Introduction
Chronic kidney disease has become a major global
public health problem, and places great burden on affected
individuals, families and societies. Despite the enormity of this
problem, current therapeutic options for chronic kidney disease in
the clinical setting are often ineffective (1). Interstitial fibrosis is considered as
the ultimate common pathway for chronic kidney diseases (2). However, the molecular mechanisms
underlying interstitial fibrosis in kidney tissues are not fully
understood.
Previous studies have demonstrated that activated
hedgehog signaling promotes renal fibrogenesis (3,4).
Hedgehog-mediated fibrotic alterations are associated with enhanced
expression of transforming growth factor (TGF)-β1 (3,5,6), and
promotes myofibroblast formation of renal tubular epithelial cells
(RTECs), endothelial cells, pericytes and activated fibroblasts. As
a result, excessive accumulation of extracellular matrix (ECM)
components in kidney tissues induces interstitial fibrogenesis.
Thus, it is of significance to search for effective therapies to
suppress the hedgehog signaling-mediated fibrotic phenotype, and
renal fibrosis.
Our previous studies demonstrated that the extract
of sedum sarmentosum Bunge (SSBE), a perennial plant that is
widely distributed on the mountain slopes of Asian countries and
contains multiple active flavonoids (such as quercetin,
isorhamnetin and kaempferide) (7–9), has
marked renal anti-fibrotic effects (10,11).
In aristolochic acid (AA)-treated RTECs, SSBE induces cellular
apoptosis and inhibits proliferation. These anti-proliferative
effects of SSBE impede myofibroblast formation, and may occur as a
result of abnormal proliferation of RTECs via
epithelial-to-mesenchymal transition (EMT). Over-activation of
hedgehog signaling is responsible for abnormal proliferation by
regulating components of the cell cycle, such as c-Myc and cyclin
D1 (12,13). Thus, it was hypothesized that SSBE
may have an inhibitory effect on hedgehog signaling. To test this
hypothesis, the present study examined the effects of SSBE on renal
fibrosis induced by ureteral obstruction in vivo, and
production of ECM components induced by AA or TGF-β1 in
vitro. Furthermore, the activity of the hedgehog signaling
pathway was evaluated.
Materials and methods
Animal model and tissue
preparation
Male Sprague-Dawley rats (weight, 180–200 g; age,
6–8 weeks; n=32) were purchased from the Experimental Animal Center
of Wenzhou Medical University (Wenzhou, China). Rats were housed
under a controlled temperature (22–25°C), humidity (40–60%) and
light environment (12-h dark/light), and fed with standard rat chow
(10–15 g twice a day) and water (20–45 ml a day), and this access
was controlled, except for one day of fasting prior to the
operation. The weight-matched rats were randomly assigned to one of
four groups: Sham-operated, treated with vehicle (saline, n=8) or
SSBE (100 mg/kg/day, n=8), and unilateral ureteral obstruction
(UUO) treated with vehicle (n=8) or SSBE (100 mg/kg/day, n=8). UUO
surgery was performed as previously described (11). All rats were sacrificed by cervical
dislocation and were anesthetized by 0.2% pentobarbital natrium
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Kidneys were
excised at day 8 for rats in the UUO SSBE and vehicle control
groups as previously described (11). SSBE (cat. no. 20101017; Xuancheng
Baicao Plant Industry and Trade Co., Ltd., Anhui, China) was
extracted according to the standard protocol (10). The animal study protocols were
approved by the Institutional Animal Care and Use Committee of
Wenzhou Medical University.
Renal histology and
immunohistochemistry
The paraffin-embedded kidney sections were stained
using standard histology procedures as previously described
(11), including hematoxylin and
eosin (H&E) and Masson's trichrome staining (both from Shanghai
Yuanye Biotechnology Co., Ltd., Shanghai, China).
Immunohistochemical analysis was performed on 4-µm-thick kidney
sections using an automatic slicing machine (YD-335; Wuxiang
Instrument, Shanghai, China) that had been dewaxed with xylene and
rehydrated using sequential ethanol (100, 95, 85 and 75%) and
distilled water. Endogenous peroxidase activity was blocked with 3%
hydrogen peroxide for 30 min. Antigen retrieval was performed by
heating the sections in 0.1% sodium citrate buffer (pH 6.0).
Immunohistochemical analysis was performed using anti-TGF-β1
(dilution 1:800, cat. no. bs0103R; BIOSS, Beijing, China),
anti-type III collagen (Col3α1; dilution 1:800, cat. no. bs-0549R;
BIOSS) and anti-proliferating cell nuclear antigen (PCNA; dilution
1:1,000, cat. no. sc-9857; Santa Cruz Biotechnology, Dallas, TX,
USA) primary antibodies at 4°C overnight and then incubated with
the appropriate horseradish peroxidase-conjugated secondary
antibody (dilution 1:10,000, cat. no. P0211; Beyotime Institute of
Biotechnology) at 37°C for 30 min. The integrated optical density
was measured using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). All samples were
semi-quantitatively or quantitatively assessed by two blind
independent investigators.
Cell culture and drug treatment
The NRK-52E renal epithelial cell line was purchased
from the Cell Bank of Chinese Academy of Sciences (Shanghai,
China), and was maintained in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5% fetal bovine serum (FBS, Invitrogen), 100 U/ml
penicillin and 100 µg/ml streptomycin (Invitrogen). NRK-52E cells
were seeded into 6-well culture plates at a density of
3×105 cell/well to confluence in complete medium
containing 5% FBS for 24 h, and then changed to serum-free medium
for 24 h before treatment with 5 ng/ml TGF-β1 (cat. no. 0312209-1;
PeproTech, Inc., Rocky Hill, NJ, USA), 10 µg/ml AA (cat. no. A5512;
Sigma-Aldrich) or 10-1,000 µg/ml SSBE.
Immunofluorescence staining
NRK-52E cells were cultured with TGF-β1, AA, and/or
SSBE in 6-well plates at a seeding density of 3×105
cells/well containing glass slides. Cells were washed with
phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde
(Sigma-Aldrich) at 4°C for 30 min. Following permeabilization with
0.1% Triton X-100 for 10 min, specimens were washed with PBS, and
the substrate was blocked with 10% FBS to eliminate nonspecific
fluorescence. Immunofluorescence staining was performed using
anti-Col3α1 (dilution 1:200), anti-E-cadherin (cat. no. ab53033,
dilution 1:400; Abcam, Cambridge, MA, USA), and anti-α-smooth
muscle actin (α-SMA; dilution 1:400; cat. no. sc-32251),
anti-protein patched homolog 1 (Ptch1; dilution 1:400; cat. no.
sc-9016), anti-smoothened (Smo; dilution 1:400; cat. no. sc-13943)
and anti-Gli family zinc finger 1 (Gli1; dilution 1:100; sc-6153),
purchased from Santa Cruz Biotechnology, primary antibodies at 4°C
overnight. Following washing with PBS three times, the cell
preparations were incubated with fluorescein isothiocyanate
(green)/tetramethylrhodamine-(red) labeled secondary antibodies
(dilution 1:2,000; Sigma-Aldrich) for 1 h at room temperature.
Following washing with PBS, cell preparations were placed in acacia
and covered with a slide. Immunofluorescence studies were
semi-quantitatively or quantitatively assessed by two blind
independent investigators.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from NRK-52E cells or kidney
tissues using TRIzol® reagent (Invitrogen). Reverse
transcription into cDNA templates were performed using a ReverTra
Ace qPCR RT kit (Toyobo, Osaka, Japan). qPCR was performed using a
SYBR-Green Real-Time PCR Master Mix Plus (Toyobo). Quality was
analyzed on agarose gels, and quantities were measured using
Varioskan Flash (Thermo Fisher). Sequence-specific primers of
α-SMA, tight junction protein 1 (ZO-1), type I collagen (Col1α1),
Col3α1, sonic hedgehog (Shh), Ptch1, Smo, Gli1, TGF-β1 and TGF-β1
receptor (TGFβ1R), all listed in Table
I, were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc., and β-actin served as an endogenous reference gene. Samples
were analyzed in triplicate. The melting curve was examined to
verify that a single product was amplified. For quantitative
analysis, all samples were analyzed using the 2−ΔΔCq
value method (14). For
semi-quantitative analysis, all samples were analyzed using gel
electrophoresis.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | GenBank accession
no. | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| Col1α1 | NM_053304.1 |
GATCCTGCCGATGTCGCTAT |
GGAGGTCTTGGTGGTTTTGTATTC |
| Col3α1 | NM_032085.1 |
AAGGCTGAAGGAAATAG |
AATGTCATAGGGTGCGATA |
| Ptch1 | NM_053566.1 |
TCCAGCCGACCCAGATTG |
ACATAGTCGTAGCCCCTGAAGTG |
| Shh | NM_017221 |
ACAAGAAACTCCGAACGATT |
ACAAGAAACTCCGAACGATT |
| Smo | NM_012807.1 |
TGTGGCTCAGGTAGATGG |
GGTGGTTGCTCTTGATGG |
| Gli1 | XM_006241443.2 |
CCTCGTGGCTTTCATCAACTCT |
GAAGCATCATTGAACCCTGAGTAGA |
| ZO-1 | NM_001106266.1 |
GGCATCCACGAAACCACCT |
CCGCCGATCCAGACAGAAT |
| α-SMA | NM_031004.2 |
AACAGAGCCGAGCAGTTAGCC |
CAACATCAGCAATCGGTCCA |
| TGF-β1 | NM_021578.2 |
AGGCGGTGCTCGCTTTGT |
GATTGCGTTGTTGCGGTCC |
| TGF-β1R | NM_012775.2 |
TGATCCATCCGTTGAAGAAA |
CTAGCTGCTCCATTGGCATA |
| β-actin | NM_031144.2 |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC |
Western blot analysis
Whole proteins from NRK-52E cells were collected
using RIPA lysis buffer (Beyotime Institute of Biotechnology) by
centrifugation at 12,900 × g for 10 min, and protein concentrations
were determined using a Bicinchoninic Acid protein assay kit
(Beyotime). Whole proteins (30 µg) from each sample were separated
by 10% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (Beijing Solarbio Science & Technology, Beijing,
China). Following blocking with 5% skimmed milk at 37°C for 1.5 h,
membranes were incubated with anti-Col3α1 (dilution 1:1,000),
anti-α-SMA (dilution 1:1,000) and anti-Smo (dilution 1:1,000)
primary antibodies at 4°C overnight, and then incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody
(dilution 1:5,000; Beyotime Institute of Biotechnology) at 37°C for
2 h. Bound antibodies were visualized using chemiluminescence
detection (ECL, cat. no. 32109; Thermo Fisher Scientific, Inc.) on
autoradiographic film. Quantification was performed by measuring
the intensity of signals using Image-Pro Plus version 6.0 software
(Media Cybernetics), and normalized to that for the anti-GAPDH
antibody (dilution 1:2,000; cat. no. AP0063; Bioworld
Technology).
Statistical analysis
All results are presented as mean ± standard error.
Statistical analyses were performed using a Statistical Package for
Social Sciences version 16.0 software (SPSS, Inc., Chicago, IL,
USA). Student's t-test was used to analyze differences between the
two groups, and one-way analysis of variance followed by least
significant difference post hoc test was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
SSBE reduces TGF-β1 expression and
alleviates interstitial fibrosis in UUO kidneys
Evidence from H&E staining revealed marked
tubular dilation and atrophy associated with interstitial fibrosis
in the obstructed kidney tissues (Fig.
1A). The total collagen deposition determined by Masson
trichrome staining was more severe as obstructive time progressed
(Fig. 1A). SSBE administration
significantly alleviated renal tubular injury and reduced total
collagen deposition (Fig. 1A).
These findings suggested that SSBE alleviated UUO-induced
interstitial fibrosis in rats.
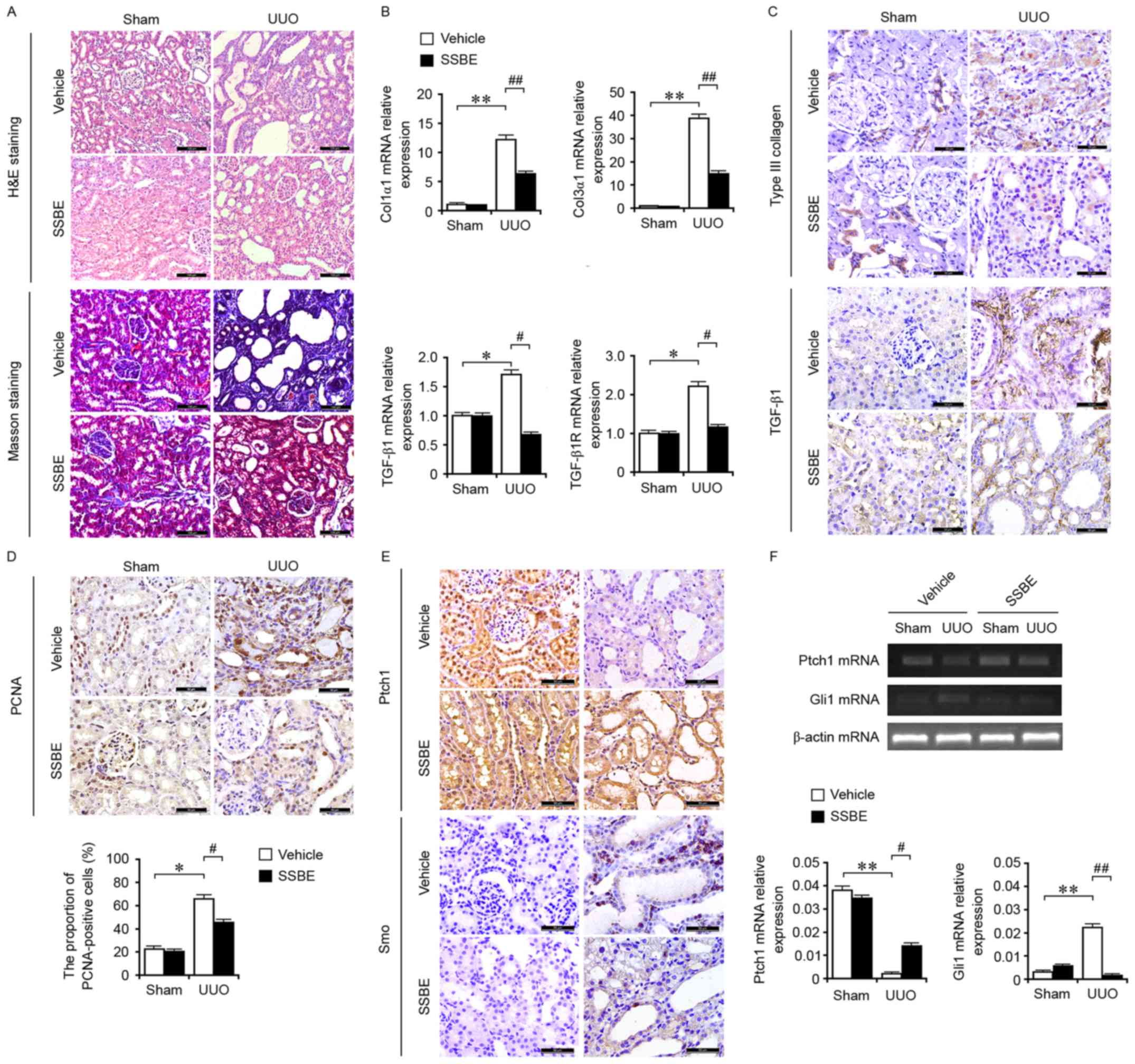 | Figure 1.SSBE inhibits hedgehog signaling
activity and alleviates interstitial fibrosis in UUO kidneys. (A)
H&E and Masson trichrome staining indicated marked kidney
injury and excessive accumulation of total collagen in UUO kidneys,
but SSBE administration alleviated this effect. Scale bar, 100 µm.
(B) Enhanced mRNA expression levels of Col1α1, Col3α1, TGF-β1 and
TGF-β1R in UUO kidneys, determined by reverse transcription
quantitative polymerase chain reaction, were inhibited by SSBE
treatment. (C) Immunochemical staining indicated upregulated
expression of Col3α1 and TGF-β1 in UUO kidneys, which were
alleviated following SSBE administration. Scale bar, 50 µm. (D)
SSBE decreased PCNA expression in kidney tissues of UUO rats. Scale
bar, 50 µm. (E) SSBE administration inhibited UUO-induced
downregulated protein expression levels of Ptch1, and upregulated
expression of Smo. Scale bar, 50 µm. (F) UUO decreased mRNA
expression levels of Ptch1 and increased expression of Smo, but
were inhibited by SSBE treatment. Data are presented as the mean ±
standard error. *P<0.05, **P<0.01 vs. sham;
#P<0.05, ##P<0.01 vs. vehicle. H&E,
hematoxylin and eosin; UUO, unilateral ureteral obstruction;
Col1α1, type I collagen; Col3α1, type III collagen; TGF-β1,
transforming growth factor-β1; TGF-β1R, transforming growth factor
β1 receptor; SSBE, Sedum sarmentosum Bunge; PCNA, proliferating
cell nuclear antigen; Ptch1, protein patched homolog 1; Smo,
smoothened. |
Compared with those in the sham-operated group, the
mRNA expression levels of Col3α1 (Fig.
1B), and the protein expression levels of Col1α1 and Col3α1
(Fig. 1C) in UUO kidneys were
significantly increased. These results supported that UUO induced
excessive ECM deposition and interstitial fibrosis in kidney
tissues. The fibrotic alterations in UUO kidneys were associated
with enhanced gene (Fig. 1B) and
protein (Fig. 1C) expression of
the profibrotic factor TGF-β1, and mRNA expression of its receptor
TGF-β1R (Fig. 1B). However, the
upregulated expression of TGF-β1, TGF-β1R and ECM components were
inhibited by the treatment of SSBE. Furthermore, SSBE suppressed
cellular proliferation in the tubules and interstitium by reducing
the numbers of PCNA-positive cells (Fig. 1D). Therefore, the inhibitory effect
of SSBE on cellular proliferation indirectly regulates the tubular
epithelial cell phenotype and myofibroblast accumulation, resulting
in the reduction of interstitial fibrogenesis.
SSBE inhibits the activation of
hedgehog signaling in UUO kidneys
UUO has been demonstrated to induce cell
proliferation in kidney tissues, which may occur via a feedback
model, and is accompanied with activation of
proliferation-associated signaling, including the hedgehog
signaling pathway (15).
Therefore, the present study examined the gene and protein
expression levels of key molecules involved in the hedgehog
signaling pathway, in the obstructed kidney. As presented in
Fig. 1E, UUO induced the synthesis
and secretion of Smo, and inhibited the expression of Ptch1, a
hedgehog inhibitor by targeting Smo. In addition, upregulated mRNA
expression levels of Gli1 and downregulated expression levels of
Ptch1 were observed in UUO kidneys (Fig. 1F). These findings suggested that
UUO induced the activation of hedgehog signaling. Previous studies
have demonstrated that in UUO rats, hedgehog signaling is activated
by a paracrine signaling loop and mediates epithelial-mesenchymal
communication and promotes renal fibrosis (3,4).
Blockade of hedgehog signaling may alleviate the extent of fibrosis
(3,16). In the present study, the activity
of hedgehog signaling in UUO rats was decreased following SSBE
treatment. Thus, it was hypothesized that SSBE exerts renal
anti-fibrotic effects via suppressing the hedgehog signaling
pathway.
SSBE inhibits EMT induction and ECM
accumulation in TGF-β1-treated RTECs
In UUO kidneys, upregulated expression levels of
molecules involved in the activation of hedgehog signaling are
primarily located around the tubules, which are rich in tubular
epithelial cells (17). However,
whether activation of hedgehog signaling is responsible for the
proliferation of epithelial cells remains unknown. The present
study investigated the effects of SSBE on EMT induction and ECM
deposition in RTECs (NRK-52E cells) treated with TGF-β1, an
important inducer triggering EMT and ECM deposition. As expected,
in TGF-β1-treated NRK-52E cells, upregulated expression of Col3α1
and the myofibroblast marker α-SMA, and downregulated expression of
the epithelial marker E-cadherin, were observed (Fig. 2A). In addition, TGF-β1 decreased
the mRNA expression levels of ZO-1, and increased the expression of
α-SMA, Col1α1 and Col3α1, compared with the control group (Fig. 2B). These TGF-β1-mediated fibrotic
alterations, including EMT induction and ECM accumulation, were
inhibited by SSBE treatment in a dose-dependent manner; SSBE at the
higher concentration (100 µg/ml) had a stronger anti-fibrotic
activity. However, SSBE at too high concentrations (>1,000
µg/ml) inhibited cellular proliferation and induced apoptosis (data
not shown), suggesting that an overdose of SSBE may have a
cytotoxic effect.
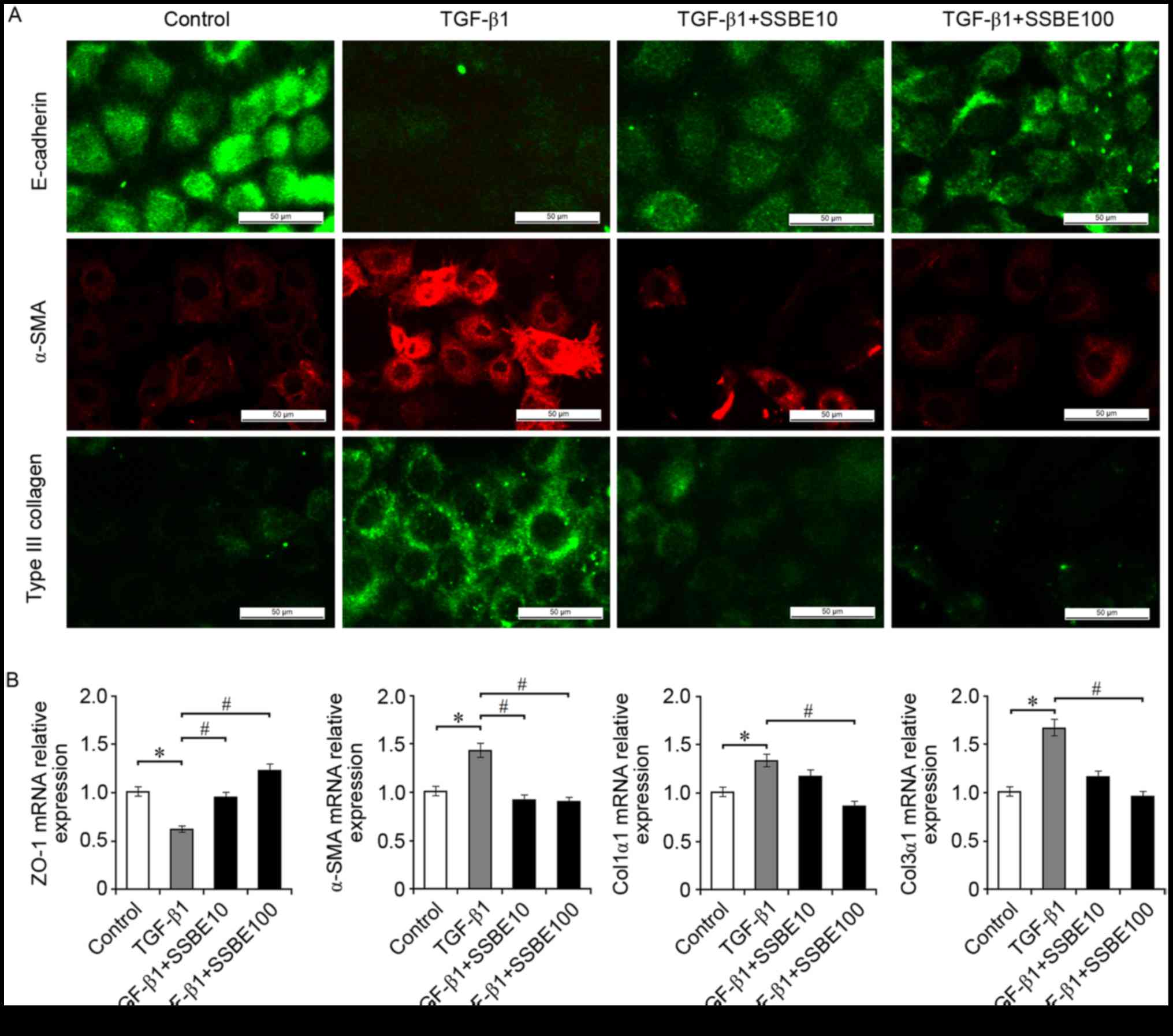 | Figure 2.SSBE inhibits extracellular matrix
accumulation in TGF-β1-treated renal tubular epithelial cells. (A)
Immunofluorescence staining indicated that SSBE treatment decreased
TGF-β1-mediated downregulated expression of E-cadherin, and
upregulated expression of α-SMA and Col3α1 in NRK-52E cells. Scale
bar, 50 µm. (B) Reverse transcription quantitative polymerase chain
reaction analysis demonstrated that mRNA expression levels of
α-SMA, Col1α1, and Col3α1 were increased, and the expression of
ZO-1 was decreased in TGF-β1-treated cells; however, this effect
was ameliorated following SSBE treatment. TGF-β1, 5 ng/ml; SSBE10,
10 µg/ml; SSBE100, 100 µg/ml. Data are presented as the mean ±
standard error. *P<0.05 vs. control; #P<0.05 vs.
TGF-β1. SSBE, Sedum sarmentosum Bunge; Col1α1, type I collagen;
Col3α1, type III collagen; TGF-β1, transforming growth factor-β1;
α-SMA, α-smooth muscle actin; ZO-1, tight junction protein 1. |
SSBE inhibits TGF-β1-induced
activation of hedgehog signaling in RTECs
In addition to the induction of EMT and deposition
of ECM, TGF-β1 enhanced the activity of hedgehog signaling in
NRK-52E cells. As presented in Fig.
3, upregulated expression of PCNA in association with activated
hedgehog signaling was observed in TGF-β1-treated NRK-52E cells.
However, SSBE treatment effectively inhibited PCNA expression.
Furthermore, SSBE downregulated the mRNA expression levels of Shh
and Gli1 in NRK-52E cells after TGF-β1 treatment (Fig. 3B and C), although the changes of
Smo expression levels were not significant. Thus, this in
vitro experiment reconfirmed that inhibiting hedgehog activity
may be an important molecular mechanism for the anti-fibrotic
effect of SSBE on renal tissues in vivo.
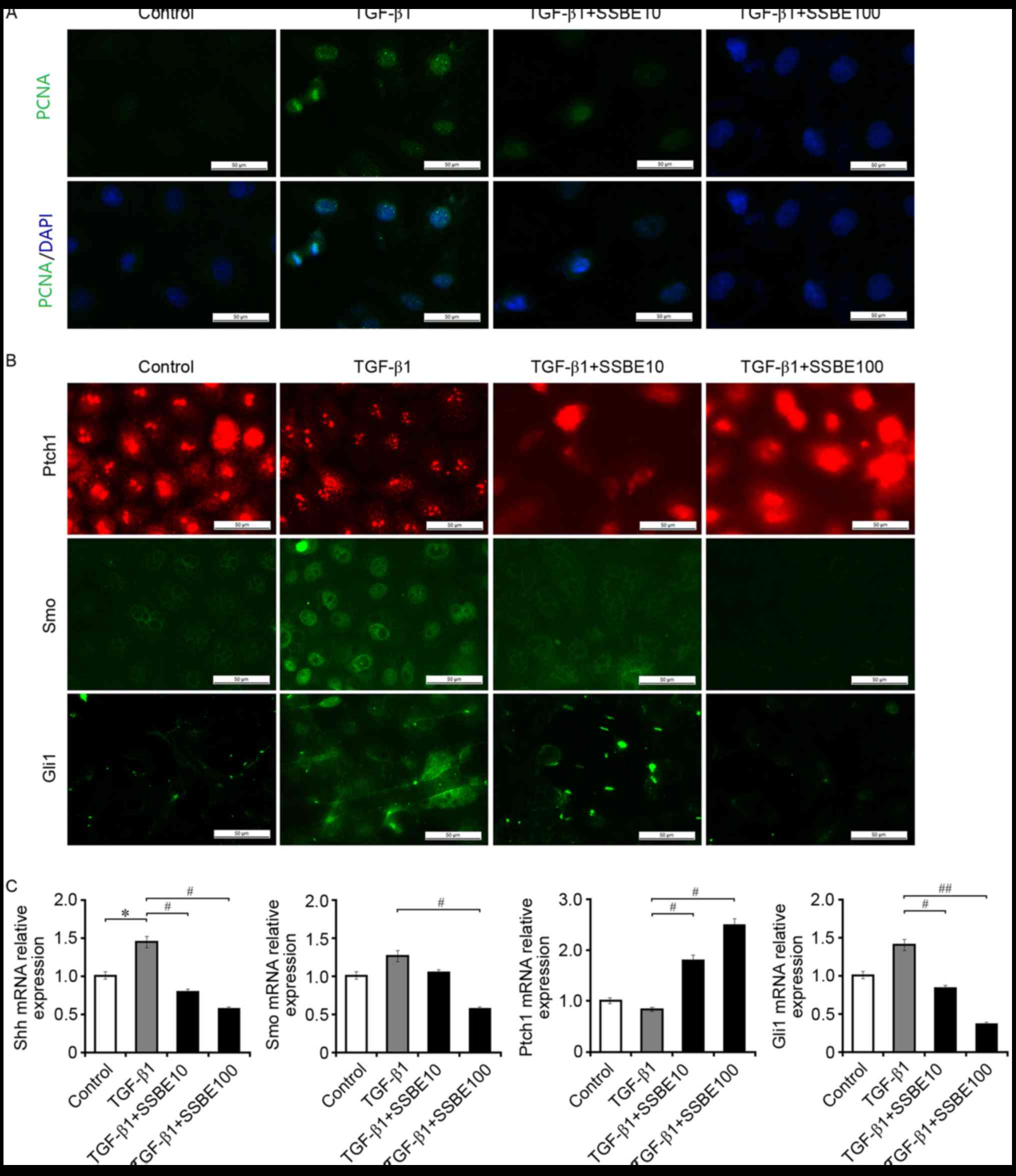 | Figure 3.SSBE inhibits TGF-β1-induced
activation of hedgehog signaling in renal tubular epithelial cells.
(A) SSBE inhibits TGF-β1-induced PCNA expression in NRK-52E cells.
Scale bar, 50 µm. (B) SSBE inhibits TGF-β1-induced upregulated
expression of Smo and Gli1 in NRK-52E cells, and downregulated
expression of Ptch1. Scale bar, 50 µm. (C) The mRNA expression
levels of Shh, Smo and Gli1 were increased, and the expression
levels of Ptch1 were decreased, in TGF-β1-treated cells. This
effect was inhibited following SSBE treatment. Data are presented
as the mean ± standard error. TGF-β1, 5 ng/ml; SSBE10, 10 µg/ml;
SSBE100, 100 µg/ml. *P<0.05 vs. control; #P<0.05,
##P<0.01 vs. TGF-β1. SSBE, Sedum sarmentosum Bunge;
TGF-β1, transforming growth factor-β1; Smo, smoothened; Shh, sonic
hedgehog; Ptch1, protein patched homolog 1; Gli1, Gli family zinc
finger 1; PCNA, proliferating cell nuclear antigen. |
SSBE inhibits AA-mediated
over-activity of hedgehog signaling and ECM deposition
AA is regarded as a potent mutagen that induces
significant cytotoxic effects on RTECs. In vivo, AA may
cause a devastating renal disease called AA nephropathy, and the
histopathology features interstitial matrix deposition and fibrosis
(18). Therefore, the present
study evaluated the effect of SSBE on RTECs following AA injury.
The results demonstrated that SSBE treatment inhibited AA-induced
overexpression of α-SMA and Col3α1 protein (Fig. 4A), but also decreased mRNA
expression levels of TGF-β1, α-SMA and Col1α1 (Fig. 4B). Furthermore, SSBE reduced the
overactivation of hedgehog signaling in RTECs following AA injury
by upregulating the mRNA expression of Ptch1 and downregulating
mRNA and protein expression of Smo (Fig. 4A and C). Therefore, in an injured
micro-environment, SSBE exhibits inhibitory activities on hedgehog
signaling, resulting in the reduction of ECM deposition and a
reduction in fibrosis.
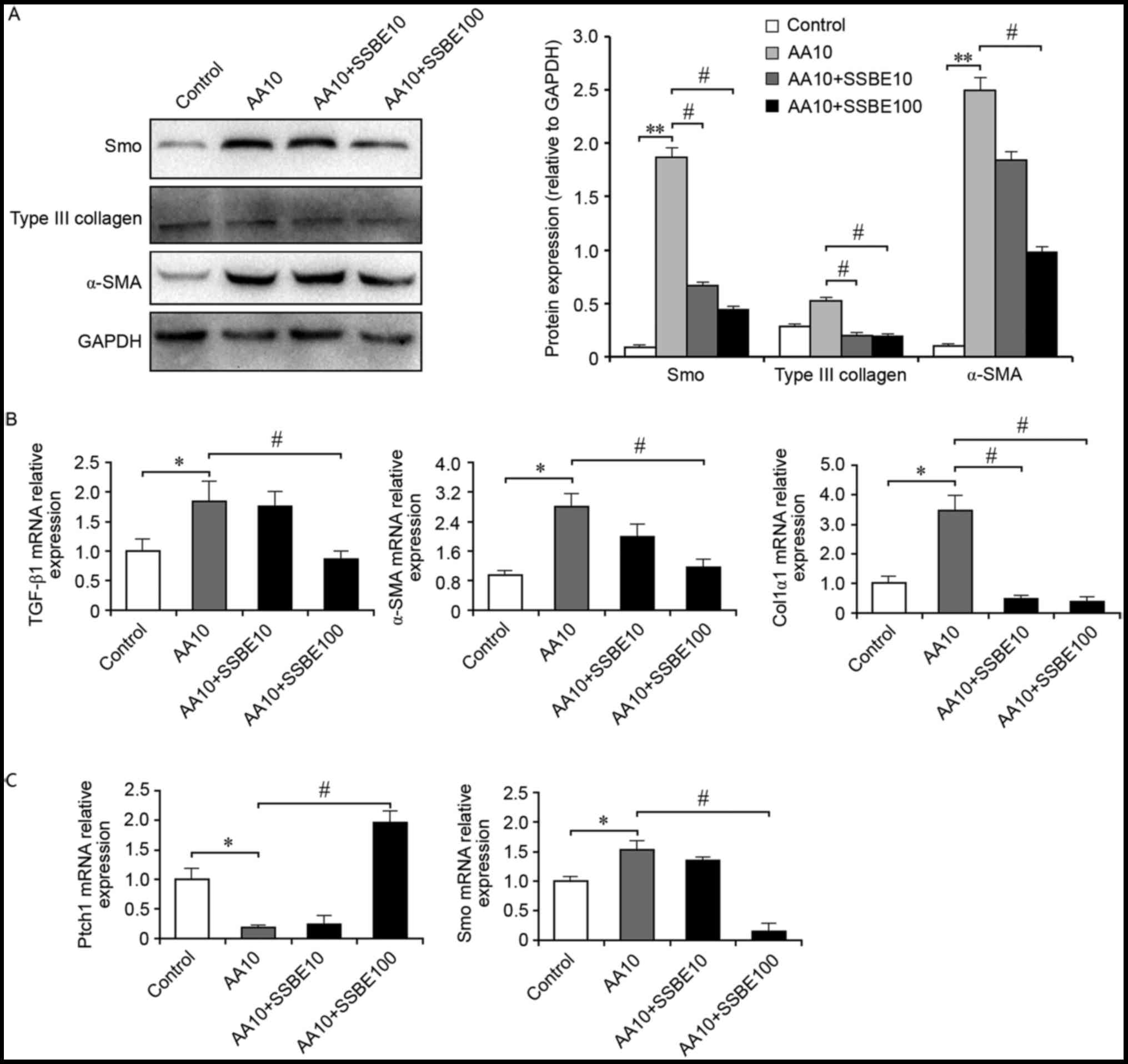 | Figure 4.SSBE inhibits AA-mediated activation
of hedgehog signaling and extracellular matrix deposition. (A) SSBE
inhibits AA-induced overexpression of Smo, α-SMA and Col3α1 protein
in NRK-52E cells, as assessed by western blot analysis. GAPDH
served as an internal control. (B) Quantification of TGF-β1, Col1α1
and α-SMA mRNA expression levels, as assessed by reverse
transcription-quantitative polymerase chain reaction. (C)
Quantification of Ptch1 and Smo mRNA expression levels. AA10, 10
µg/ml; SSBE10, 10 µg/ml; SSBE100, 100 µg/ml. Data are presented as
the mean ± standard error. *P<0.05, **P<0.01 vs. control;
#P<0.05 vs. AA10. SSBE, Sedum sarmentosum Bunge;
TGF-β1, transforming growth factor-β1; Smo, smoothened; Ptch1,
protein patched homolog 1; Col1α1, type I collagen; α-SMA, α-smooth
muscle actin; AA, aristolochic acid. |
Discussion
The present study examined the anti-renal fibrotic
effects of SSBE in vivo and in vitro. In the kidney
tissues of UUO rats, SSBE administration significantly alleviated
tubular damage and interstitial fibrosis. In addition, SSBE
effectively inhibited the formation of α-SMA-positive
myofibroblasts and reduced excessive accumulation of ECM components
in RTECs following exposure to the profibrotic factor TGF-β1, or
the noxious chemical AA.
These results indicated that the anti-fibrotic
effect of SSBE on renal tissues may be associated with the
inhibition of proliferation. SSBE treatment significantly decreased
the expression levels of PCNA, a reference biomarker for cellular
proliferation, not only in kidney tissues of UUO rats, but also in
TGF-β1-treated RTECs, suggesting that the inhibitory effect of SSBE
on proliferation includes epithelial cells. The proliferation
process of RTEC after injury involves regeneration and the EMT
response (19,20). When the injury is moderate, limited
and short-term cell death is controlled by the regenerative
process, in which functional RTECs are replaced by cells of the
same lineage. When regeneration fails to keep pace with cell death,
the injured RTECs may lose their polarity, develop the ability to
migrate, and acquire improved plasticity. As a result, the
trans-differentiation of RTECs is induced and the cells form
α-SMA-positive myofibroblasts, leading to excessive accumulation of
the fibrous matrix and the formation of a scar (21). The inhibition of proliferation in
RTECs following SSBE treatment resulted in reduction of ECM
accumulation and a decrease in fibrotic alterations. Thus, it is
hypothesized that SSBE may be a potential agent for the treatment
of fibrotic kidney disease. In addition, the inactivation of
proliferation-associated signaling may be an important factor that
mediates the inhibition of cellular proliferation.
Hedgehog signaling is a proliferation-associated
pathway that serves a crucial role in the genesis and the
development of several malignancies (22,23).
Aberrant activation of hedgehog signaling induces the expression of
c-Myc and cyclin D1, resulting in the disorder in regulation of
cell cycle (24,25). In tumor cells, hedgehog signaling
is activated for various reasons, and induces cellular
over-proliferation and malignant alterations. Similar to
cancerogenesis, fibrogenesis may be an outcome of abnormal
proliferation in certain tissue cells, such as RTECs, and
activation of hedgehog signaling may contribute to this fibrotic
fate. Numerous studies have confirmed that activated hedgehog
signaling is involved in fibrogenesis of many tissues, including
the liver and kidney (4,15). The present study also supported the
conclusion that hedgehog signaling is activated during renal
fibrogenesis, and then promotes the formation of myofibroblasts
from RTECs via the EMT process. Treatment with SSBE downregulated
the hedgehog signaling activity, resulting in the abolishment of
EMT induction and ECM deposition. Thus, the renal anti-fibrotic
effect of SSBE may occur through suppressing the activation of
hedgehog signaling.
Previous pharmacological studies have revealed that
SSBE possesses significant anti-inflammatory, antitumor and
anti-viral infection activities (26,27).
SSBE treatment exerts a marked inhibitory effect on
lipopolysaccharide-induced nitric oxide production in RAW264.7
macrophage cells (26). In
addition, in a hepatoma cell line, SSBE treatment inhibited
proliferation and induced apoptosis through suppressing signal
transducer and activator of transcription (STAT) phosphorylation
(28). Furthermore, SSBE may
relieve the symptoms of trinitrobenzene sulphonic acid-induced
experimental colitis through reducing TGF-β1 levels in T cells
(29). STAT is a key transcription
factor that regulates activation of the hedgehog signaling pathway
(30,31). As a profibrotic factor in
vivo, TGF-β1 is regarded as an important inducer that triggers
hedgehog signaling activation (19,21,32).
Thus, these findings reconfirmed that hedgehog signaling may be
involved in the anti-fibrotic effect of SSBE.
However, it should be noted that SSBE is a complex
Chinese herb consisting of multiple active chemical constituents.
The pharmacological function of SSBE may depend largely on the
activities of these chemical constituents. Thus, further studies
are required to clarify the potential molecular mechanism for each
chemical constituent of SSBE, and to screen effective agents for
fibrotic kidney disease.
In conclusion, these in vitro and in
vivo experiments preliminarily demonstrated that SSBE treatment
inhibited the hedgehog signaling pathway by reducing TGF-β1
expression and abolishing the induction of EMT, resulting reduced
accumulation of ECM components in the cortical interstitium. These
results implicate SSBE as a potential therapeutic agent for the
prevention of kidney fibrosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LY17H050005) and
the Natural Science Foundation of China (grant no. 81572087). The
project was also supported by the Wenzhou Municipal Science and
Technology Plan Project (grant no. Y20150037).
References
|
1
|
Declèves AE and Sharma K: Novel targets of
antifibrotic and anti-inflammatory treatment in CKD. Nat Rev
Nephrol. 10:257–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meran S and Steadman R: Fibroblasts and
myofibroblasts in renal fibrosis. Int J Exp Pathol. 92:158–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding H, Zhou D, Hao S, Zhou L, He W, Nie
J, Hou FF and Liu Y: Sonic hedgehog signaling mediates
epithelial-mesenchymal communication and promotes renal fibrosis. J
Am Soc Nephrol. 23:801–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabian SL, Penchev RR, St-Jacques B, Rao
AN, Sipilä P, West KA, McMahon AP and Humphreys BD: Hedgehog-Gli
pathway activation during kidney fibrosis. Am J Pathol.
180:1441–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gill PS and Rosenblum ND: Control of
murine kidney development by sonic hedgehog and its GLI effectors.
Cell Cycle. 5:1426–1430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi SS, Omenetti A, Witek RP, Moylan CA,
Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, et
al: Hedgehog pathway activation and epithelial-to-mesenchymal
transitions during myofibroblastic transformation of rat hepatic
cells in culture and cirrhosis. Am J Physiol Gastrointest Liver
Physiol. 297:G1093–G1106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ninomiya K, Morikawa T, Zhang Y, Nakamura
S, Matsuda H, Muraoka O and Yoshikawa M: Bioactive constituents
from Chinese natural medicines. XXIII. Absolute structures of new
megastigmane glycosides, sedumosides A(4), A(5), A(6), H, and I,
and hepatoprotective megastigmanes from Sedum sarmentosum.
Chem Pharm Bull (Tokyo). 55:1185–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morikawa T, Zhang Y, Nakamura S, Matsuda
H, Muraoka O and Yoshikawa M: Bioactive constituents from Chinese
natural medicines. XXII. Absolute structures of new megastigmane
glycosides, sedumosides E1, E2,
E3, F1, F2 and G, from Sedum
sarmentosum (Crassulaceae). Chem Pharm Bull (Tokyo).
55:435–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh H, Kang DG, Kwon JW, Kwon TO, Lee SY,
Lee DB and Lee HS: Isolation of angiotensin converting enzyme (ACE)
inhibitory flavonoids from Sedum sarmentosum. Biol Pharm
Bull. 27:2035–2037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai Y, Lu H, Hu L, Hong D, Ding L and Chen
B: Effect of Sedum sarmentosum BUNGE extract on aristolochic
acid-induced renal tubular epithelial cell injury. J Pharmacol Sci.
124:445–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai Y, Lu H, Zhang G, Wu C, Lin C, Liang Y
and Chen B: Sedum sarmentosum Bunge extract exerts renal
anti-fibrotic effects in vivo and in vitro. Life Sci. 105:22–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cutcliffe C, Kersey D, Huang CC, Zeng Y,
Walterhouse D and Perlman EJ: Renal Tumor Committee of the
Children's Oncology Group: Clear cell sarcoma of the kidney:
Up-regulation of neural markers with activation of the sonic
hedgehog and Akt pathways. Clin Cancer Res. 11:7986–7994. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Besschetnova TY, Brooks CR, Shah
JV and Bonventre JV: Epithelial cell cycle arrest in G2/M mediates
kidney fibrosis after injury. Nat Med. 16:535–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhardwaj G, Murdoch B, Wu D, Baker DP,
Williams KP, Chadwick K, Ling LE, Karanu FN and Bhatia M: Sonic
hedgehog induces the proliferation of primitive human hematopoietic
cells via BMP regulation. Nat Immunol. 2:172–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omenetti A, Porrello A, Jung Y, Yang L,
Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, et
al: Hedgehog signaling regulates epithelial-mesenchymal transition
during biliary fibrosis in rodents and humans. J Clin Invest.
118:3331–3342. 2008.PubMed/NCBI
|
|
17
|
Lu H, Chen B, Hong W, Liang Y and Bai Y:
Transforming growth factor-β1 stimulates hedgehog signaling to
promote epithelial-mesenchymal transition after kidney injury. FEBS
J. 283:3771–3790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baudoux TE, Pozdzik AA, Arlt VM, De Prez
EG, Antoine MH, Quellard N, Goujon JM and Nortier JL: Probenecid
prevents acute tubular necrosis in a mouse model of aristolochic
acid nephropathy. Kidney Int. 82:1105–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: Pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Humphreys BD, Valerius MT, Kobayashi A,
Mugford JW, Soeung S, Duffield JS, McMahon AP and Bonventre JV:
Intrinsic epithelial cells repair the kidney after injury. Cell
Stem Cell. 2:284–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
di Magliano Pasca M and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hooper JE and Scott MP: Communicating with
hedgehogs. Nat Rev Mol Cell Biol. 6:306–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang L, Walter V, Hayes DN and Onaitis M:
Hedgehog-GLI signaling inhibition suppresses tumor growth in
squamous lung cancer. Clin Cancer Res. 20:1566–1575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung HJ, Kang HJ, Song YS, Park EH, Kim YM
and Lim CJ: Anti-inflammatory, anti-angiogenic and anti-nociceptive
activities of Sedum sarmentosum extract. J Ethnopharmacol.
116:138–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johari J, Kianmehr A, Mustafa MR, Abubakar
S and Zandi K: Antiviral activity of baicalein and quercetin
against the Japanese encephalitis virus. Int J Mol Sci.
13:16785–16795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang D, Zhang W, Huang D and Wu J:
Antitumor activity of the aqueous extract from Sedum
sarmentosum Bunge in vitro. Cancer Biother Radiopharm.
25:81–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ge X, Wu Z, Wu Q, Yang F, Yang C and Yao
X: Study of the effect and mechanism of Sedum sarmentosum
Bunge on TNBS-induced colitis in rats. Chin J Integr Trad West Med
Dig. 15:391–394. 2007.
|
|
30
|
Dong W, Cui J, Tian X, He L, Wang Z, Zhang
P and Zhang H: Aberrant sonic hedgehog signaling pathway and STAT3
activation in papillary thyroid cancer. Int J Clin Exp Med.
7:1786–1793. 2014.PubMed/NCBI
|
|
31
|
Yang Q, Shen SS, Zhou S, Ni J, Chen D,
Wang G and Li Y: STAT3 activation and aberrant ligand-dependent
sonic hedgehog signaling in human pulmonary adenocarcinoma. Exp Mol
Pathol. 93:227–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maitah MY, Ali S, Ahmad A, Gadgeel S and
Sarkar FH: Up-regulation of sonic hedgehog contributes to
TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells.
PLoS One. 6:e160682011. View Article : Google Scholar : PubMed/NCBI
|


















