
63
|
Tao K, Liu J, Liang J, Xu X, Xu L and Mao
W: Vascular endothelial cell-derived exosomal miR-30a-5p inhibits
lung adenocarcinoma malignant progression by targeting CCNE2.
Carcinogenesis. 42:1056–1067. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang G, Wu YJ and Yan F: MicroRNA-130-5p
promotes invasion as well as migration of lung adenocarcinoma cells
by targeting the EZH2 signaling pathway. Eur Rev Med Pharmacol Sci.
23:9480–9488. 2019.PubMed/NCBI
|
|
65
|
Dai B, Kong DL, Tian J, Liu TW, Zhou H and
Wang ZF: microRNA-1205 promotes cell growth by targeting APC2 in
lung adenocarcinoma. Eur Rev Med Pharmacol Sci. 23:1125–1133.
2019.PubMed/NCBI
|
|
66
|
Bai J, Hu Y, Chen X, Chen L, Zhang L, Yin
C and Li H: miR-144-3p inhibits the invasion and metastasis of lung
adenocarcinoma cells by targeting IRS1. Zhongguo Fei Ai Za Zhi.
24:323–330. 2021.(In Chinese). PubMed/NCBI
|
|
67
|
Liu K, Zhang W, Tan J, Ma J and Zhao J:
MiR-200b-3p functions as an oncogene by targeting ABCA1 in lung
adenocarcinoma. Technol Cancer Res Treat. 18:15330338198925902019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo ZZ, Ma ZJ, He YZ, Jiang W, Xia Y, Pan
CF, Wei K, Shi YJ, Chen L and Chen YJ: miR-550a-5p functions as a
tumor promoter by targeting LIMD1 in lung adenocarcinoma. Front
Oncol. 10:5707332020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun Y, Zhao J, Yin X, Yuan X, Guo J and Bi
J: miR-297 acts as an oncogene by targeting GPC5 in lung
adenocarcinoma. Cell Prolif. 49:636–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen Y and Yang C: miR-197-3p-induced
downregulation of lysine 63 deubiquitinase promotes cell
proliferation and inhibits cell apoptosis in lung adenocarcinoma
cell lines. Mol Med Rep. 17:3921–3927. 2018.PubMed/NCBI
|
|
71
|
Fang H, Liu Y, He Y, Jiang Y, Wei Y, Liu
H, Gong Y and An G: Extracellular vesicle-delivered miR-505-5p, as
a diagnostic biomarker of early lung adenocarcinoma, inhibits cell
apoptosis by targeting TP53AIP1. Int J Oncol. 54:1821–1832.
2019.PubMed/NCBI
|
|
72
|
Qian T, Shi S, Xie L and Zhu Y: miR-938
promotes cell proliferation by regulating RBM5 in lung
adenocarcinoma cells. Cell Biol Int. 44:295–305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cao J, Geng J, Chu X, Wang R, Huang G and
Chen L: miRNA-885-3p inhibits docetaxel chemoresistance in lung
adenocarcinoma by downregulating Aurora A. Oncol Rep. 41:1218–1230.
2019.PubMed/NCBI
|
|
74
|
Bao B, Yu X and Zheng W: MiR-139-5p
targeting CCNB1 modulates proliferation, migration, invasion and
cell cycle in lung adenocarcinoma. Mol Biotechnol. 64:852–860.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li J, He X, Wu X, Liu X, Huang Y and Gong
Y: miR-139-5p inhibits lung adenocarcinoma cell proliferation,
migration, and invasion by targeting MAD2L1. Comput Math Methods
Med. 2020:29535982020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Z, Zhou L, Chen B, Li X, Zou Q, Xu W,
Fang L, Wu A, Li Z and Chen Y: microRNA-660 enhances cisplatin
sensitivity via decreasing SATB2 expression in lung adenocarcinoma.
Genes. 14:9112023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Feng YY, Liu CH, Xue Y, Chen YY, Wang YL
and Wu XZ: MicroRNA-147b promotes lung adenocarcinoma cell
aggressiveness through negatively regulating microfibril-associated
glycoprotein 4 (MFAP4) and affects prognosis of lung adenocarcinoma
patients. Gene. 730:1443162020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wan S, Liu Z, Chen Y, Mai Z, Jiang M, Di Q
and Sun B: MicroRNA-140-3p represses the proliferation, migration,
invasion and angiogenesis of lung adenocarcinoma cells via
targeting TYMS (thymidylate synthetase). Bioengineered.
12:11959–11977. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang H, Kanmangne D, Li R, Qian Z, Xia X,
Wang X and Wang T: miR-30a-3p suppresses the proliferation and
migration of lung adenocarcinoma cells by downregulating CNPY2.
Oncol Rep. 43:646–654. 2020.PubMed/NCBI
|
|
80
|
Chen L, Chen X, Liu L, Zhao Y, Zuo W, Yin
C and Li H: miR-30b-3p inhibits the proliferation and invasion of
lung adenocarcinoma by targeting COX6B1. Zhongguo Fei Ai Za Zhi.
25:567–574. 2022.(In Chinese). PubMed/NCBI
|
|
81
|
Tu Y and Mei F: miR-3648 promotes lung
adenocarcinoma-genesis by inhibiting SOCS2 (suppressor of cytokine
signaling 2). Bioengineered. 13:3044–3056. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu Z, Cui Y, Wang S, Wu C, Mei F, Han E,
Hu Z and Zhou B: MiR-96-5p is an oncogene in lung adenocarcinoma
and facilitates tumor progression through ARHGAP6 downregulation. J
Appl Genet. 62:631–638. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou F, Qian C, Chen T and Zang X:
MiR-96-5p facilitates lung adenocarcinoma cell phenotypes by
inhibiting FHL1. Comput Math Methods Med. 2022:78912222022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen G, Wang Q and Wang K: MicroRNA-218-5p
affects lung adenocarcinoma progression through targeting
endoplasmic reticulum oxidoreductase 1 alpha. Bioengineered.
13:10061–10070. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Miao H, Zeng Q, Xu S and Chen Z:
miR-1-3p/CELSR3 participates in regulating malignant phenotypes of
lung adenocarcinoma cells. Curr Gene Ther. 21:304–312. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li T, Wang X, Jing L and Li Y: MiR-1-3p
inhibits lung adenocarcinoma cell tumorigenesis via targeting
protein regulator of cytokinesis 1. Front Oncol. 9:1202019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
An JC, Shi HB, Hao WB, Zhu K and Ma B:
miR-944 inhibits lung adenocarcinoma tumorigenesis by targeting
STAT1 interaction. Oncol Lett. 17:3790–3798. 2019.PubMed/NCBI
|
|
88
|
Feng H, Zhang Z, Qing X, French SW and Liu
D: miR-186-5p promotes cell growth, migration and invasion of lung
adenocarcinoma by targeting PTEN. Exp Mol Pathol. 108:105–113.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu Q and Xu Z: miR-196b-5p promotes
proliferation, migration and invasion of lung adenocarcinoma cells
via targeting RSPO2. Cancer Manag Res. 12:13393–13402. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lin R, Li GS, Gan XY, Peng JX, Feng Y,
Wang LT, Zhang CY, Huang KY, Huang SH, Yang L, et al: The clinical
significance and mechanism of microRNA-22-3p targeting TP53 in lung
adenocarcinoma. Technol Health Care. 31:1691–1707. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Goto A, Tanaka M, Yoshida M, Umakoshi M,
Nanjo H, Shiraishi K, Saito M, Kohno T, Kuriyama S, Konno H, et al:
The low expression of miR-451 predicts a worse prognosis in
non-small cell lung cancer cases. PLoS One. 12:e01812702017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang G, Zhou Y, Chen W, Yang Y, Ye J, Ou H
and Wu H: miR-21-5p promotes lung adenocarcinoma cell
proliferation, migration and invasion via targeting WWC2. Cancer
Biomark. 28:549–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wei D: MiR-486-5p specifically suppresses
SAPCD2 expression, which attenuates the aggressive phenotypes of
lung adenocarcinoma cells. Histol Histopathol. 37:909–917.
2022.PubMed/NCBI
|
|
94
|
Yang W, Bai J, Liu D, Wang S, Zhao N, Che
R and Zhang H: MiR-93-5p up-regulation is involved in non-small
cell lung cancer cells proliferation and migration and poor
prognosis. Gene. 647:13–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shao L, He Q, Wang J, He F, Lin S, Wu L,
Gao Y, Ma W, Dong J, Yang X and Li F: MicroRNA-326 attenuates
immune escape and prevents metastasis in lung adenocarcinoma by
targeting PD-L1 and B7-H3. Cell Death Discov. 7:1452021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhao J, Yu H, Han T and Zhu X: Prognosis
value of microRNA-3677-3p in lung adenocarcinoma and its regulatory
effect on tumor progression. Cancer Manag Res. 13:9261–9270. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ling DJ, Chen ZS, Zhang YD, Liao QD, Feng
JX, Zhang XY and Shi TS: MicroRNA-145 inhibits lung cancer cell
metastasis. Mol Med Rep. 11:3108–3114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang HB, Shen B, Ma ZC, Xu YY, Lou YL and
Chen M: MiR-593-5p inhibited proliferation and migration of lung
adenocarcinoma by targeting ICAM-1. Eur Rev Med Pharmacol Sci.
24:4298–4305. 2020.PubMed/NCBI
|
|
99
|
Huang JY, Cui SY, Chen YT, Song HZ, Huang
GC, Feng B, Sun M, De W, Wang R and Chen LB: MicroRNA-650 was a
prognostic factor in human lung adenocarcinoma and confers the
docetaxel chemoresistance of lung adenocarcinoma cells via
regulating Bcl-2/Bax expression. PLoS One. 8:e726152013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhuo E, Cai C, Liu W, Li K and Zhao W:
Downregulated microRNA-140-5p expression regulates apoptosis,
migration and invasion of lung cancer cells by targeting zinc
finger protein 800. Oncol Lett. 20:3902020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang X, Xiao H, Wu D, Zhang D and Zhang Z:
miR-335-5p regulates cell cycle and metastasis in lung
adenocarcinoma by targeting CCNB2. Onco Targets Ther. 13:6255–6263.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen L, Heikkinen L, Wang C, Yang Y, Sun H
and Wong G: Trends in the development of miRNA bioinformatics
tools. Brief Bioinform. 20:1836–1852. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Petkova V, Marinova D, Kyurkchiyan S,
Stancheva G, Mekov E, Kachakova-Yordanova D, Slavova Y, Kostadinov
D, Mitev V and Kaneva R: MiRNA expression profiling in
adenocarcinoma and squamous cell lung carcinoma reveals both common
and specific deregulated microRNAs. Medicine (Baltimore).
101:e300272022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jin X, Chen Y, Chen H, Fei S, Chen D, Cai
X, Liu L, Lin B, Su H, Zhao L, et al: Evaluation of tumor-derived
exosomal miRNA as potential diagnostic biomarkers for early-stage
non-small cell lung cancer using next-generation sequencing. Clin
Cancer Res. 23:5311–5319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Seijo LM, Peled N, Ajona D, Boeri M, Field
JK, Sozzi G, Pio R, Zulueta JJ, Spira A, Massion PP, et al:
Biomarkers in lung cancer screening: Achievements, promises, and
challenges. J Thorac Oncol. 14:343–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kim Y, Sim J, Kim H, Bang SS, Jee S, Park
S and Jang K: MicroRNA-374a expression as a prognostic biomarker in
lung adenocarcinoma. J Pathol Transl Med. 53:354–360. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tong L, Han WZ, Wang JL, Sun NN and Zhuang
M: MicroRNA-365 inhibits the progression of lung adenocarcinoma
through targeting ETS1 and inactivating AKT/mTOR pathway. Eur Rev
Med Pharmacol Sci. 24:4836–4845. 2020.PubMed/NCBI
|
|
112
|
Kim Y, Kim H, Bang S, Jee S and Jang K:
MicroRNA-130b functions as an oncogene and is a predictive marker
of poor prognosis in lung adenocarcinoma. Lab Invest. 101:155–164.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun Y, Mei H, Xu C, Tang H and Wei W:
Circulating microRNA-339-5p and −21 in plasma as an early detection
predictors of lung adenocarcinoma. Pathol Res Pract. 214:119–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li X and Wu X: MiR-21-5p promotes the
progression of non-small-cell lung cancer by regulating the
expression of SMAD7. Onco Targets Ther. 11:8445–8454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen J, Li X, Yang L and Zhang J: Long
non-coding RNA LINC01969 promotes ovarian cancer by regulating the
miR-144-5p/LARP1 axis as a competing endogenous RNA. Front Cell Dev
Biol. 8:6257302021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: A
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Verma V and Lautenschlaeger T: MicroRNAs
in non-small cell lung cancer invasion and metastasis: From the
perspective of the radiation oncologist. Expert Rev Anticancer
Ther. 16:767–774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21:32332020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Jiang N, Zou C, Zhu Y, Luo Y, Chen L, Lei
Y, Tang K, Sun Y, Zhang W, Li S, et al: HIF-1α-regulated miR-1275
maintains stem cell-like phenotypes and promotes the progression of
LUAD by simultaneously activating Wnt/β-catenin and Notch
signaling. Theranostics. 10:2553–2570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Du X, Wang S, Liu X, He T, Lin X, Wu S,
Wang D, Li J, Huang W and Yang H: MiR-1307-5p targeting TRAF3
upregulates the MAPK/NF-κB pathway and promotes lung adenocarcinoma
proliferation. Cancer Cell Int. 20:5022020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
He T, Shen H, Wang S, Wang Y, He Z, Zhu L,
Du X, Wang D, Li J, Zhong S, et al: MicroRNA-3613-5p promotes lung
adenocarcinoma cell proliferation through a RELA and AKT/MAPK
positive feedback loop. Mol Ther Nucleic Acids. 22:572–583. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ma DB, Qin MM, Shi L and Ding XM:
MicroRNA-6077 enhances the sensitivity of patients-derived lung
adenocarcinoma cells to anlotinib by repressing the activation of
glucose transporter 1 pathway. Cell Signal. 64:1093912019.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Song M and Xing X: miR-6742-5p regulates
the invasion and migration of lung adenocarcinoma cells via
mediating FGF8/ERK12/MMP9/MMP2 signaling pathway. Aging (Albany
NY). 15:53–69. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fang H, Wu W and Wu Z: miR-382-3p
downregulation contributes to the carcinogenesis of lung
adenocarcinoma by promoting AKT SUMOylation and phosphorylation.
Exp Ther Med. 24:4402022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin Q: MicroRNA-1-3p affects lung
adenocarcinoma progression through E2F8 and regulating NF-кB
pathway. Cytokine. 156:1559222022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sun Y, Liu W, Zhao Q, Zhang R, Wang J, Pan
P, Shang H, Liu C and Wang C: Down-regulating the expression of
miRNA-21 inhibits the glucose metabolism of A549/DDP cells and
promotes cell death through the PI3K/AKT/mTOR/HIF-1α pathway. Front
Oncol. 11:6535962021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Edmonds MD, Boyd KL, Moyo T, Mitra R,
Duszynski R, Arrate MP, Chen X, Zhao Z, Blackwell TS, Andl T and
Eischen CM: MicroRNA-31 initiates lung tumorigenesis and promotes
mutant KRAS-driven lung cancer. J Clin Invest. 126:349–364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Qu J, Li M, An J, Zhao B, Zhong W, Gu Q,
Cao L, Yang H and Hu C: MicroRNA-33b inhibits lung adenocarcinoma
cell growth, invasion, and epithelial-mesenchymal transition by
suppressing Wnt/β-catenin/ZEB1 signaling. Int J Oncol.
47:2141–2152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhou Y, Zhang Y, Li Y, Liu L, Li Z, Liu Y
and Xiao Y: MicroRNA-106a-5p promotes the proliferation, autophagy
and migration of lung adenocarcinoma cells by targeting LKB1/AMPK.
Exp Ther Med. 22:14222021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fan X, Liang Y, Liu Y, Bai Y, Yang C and
Xu S: The upregulation of TMPRSS4, partly ascribed to the
downregulation of miR-125a-5p, promotes the growth of human lung
adenocarcinoma via the NF-κB signaling pathway. Int J Oncol.
53:148–158. 2018.PubMed/NCBI
|
|
135
|
Ghoshal-Gupta S, Kutiyanawalla A, Lee BR,
Ojha J, Nurani A, Mondal AK, Kolhe R, Rojiani AM and Rojiani MV:
TIMP-1 downregulation modulates miR-125a-5p expression and triggers
the apoptotic pathway. Oncotarget. 9:8941–8956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Meng H, Li B, Xu W, Ding R, Xu S, Wu Q and
Zhang Y: miR-140-3p enhances the sensitivity of LUAD cells to
antitumor agents by targeting the ADAM10/Notch pathway. J Cancer.
13:3660–3673. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jiang WS, Huang CL, Zhang J, Xu F and Dai
XH: MicroRNA-149 inhibits the progression of lung adenocarcinoma
through targeting RAP1B and inactivating Wnt/β-catenin pathway. Eur
Rev Med Pharmacol Sci. 24:4846–4854. 2020.PubMed/NCBI
|
|
138
|
Liu J, Xing Y and Rong L: miR-181
regulates cisplatin-resistant non-small cell lung cancer via
downregulation of autophagy through the PTEN/PI3K/AKT pathway.
Oncol Rep. 39:1631–1639. 2018.PubMed/NCBI
|
|
139
|
Seidl C, Panzitt K, Bertsch A, Brcic L,
Schein S, Mack M, Leithner K, Prinz F, Olschewski H, Kornmueller K
and Hrzenjak A: MicroRNA-182-5p regulates hedgehog signaling
pathway and chemosensitivity of cisplatin-resistant lung
adenocarcinoma cells via targeting GLI2. Cancer Lett. 469:266–276.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Guo L, Wang J, Yang P, Lu Q, Zhang T and
Yang Y: MicroRNA-200 promotes lung cancer cell growth through
FOG2-independent AKT activation. IUBMB Life. 67:720–725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liu X, Gao X, Zhang W, Zhu T, Bi W and
Zhang Y: MicroRNA-204 deregulation in lung adenocarcinoma controls
the biological behaviors of endothelial cells potentially by
modulating Janus kinase 2-signal transducer and activator of
transcription 3 pathway. IUBMB Life. 70:81–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Watt K, Newsted D, Voorand E, Gooding RJ,
Majewski A, Truesdell P, Yao B, Tuschl T, Renwick N and Craig AW:
MicroRNA-206 suppresses TGF-β signalling to limit tumor growth and
metastasis in lung adenocarcinoma. Cell Signal. 50:25–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lv Q, Hu JX, Li YJ, Xie N, Song DD, Zhao
W, Yan YF, Li BS, Wang PY and Xie SY: MiR-320a effectively
suppresses lung adenocarcinoma cell proliferation and metastasis by
regulating STAT3 signals. Cancer Biol Ther. 18:142–151. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhou QY, Gui SY, Zhang P and Wang M:
Upregulation of miR-345-5p suppresses cell growth of lung
adenocarcinoma by regulating ras homolog family member A (RhoA) and
Rho/Rho associated protein kinase (Rho/ROCK) pathway. Chin Med J
(Engl). 134:2619–2628. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang Y, Chen T, Huang H, Jiang Y, Yang L,
Lin Z, He H, Liu T, Wu B, Chen J, et al: miR-363-3p inhibits tumor
growth by targeting PCNA in lung adenocarcinoma. Oncotarget.
8:20133–20144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wang Y, Zhang S, Bao H, Mu S, Zhang B, Ma
H and Ma S: MicroRNA-365 promotes lung carcinogenesis by
downregulating the USP33/SLIT2/ROBO1 signalling pathway. Cancer
Cell Int. 18:642018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xuan YW, Liao M, Zhai WL, Peng LJ and Tang
Y: MicroRNA-381 inhibits lung adenocarcinoma cell biological
progression by directly targeting LMO3 through regulation of the
PI3K/Akt signaling pathway and epithelial-to-mesenchymal
transition. Eur Rev Med Pharmacol Sci. 23:8411–8421.
2019.PubMed/NCBI
|
|
148
|
He B, Wu C, Sun W, Qiu Y, Li J, Liu Z,
Jing T, Wang H and Liao Y: miR-383 increases the cisplatin
sensitivity of lung adenocarcinoma cells through inhibition of the
RBM24-mediated NF-κB signaling pathway. Int J Oncol. 59:872021.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wan L, Zhu L, Xu J, Lu B, Yang Y, Liu F
and Wang Z: MicroRNA-409-3p functions as a tumor suppressor in
human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem.
34:1273–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ma J, Huang W, Zhu C, Sun X, Zhang Q,
Zhang L, Qi Q, Bai X, Feng Y and Wang C: miR-423-3p activates FAK
signaling pathway to drive EMT process and tumor growth in lung
adenocarcinoma through targeting CYBRD1. J Clin Lab Anal.
35:e240442021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Liu R, Wang F, Guo Y, Yang J, Chen S, Gao
X and Wang X: MicroRNA-425 promotes the development of lung
adenocarcinoma via targeting A disintegrin and metalloproteinases 9
(ADAM9). Onco Targets Ther. 11:4065–4073. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chen D, Huang J, Zhang K, Pan B, Chen J,
De W, Wang R and Chen L: MicroRNA-451 induces
epithelial-mesenchymal transition in docetaxel-resistant lung
adenocarcinoma cells by targeting proto-oncogene c-Myc. Eur J
Cancer. 50:3050–3067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li Z, Jiang D and Yang S: MiR-490-3p
inhibits the malignant progression of lung adenocarcinoma. Cancer
Manag Res. 12:10975–10984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Duan J, Wang L, Shang L, Yang S, Wu H,
Huang Y and Miao Y: miR-152/TNS1 axis inhibits non-small cell lung
cancer progression through Akt/mTOR/RhoA pathway. Biosci Rep.
41:BSR202015392021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhijun Z and Jingkang H: MicroRNA-520e
suppresses non-small-cell lung cancer cell growth by targeting
Zbtb7a-mediated Wnt signaling pathway. Biochem Biophys Res Commun.
486:49–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Jiang Z, Zhang J, Chen F and Sun Y:
MiR-148b suppressed non-small cell lung cancer progression via
inhibiting ALCAM through the NF-κB signaling pathway. Thorac
Cancer. 11:415–425. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Jiang W, Wei K, Pan C, Li H, Cao J, Han X,
Tang Y, Zhu S, Yuan W, He Y, et al: MicroRNA-1258 suppresses tumour
progression via GRB2/Ras/Erk pathway in non-small-cell lung cancer.
Cell Prolif. 51:e125022018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wu T, Hu H, Zhang T, Jiang L, Li X, Liu S,
Zheng C, Yan G, Chen W, Ning Y, et al: miR-25 promotes cell
proliferation, migration, and invasion of non-small-cell lung
cancer by targeting the LATS2/YAP signaling pathway. Oxid Med Cell
Longev. 2019:97197232019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ding X, Zhong T, Jiang L, Huang J, Xia Y
and Hu R: miR-25 enhances cell migration and invasion in
non-small-cell lung cancer cells via ERK signaling pathway by
inhibiting KLF4. Mol Med Rep. 17:7005–7016. 2018.PubMed/NCBI
|
|
160
|
Hu Z, Xiao D, Qiu T, Li J and Liu Z:
MicroRNA-103a curtails the stemness of non-small cell lung cancer
cells by binding OTUB1 via the hippo signaling pathway. Technol
Cancer Res Treat. 19:15330338209716432020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Jiang K, Shen M, Chen Y and Xu W: miR-150
promotes the proliferation and migration of non-small cell lung
cancer cells by regulating the SIRT2/JMJD2A signaling pathway.
Oncol Rep. 40:943–951. 2018.PubMed/NCBI
|
|
162
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang JX, Zhai JF, Yang XT and Wang J:
MicroRNA-132 inhibits migration, invasion and
epithelial-mesenchymal transition by regulating TGFβ1/Smad2 in
human non-small cell lung cancer. Eur Rev Med Pharmacol Sci.
20:3793–3801. 2016.PubMed/NCBI
|
|
164
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ward SG, Westwick J and Harris S: Sat-Nav
for T cells: Role of PI3K isoforms and lipid phosphatases in
migration of T lymphocytes. Immunol Lett. 138:15–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Akbarzadeh M, Mihanfar A, Akbarzadeh S,
Yousefi B and Majidinia M: Crosstalk between miRNA and
PI3K/AKT/mTOR signaling pathway in cancer. Life Sci.
285:1199842021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zou S, Tong Q, Liu B, Huang W, Tian Y and
Fu X: Targeting STAT3 in Cancer Immunotherapy. Mol Cancer.
19:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Huang S: mTOR signaling in metabolism and
cancer. Cells. 9:22782020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kryczka J, Kryczka J, Czarnecka-Chrebelska
KH and Brzeziańska-Lasota E: Molecular mechanisms of
chemoresistance induced by cisplatin in NSCLC cancer therapy. Int J
Mol Sci. 22:88852021. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21:11022020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Asl ER, Amini M, Najafi S, Mansoori B,
Mokhtarzadeh A, Mohammadi A, Lotfinejad P, Bagheri M, Shirjang S,
Lotfi Z, et al: Interplay between MAPK/ERK signaling pathway and
MicroRNAs: A crucial mechanism regulating cancer cell metabolism
and tumor progression. Life Sci. 278:1194992021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Arancio W, Pizzolanti G, Genovese SI,
Baiamonte C and Giordano C: Competing endogenous RNA and
interactome bioinformatic analyses on human telomerase.
Rejuvenation Res. 17:161–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Yang S, Liu T, Sun Y and Liang X: The long
noncoding RNA LINC00483 promotes lung adenocarcinoma progression by
sponging miR-204-3p. Cell Mol Biol Lett. 24:702019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Qian Y, Zhang Y, Ji H, Shen Y, Zheng L,
Cheng S and Lu X: LINC01089 suppresses lung adenocarcinoma cell
proliferation and migration via miR-301b-3p/STARD13 axis. BMC Pulm
Med. 21:2422021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Cai Y, Sheng Z, Chen Y and Wang J: LncRNA
HMMR-AS1 promotes proliferation and metastasis of lung
adenocarcinoma by regulating MiR-138/sirt6 axis. Aging (Albany NY).
11:3041–3054. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Chen W, Li X, Du B, Cui Y, Ma Y and Li Y:
The long noncoding RNA HOXA11-AS promotes lung adenocarcinoma
proliferation and glycolysis via the microRNA-148b-3p/PKM2 axis.
Cancer Med. 12:4421–4433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chen X, Chen H, Liu M, Xiong J and Song Z:
Long noncoding RNA LINC00520 accelerates lung adenocarcinoma
progression via miR-1252-5p/FOXR2 pathway. Hum Cell. 34:478–490.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhang Y, Li W, Lin Z, Hu J, Wang J, Ren Y,
Wei B, Fan Y and Yang Y: The long noncoding RNA Linc01833 enhances
lung adenocarcinoma progression via MiR-519e-3p/S100A4 axis. Cancer
Manag Res. 12:11157–11167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Dong HX, Wang R, Jin XY, Zeng J and Pan J:
LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via
inhibiting hsa-mir-22-3p. J Cell Physiol. 233:4126–4136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Bai J, Li H, Chen X, Chen L, Hu Y, Liu L,
Zhao Y, Zuo W, Zhang B and Yin C: LncRNA-AC009948.5 promotes
invasion and metastasis of lung adenocarcinoma by binding to
miR-186-5p. Front Oncol. 12:9499512022. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Huang J, Yu Q, Zhou Y, Chu Y, Jiang F and
Wang Q: FAM201A knockdown inhibits proliferation and invasion of
lung adenocarcinoma cells by regulating miR-7515/GLO1 axis. J Cell
Physiol. 236:5620–5632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Ge Z, Liu H, Ji T, Liu Q, Zhang L, Zhu P,
Li L and Zhu L: Long non-coding RNA 00960 promoted the
aggressiveness of lung adenocarcinoma via the miR-124a/SphK1 axis.
Bioengineered. 13:1276–1287. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Tai G, Fu H, Bai H, Liu H, Li L and Song
T: Long non-coding RNA GLIDR accelerates the tumorigenesis of lung
adenocarcinoma by miR-1270/TCF12 axis. Cell Cycle. 20:1653–1662.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Mu X, Wu H, Liu J, Hu X, Wu H, Chen L, Liu
W, Luo S and Zhao Y: Long noncoding RNA TMPO-AS1 promotes lung
adenocarcinoma progression and is negatively regulated by
miR-383-5p. Biomed Pharmacother. 125:1099892020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Liu T, Yang C, Wang W and Liu C: LncRNA
SGMS1-AS1 regulates lung adenocarcinoma cell proliferation,
migration, invasion, and EMT progression via miR-106a-5p/MYLI9
axis. Thorac Cancer. 12:2104–2112. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Xu Q, Xu Z, Zhu K, Lin J and Ye B:
LINC00346 sponges miR-30c-2-3p to promote the development of lung
adenocarcinoma by targeting MYBL2 and regulating CELL CYCLE
signaling pathway. Front Oncol. 11:6872082021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yao Y, Hua Q and Zhou Y: CircRNA
has_circ_0006427 suppresses the progression of lung adenocarcinoma
by regulating miR-6783-3p/DKK1 axis and inactivating Wnt/β-catenin
signaling pathway. Biochem Biophys Res Commun. 508:37–45. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Du J, Zhang G, Qiu H, Yu H and Yuan W: The
novel circular RNA circ-CAMK2A enhances lung adenocarcinoma
metastasis by regulating the miR-615-5p/fibronectin 1 pathway. Cell
Mol Biol Lett. 24:722019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Li X, Su S, Ye D, Yu Z, Lu W and Liu L:
Hsa_circ_0020850 promotes the malignant behaviors of lung
adenocarcinoma by regulating miR-326/BECN1 axis. World J Surg
Oncol. 20:132022. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ma D, Liu H, Qin Y, Li D, Cui Y, Li L, He
J, Chen Y and Zhou X: Circ_0007142/miR-186/FOXK1 axis promoted lung
adenocarcinoma progression. Am J Transl Res. 12:4728–4738.
2020.PubMed/NCBI
|
|
199
|
Huang C, Yue W, Li L, Li S, Gao C, Si L,
Qi L, Cheng C, Lu M, Chen G, et al: Circular RNA hsa-circ-000881
suppresses the progression of lung adenocarcinoma in vitro via a
miR-665/PRICKLE2 axis. Ann Transl Med. 9:4982021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Shi Q and Ju JG: Circ_0001998 regulates
the proliferation, invasion, and apoptosis of lung adenocarcinoma
via sponging miR-145. Evid Based Complement Alternat Med.
2022:64461502022.PubMed/NCBI
|
|
201
|
Fan J, Xia X and Fan Z: Hsa_circ_0129047
regulates the miR-375/ACVRL1 axis to attenuate the progression of
lung adenocarcinoma. J Clin Lab Anal. 36:e245912022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhang B, Chen M, Jiang N, Shi K and Qian
R: A regulatory circuit of circ-MTO1/miR-17/QKI-5 inhibits the
proliferation of lung adenocarcinoma. Cancer Biol Ther.
20:1127–1135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Wei W, Wang C, Wang L and Zhang J:
circ_0020123 promotes cell proliferation and migration in lung
adenocarcinoma via PDZD8. Open Med (Wars). 17:536–549. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Sun Z: Circular RNA hsa_circ_0001588
promotes the malignant progression of lung adenocarcinoma by
modulating miR-524-3p/NACC1 signaling. Life Sci. 259:1181572020.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Cao F, Liu S, Li Z, Meng L, Sang M and
Shan B: Activation of circ_0072088/miR-1261/PIK3CA pathway
accelerates lung adenocarcinoma progression. Thorac Cancer.
13:1548–1557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Panda AC: Circular RNAs Act as miRNA
sponges. Adv Exp Med Biol. 1087:67–79. 2018. View Article : Google Scholar : PubMed/NCBI
|