Introduction
Lung cancer remains a leading cause of
cancer-associated mortality worldwide, with an estimated 1.61
million new occurrences and 1.38 million fatalities occurring each
year worldwide. In addition, the majority of patients are diagnosed
at an advanced stage (1).
Therefore, despite therapeutic progress, prognosis remains poor,
with a 5-year survival rate of <15% (2).
Cisplatin is a traditional first-line chemotherapy
reagent (3) that causes DNA damage
and apoptosis (4); however,
cisplatin resistance remains an issue that contributes to
recurrence and therapeutic failure. Various mechanisms underlying
cisplatin resistance have been reported, including activation of
DNA repair, decreased drug influx and increased efflux, and
resistance to apoptosis (5).
Therefore, restoring cisplatin-induced apoptosis may prevent
cisplatin-acquired resistance.
Previous studies have suggested that certain
chemotherapeutics induce both apoptosis and autophagy in tumor
cells, and that the balance between autophagy and apoptosis may
determine cell fate (6–8). Autophagy is a homeostatic, cellular
process of catabolic degradation, which eliminates damaged
organelles and recycles essential components during cellular stress
responses (9). Autophagy serves a
dual role in the tumorigenesis of lung cancer (7,10);
as an adaptive response, autophagy may contribute to the
acquisition of cisplatin resistance in lung cancer cells (11), whereas inhibition of autophagy may
resensitize tumor cells to diverse cancer therapies (12,13).
Conversely, autophagy-mediated cell death and autophagy-induced
apoptosis have also been reported under various circumstances and
among different cell types (14,15).
Therefore, the autophagic response may be associated with cisplatin
resistance, or it may be associated with apoptotic or autophagic
cell death (16); the function and
mechanism of autophagy in cisplatin resistance and apoptosis remain
to be elucidated.
In the present study, the function and mechanism of
cisplatin-induced autophagy were investigated. Beclin 1,
serine/threonine-protein kinase ULK1 (ULK1), autophagy protein
(Atg)5, Atg3, Atg7, Atg12 and sequestosome-1 (SQSTM1) transcription
and expression were analyzed following cisplatin treatment.
Knockdown of Atg5 and Beclin 1 by small interfering (si)RNA
transfection was used to determine the association between
cisplatin-induced apoptosis and autophagy. The results indicated
that specific disruption of the autophagic response may be
considered a rationale for the restoration of cisplatin
sensitivity, and may provide a target for anti-lung cancer
therapy.
Materials and methods
Cell culture and reagents
Human lung cancer A549 cells (ATCC®
CCL-185™) were purchased from American Type Culture Collection
(Manassas, VA, USA) and cultured as previously described (11). Cisplatin (20 µM) treatment was at
37°C in an atmosphere containing 5% CO2 for the
indicated time points in Fig. 1.
Cisplatin, MTT and the lactate dehydrogenase (LDH)-dependent
Cytotoxic Non-Radioactive Cytotoxicity assay were purchased from
Promega Corp. (Madison, WI, USA). The Caspase-3 Fluorometric Assay
kit was purchased from BioVision, Inc. (Milpitas, CA, USA).
Microtubule-associated protein 1 light chain 3β (LC3B) antibody was
obtained from Novus Biologicals, LLC, Littleton, CO, USA (cat. no.
NB100-2220), and Beclin 1 (cat. no. 3738), Atg5 (cat. no. 2630),
cleaved caspase-3 (cat. no. 9661), Atg3 (cat. no. 3415), Atg7 (cat.
no. 2631) and Atg12 (cat. no. 4180) antibodies were from Cell
Signaling Technology, Inc. (Danvers, MA, USA). SQSTM1 antibody
(cat. no. M217-3) was purchased from MBL International Co. (Woburn,
MA, USA), and ULK1 (cat. no. A7481) and β-actin (cat. no. A5441)
antibodies were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Goat anti-rabbit secondary antibody (cat. no. A-11037)
was from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted by using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. CDNA was synthesized using a PrimeScript
RT Reagent kit (Takara Bio, Inc., Otsu, Japan). The PCR reaction
was performed with SYBR® Green Master Mix (Ambion;
Thermo Fisher Scientific, Inc.) in an ABI 7500 RT-PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.; listed
in Table I), as described
previously (17). Amplification
was performed under the following conditions: 95°C for 5 min in the
holding stage; 40 cycles of 95°C for 10 sec and 60°C for 30 sec in
the cycling stage; and 95°C for 15 sec, 60°C for 1 min and 60°C for
15 sec in the melt curve stage. Relative gene expression was
calculated using the comparative 2−ΔΔCq method (18). The mean Cq value of the target gene
was normalized to the averaged Cq values of GAPDH to obtain a ΔCq
value, which was subsequently normalized to control samples to
obtain a ΔΔCq value. Each measurement was assessed in triplicate.
The gene expression ratio was presented as the mean ± standard
deviation of three independent experiments.
 | Table I.Primer pairs for quantitative
polymerase chain reaction. |
Table I.
Primer pairs for quantitative
polymerase chain reaction.
|
| Primer sequence
(5′→3′) |
|---|
|
|
|
|---|
| Target name | Forward | Reverse |
|---|
| Beclin 1 |
CAAGATCCTGGACCGTGTACA |
TGGCACTTTCTGTGGACATCA |
| Atg12 |
TCTATGAGTGTTTTGGCAGTG |
ATCACATCTGTTAAGTCTCTTGC |
| Atg7 |
AGGAGATTCAACCAGAGACC |
GCACAAGCCCAAGAGAGG |
| Atg5 |
GGGAAGCAGAACCATACTATTTG |
AAATGTACTGTGATGTTCCAAGG |
| Atg3 |
TCACAACACAGGTATTACAGG |
TCACCGCCAGCATCAG |
| ULK1 |
CGCCTGTTCTACGAGAAGAAC |
GAAGTCCATGCGGTCCTTGTG |
| SQSTM1 |
AAGCCGGGTGGGAATGTTG |
GCTTGGCCCTTCGGATTCT |
| GAPDH |
GGGAAGCTTGTCATCAATGG |
CATCGCCCCACTTGATTTTG |
Western blot analysis
Cytoplasmic protein expression in cultured cells was
detected using western blotting as previously described (19). Membranes were incubated overnight
at 4°C using primary antibodies diluted in 1% bovine serum albumin.
LC3B, Beclin 1, ULK1, Atg5, cleaved caspase-3, Atg3, Atg7 and Atg12
primary antibodies were diluted at 1:1,000. SQSTM1 primary antibody
was diluted at 1:2,000. The secondary antibodies were diluted at
1:5,000 and were incubated at room temperature for 1 h.
siRNA transfection
Beclin 1 and Atg5 siRNA were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The recombinant
lentiviral vectors, empty lentiviral vectors and secondary
packaging plasmids were co-transfected into the 293T cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. The
obtained Beclin 1 and Atg 5 siRNA particle solutions were
designated as Lv-si8678 and Lv-si9474 and the Lv-control and were
stored at −80°C until use. The sequences of Beclin1, Atg5 and
negative control were: 5′-CAGTTTGGCACAATCAATA-3′,
5′-AUCCAUGAGUUUCCGAUUC-3′ and 5′-UUCUCCGAACGUGUCAGUT-3′,
respectively. Transfection of cells with 100 nM siRNA was performed
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to a previously published method
(20).
Cell viability, LDH release and
caspase-3 activity assays
Cell viability was measured using the MTT assay
according to the manufacturer's protocol (Promega Corp.). LDH
release into the culture medium was measured using the
LDH-dependent Cytotoxic Non-Radioactive Cytotoxicity Assay and
caspase-3 activity was measured with a Caspase-3 Fluorometric Assay
kit, both according to manufacturers' protocols.
Transmission electron microscopy
(TEM)
For TEM, cells were embedded, sectioned, double
stained and analyzed using a JEM-1200EX transmission electron
microscope (JEOL, Ltd., Tokyo, Japan), as previously described
(20).
Immunofluorescence staining
Human lung cancer A549 cells (ATCC®
CCL-185™) were treated with cisplatin (20 µM) for 96 h at 37°C in
an atmosphere containing 5% CO2. LC3B puncta were
measured using immunofluorescence, as previously described
(20).
Statistical analysis
All values are presented as the means ± standard
deviation (n=3). Quantitative data were analyzed using a Student's
t test or two-way analysis of variance (ANOVA), with a Tukey post
hoc test used following ANOVA, by using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
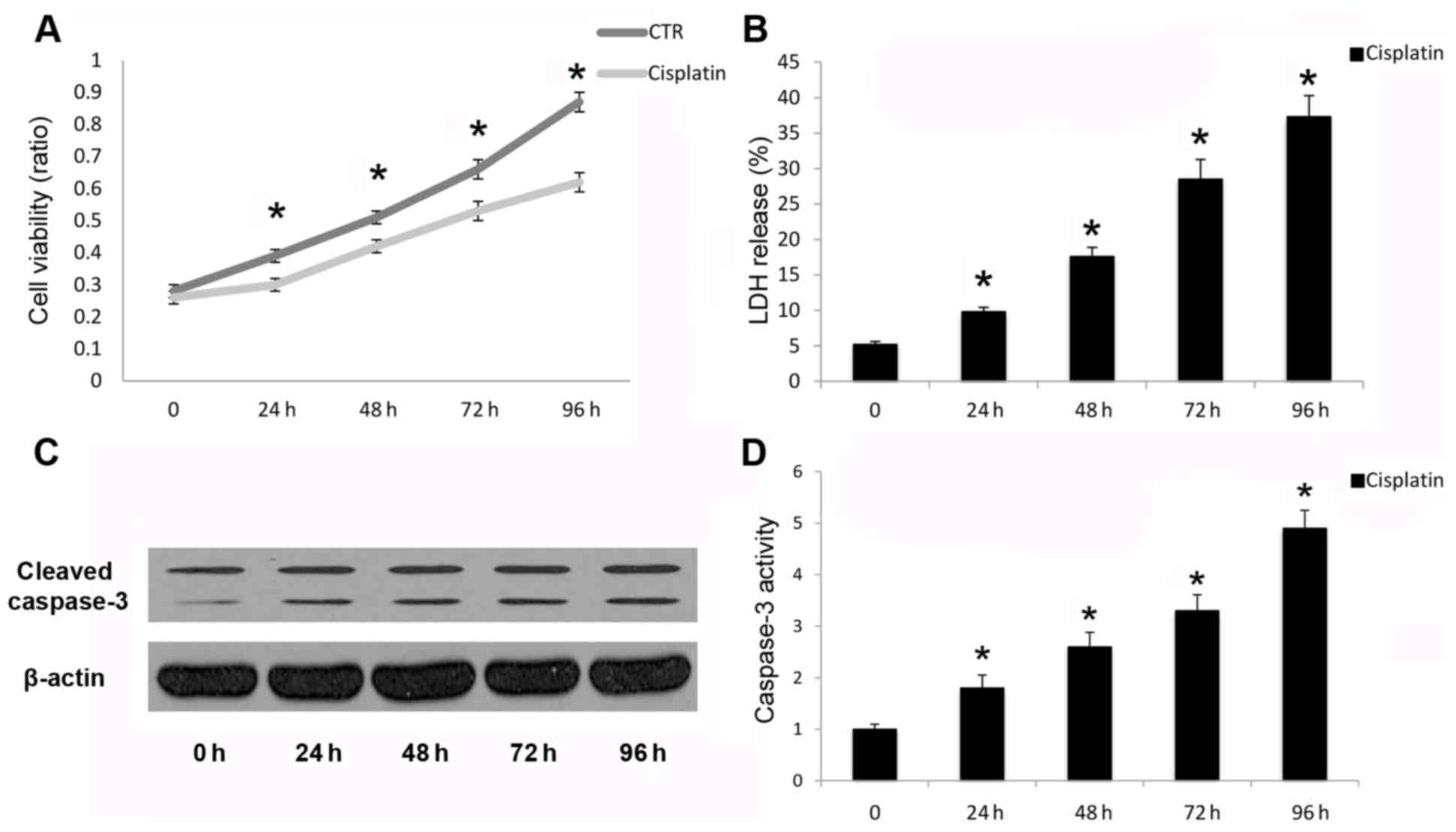
Cisplatin leads to apoptotic cell
death in A549 cells
Cleavage and activation of caspase-3 is necessary
for the execution phase of intrinsic and extrinsic apoptotic
signaling pathways (21). In the
present study, cisplatin inhibited A549 human cancer cell viability
(Fig. 1A) and increased LDH
release (Fig. 1B). Cisplatin also
promoted cleavage (Fig. 1C) and
activation (Fig. 1D) of capase-3
following cisplatin treatment in a time-dependent manner up to 96
h. Reduced cell viability and promotion of cell death were due to
the induction of apoptosis, as demonstrated by caspase-3 cleavage
and activation. Therefore, cisplatin may induce apoptotic cell
death in A549 cells in a time-dependent manner.
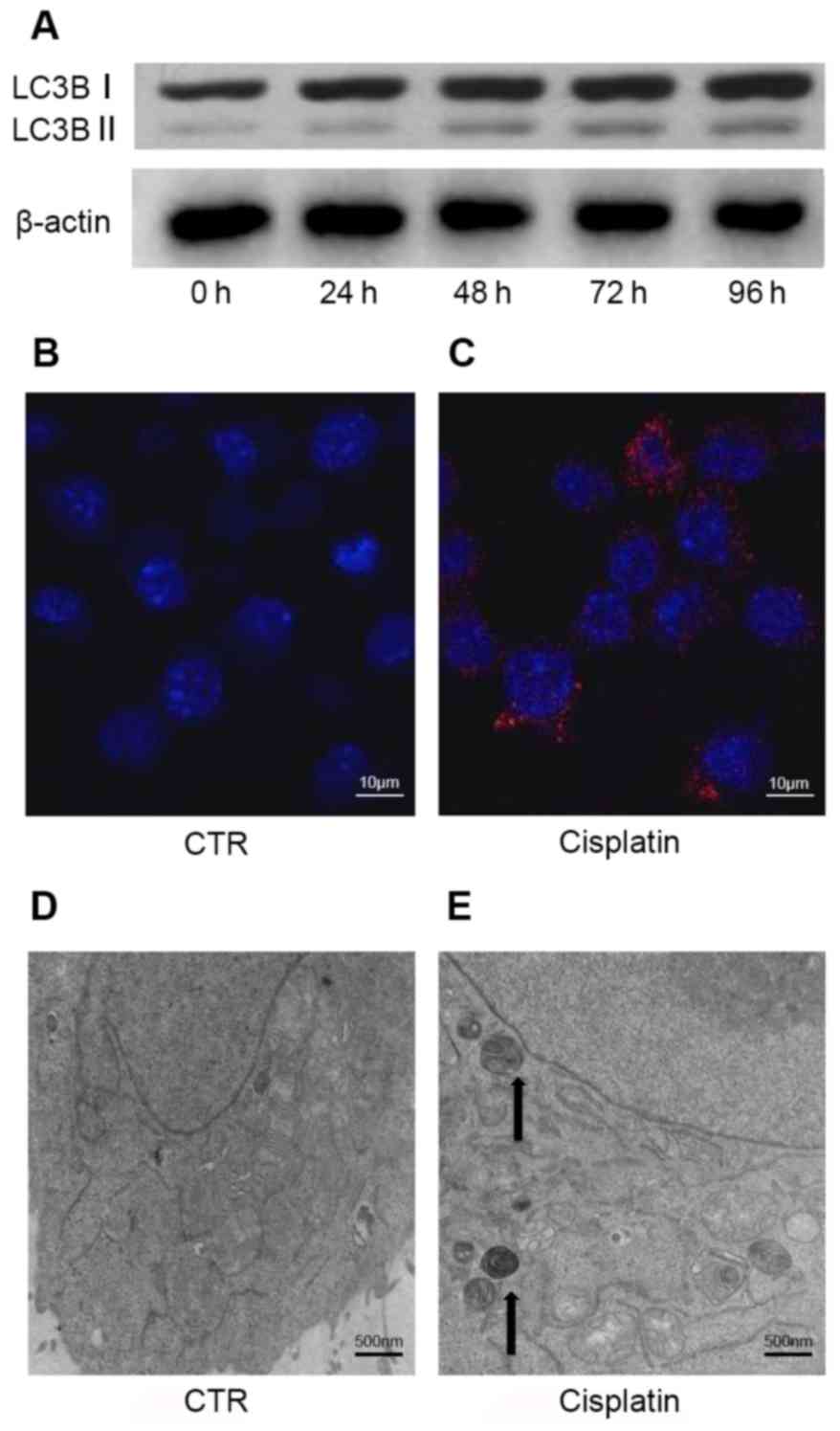
Cisplatin initiates the autophagic
response in A549 cells
Cellular stress responses, caused by factors
including exposure to anticancer drugs, can trigger the autophagic
response (22). Kinetic analysis
of autophagosome formation in A549 cells demonstrated that
cisplatin-induced autophagy occurs in a time-dependent manner.
Western blot analysis indicated an increased LC3B-I/II conversion
following cisplatin treatment up to 96 h (Fig. 2A). Densitometry was performed by
Image J to quantify OD; increased LC3B-I/II conversion indicated
that autophagosome formation was upregulated. The ratio of
LC3B-I/II conversion for each group was 0.34 (control), 0.45 (24
h), 0.56 (48 h), 0.69 (72 h) and 0.78 (96 h).
Autophagy occurred in A549 cells treated with
cisplatin, as demonstrated by increased LC3B puncta (Fig. 2B and C). In addition,
cisplatin-induced morphological alterations were observed by TEM,
including the formation of double membrane-bound autophagosomes and
mitochondrial damage (Fig. 2D and
E). These specific alterations in LC3B have been characterized
as autophagosome markers. Therefore, the results of the present
study indicated that cisplatin triggers the autophagic response in
A549 cells.
Cisplatin induces the autophagic
response in A549 cells by upregulating mRNA and protein levels of
Atg5 and Beclin 1
The signaling pathways that mediate autophagic
induction differ according to cell type and stimulus (23). The mechanism underlying
cisplatin-mediated autophagy in A549 cells remains to be
elucidated. Therefore, the present study investigated the mechanism
underlying cisplatin-induced autophagy in A549 cells. Beclin 1,
Atg5, ULK1, Atg3, Atg7, Atg12 and SQSTM1 mRNA and protein levels
were measured following cisplatin treatment. Induction of autophagy
following cisplatin treatment resulted in increased transcription
of certain autophagy-associated genes, including Atg5 and Beclin 1
(Fig. 3A). However, the expression
of other autophagy-associated genes was not significantly altered
(Fig. 3A). Autophagy-associated
gene expression analysis indicated that cisplatin induced autophagy
through upregulation of Beclin 1 and Atg5 (Fig. 3B), without altering the expression
of other autophagy-associated proteins (Fig. 3C). Atg5 and Beclin 1 expression
levels were continuously increased following cisplatin treatment.
Therefore, Atg5 and Beclin1 transcriptional and translational
upregulation may be involved in cisplatin-induced autophagy in A549
lung cancer cells.
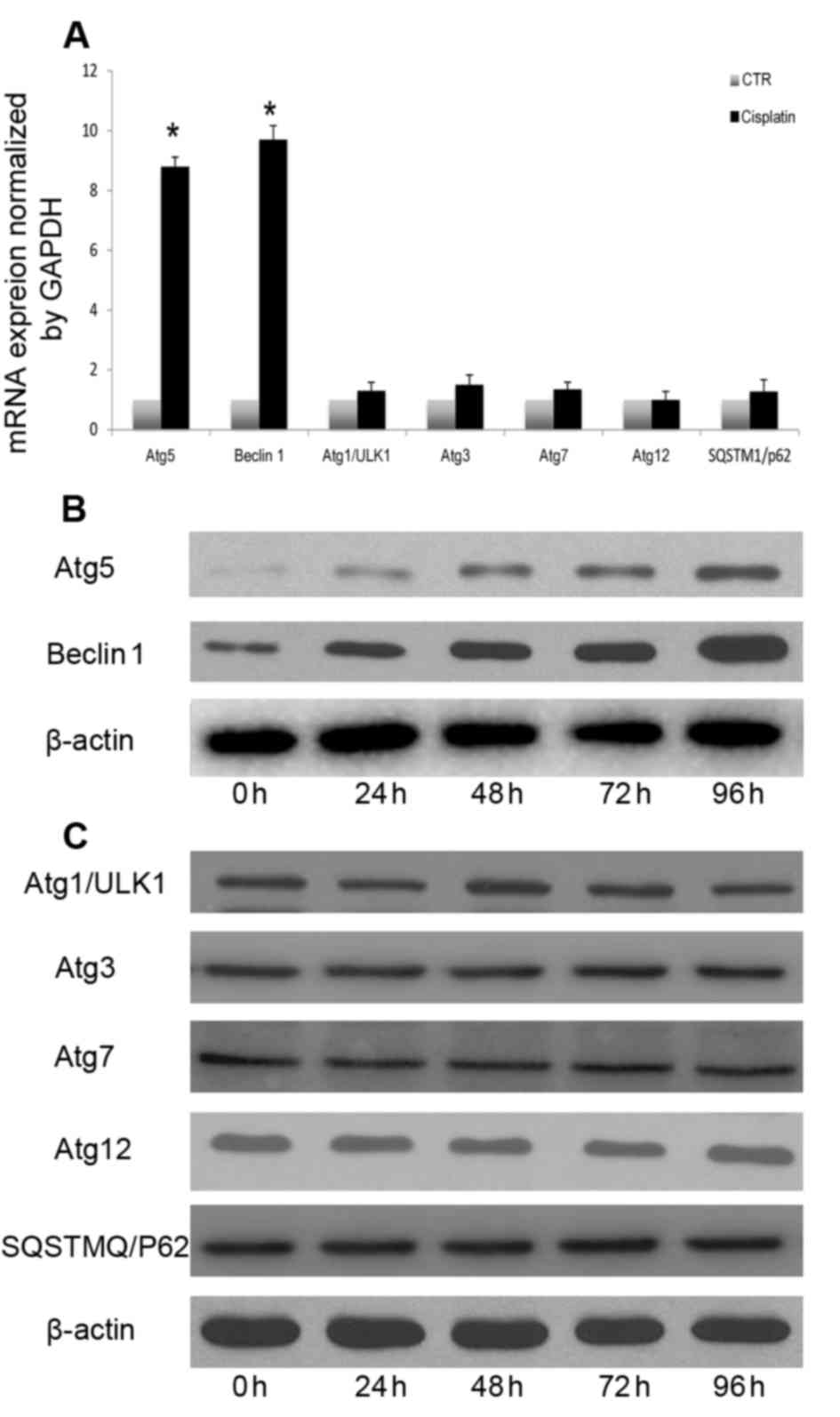 | Figure 3.Cisplatin-induced autophagic response
involves upregulation of Atg5 and Beclin 1. (A) A549 cells were
treated with cisplatin (20 µM) for 24 h, and Beclin 1, Atg5, ULK1,
Atg3, Atg7, Atg12 and SQSTM1 transcription were analyzed by reverse
transcription-quantitative polymerase chain reaction. A549 cells
were treated with cisplatin (20 µM) at the indicated time points,
and (B) Beclin 1 and Atg5, and (C) ULK1, Atg3, Atg7, Atg12 and
SQSTM1 expression levels were measured by western blotting.
*P<0.05 vs. the CTR group. Atg, autophagy protein; CTR, control;
SQSTM1, sequestosome-1; ULK1, serine/threonine-protein kinase
ULK1. |
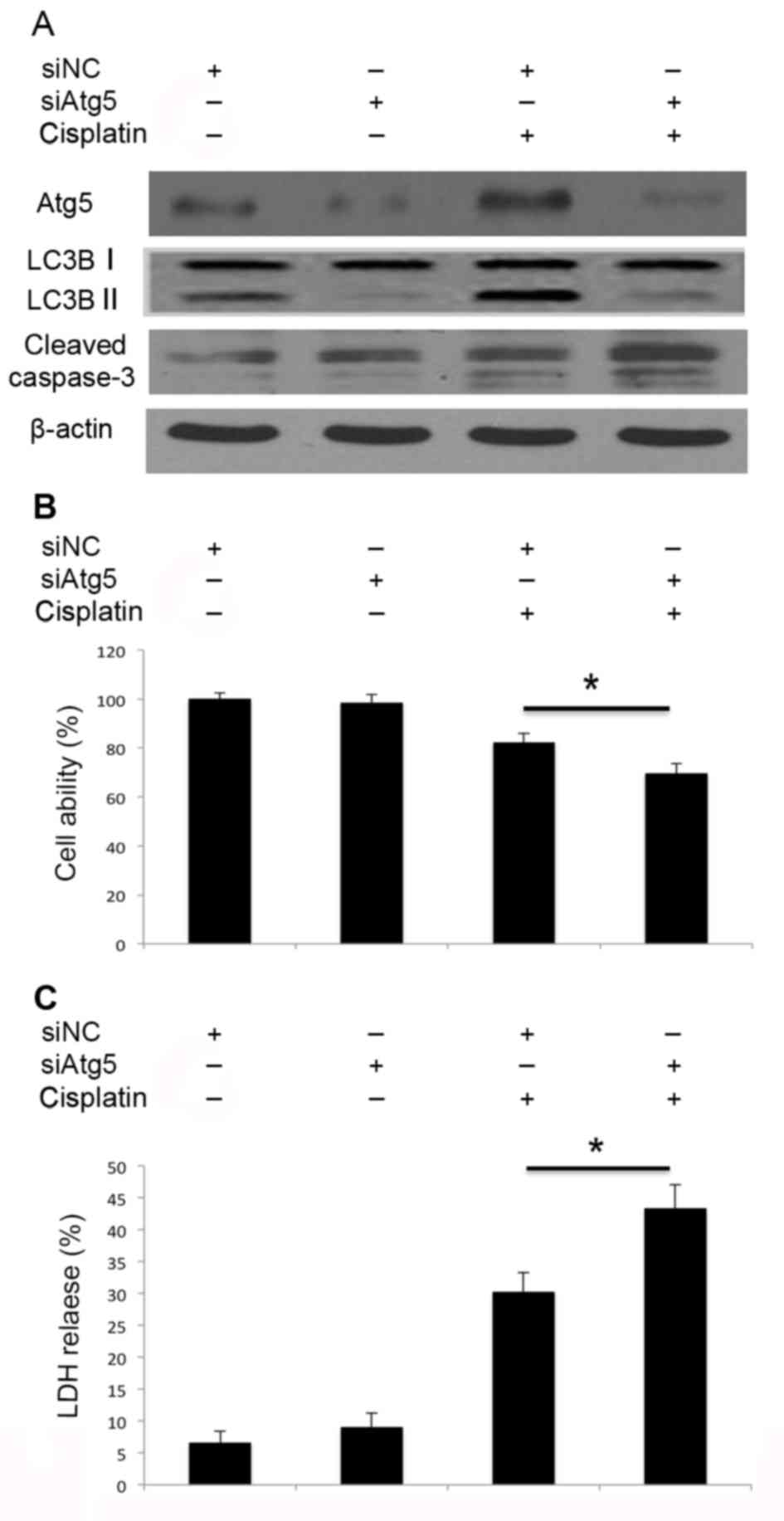
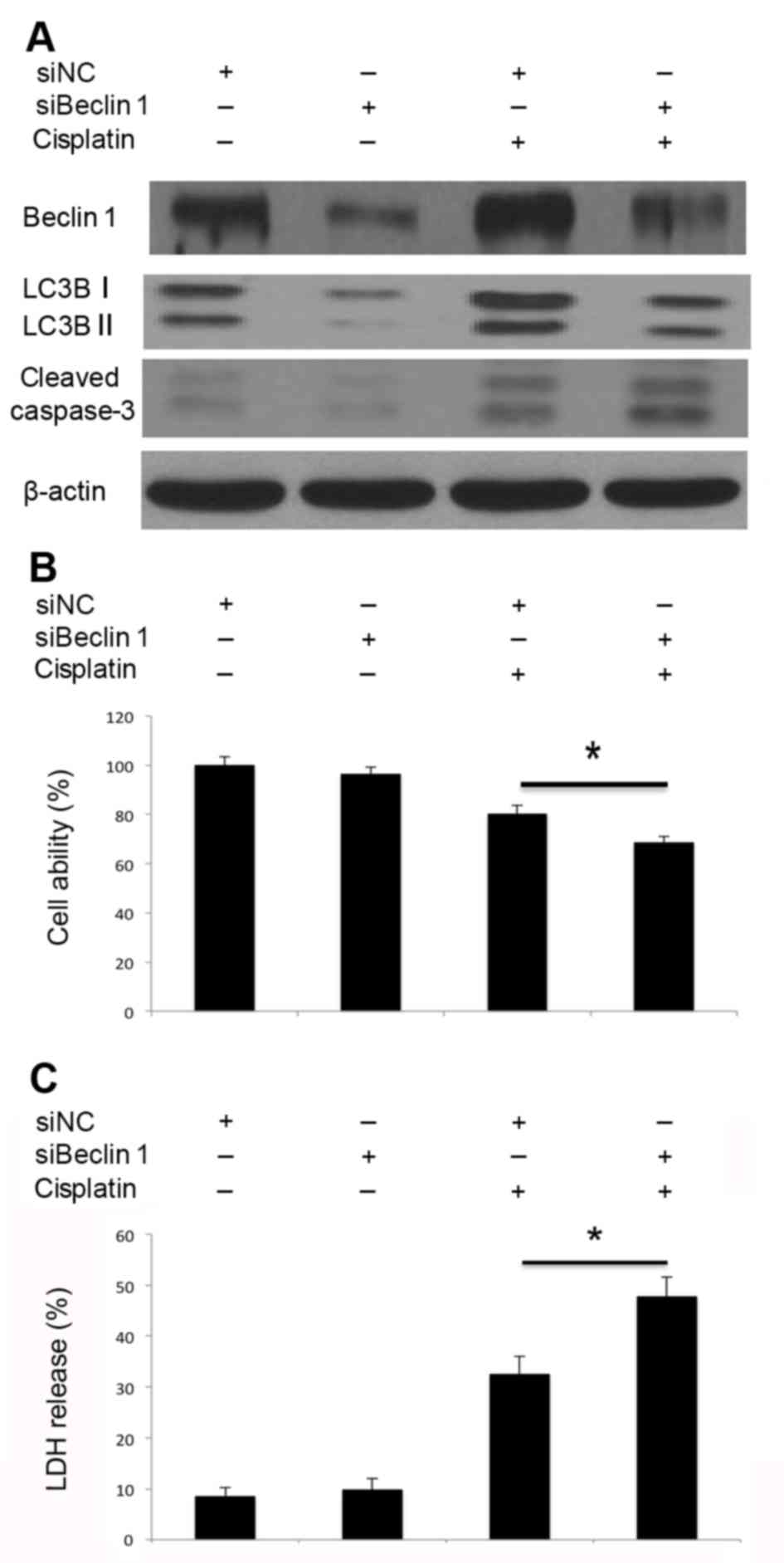
Inhibition of autophagy by Atg5 and
Beclin 1 siRNA transfection promotes cisplatin-induced apoptosis of
A549 cells
Subsequently, it was investigated whether autophagy
resulted in cell death, or exerted protective effects following
cisplatin treatment in A549 cells. Knockdown of Atg5 by siRNA
transfection impaired cisplatin-induced Atg5 activation and
therefore inhibited the activation of autophagy, and promoted
caspase-3 cleavage (Fig. 4A).
Inhibition of autophagy through knockdown of Atg5 (Fig. 4B) also inhibited cell viability and
led to increased LDH release (Fig.
4C). Similarly, knockdown of Beclin 1 inhibited
cisplatin-induced Beclin 1 activation and autophagosome formation
(Fig. 5A). Decreased LC3B-I/II
conversion indicated that autophagosome formation was inhibited.
Beclin 1 knockdown markedly promoted cisplatin-induced caspase-3
cleavage (Fig. 5A), inhibited cell
viability (Fig. 5B) and led to
increased LDH release (Fig. 5C).
Therefore, knockdown of Atg5 and Beclin 1 by siRNA may impair the
cisplatin-induced activation of autophagy, promote caspase-3
cleavage and lead to increased cell death. Disruption of autophagy
through the knockdown of Atg5 and Beclin 1 may promote
cisplatin-induced apoptotic cell death in A549 cells.
Discussion
A limited number of patients with lung cancer
diagnosed at a metastatic stage survive >5 years.
Cisplatin-based combination chemotherapy is used to extend the
survival of patients with advanced lung cancer; however, the
majority of patients relapse within 1 year, primarily due to
acquired resistance (24,25). Therefore, increasing the
sensitivity of cancer cells to cisplatin is desired. Restoring
cisplatin-induced apoptosis may be an effective strategy for
overcoming chemotherapeutic resistance. The present study aimed to
elucidate the mechanism and function underlying cisplatin-induced
autophagy in A549 lung cancer cells, using genetic knockdown of
autophagy-associated genes to provide a potential target for
improvement of sensitivity to chemotherapeutic drugs.
Previous studies indicated reciprocal regulation
between autophagy and apoptosis in tumor cell survival following
chemotherapy (11,26). Modulation of autophagy may affect
apoptosis of cancer cells. The present study demonstrated that
cisplatin induced apoptosis and autophagy in human lung cancer
cells. Subsequently, it was further investigated whether inhibition
of autophagy would result in an increased rate of apoptosis.
A previous study demonstrated that upregulation of
autophagy contributes to cisplatin resistance in human lung cancer
cells (11). Inhibition of
autophagy, either by pharmacological inhibitors or by genetic
knockdown of autophagy-associated genes, may enhance
chemotherapy-induced cytotoxicity. In addition, inhibition of
autophagy may lead to the accumulation of reactive oxygen species
in cisplatin-treated lung cancer cells, and suppression of
autophagy may sensitize cells to cisplatin-induced
caspase-dependent and -independent apoptosis (27). Combination treatment using
autophagic inhibitors can potentiate the efficacy of epidermal
growth factor receptor-targeted cancer therapeutics (28). Furthermore, inhibition of autophagy
promotes paclitaxel-induced apoptosis (29). In all of these aforementioned
cases, autophagy was protective and prevented lung cancer cells
from undergoing apoptosis; however, if cellular damage is
extensive, or if apoptosis is compromised, autophagic cell death
may occur (14,15). Previous studies presented
conflicting results regarding the function of autophagy in cancer
chemotherapy; in certain studies autophagy promotes cell survival,
and in other, induces autophagic cell death (22,30).
The present study demonstrated that inhibition of autophagy
enhanced the cytotoxicity of cisplatin. The results of the present
study indicated that autophagy may serve a protective role and may
participate in cisplatin-acquired resistance. Cisplatin upregulated
Atg5 and Beclin 1 mRNA and protein expression; however, the
expression levels of other autophagy-associated genes were not
altered by cisplatin. Therefore, the present study further
investigated the role of Atg5 and Beclin 1 in cisplatin-induced
autophagy and apoptosis.
Apoptosis and autophagy interact via crosstalk
(31) and each may occur
simultaneously or sequentially (32,33).
Autophagy and apoptosis are distinct processes but an overlapping
mechanism may regulate both. Beclin 1, Atg5, Atg7 and high-mobility
group box 1 are involved in the regulation of apoptosis and
autophagy (17,34). Formation of the Atg12-Atg5
conjugate is necessary for autophagosome formation and autophagy
mediates cisplatin resistance under hypoxia through Atg5 (35). Tissue-specific knockdown of Atg5
markedly impairs mitochondrial energy homoeostasis and leads to
oxidative stress and constitutively active DNA damage (10). Inhibition of Atg5 sensitizes
resistant carcinoma cells to radiotherapy and certain
chemotherapeutic reagents (31,36,37).
However, Atg5 is not involved in gefitinib- or erlotinib-induced
autophagy (38). The present study
demonstrated that Atg5 is involved in cisplatin-induced autophagy,
and inhibition of autophagy by knocking down Atg5 significantly
enhanced cisplatin-induced apoptosis.
Beclin 1 initiates autophagy by forming a Beclin
1-phosphatidylinositol 3-kinase III/Vps34 complex. Previous studies
demonstrated that decreased expression of Beclin 1 is associated
with tumor progression in lung, colon and ovarian cancer (39–41),
and reduced Beclin 1 expression is a predictor of poor prognosis
for gastric and lung cancer (42,43).
The carcinogenic mechanism of Beclin 1 downregulation in cancer may
be associated with apoptosis. In a previous study, eliminating
Beclin 1 protein resulted in stimulation of the apoptotic pathway
in DNA-damaged breast cancer cells (44). Beclin 1 overexpression may also
contribute to inhibition of lung cancer cell growth, angiogenesis
and inhibition of apoptosis during radiotherapy (45). Beclin 1 plays a dual role in
tumorigenesis. In the present study, Beclin 1 knockdown inhibited
cisplatin-induced autophagy and promoted cisplatin-induced
apoptotic cell death. However, the function of Beclin 1
overexpression, whether protective or detrimental, is dependent on
the specific tumor type, stage and circumstances. The present study
demonstrated that knockdown of Beclin 1 promoted sensitivity of
lung cancer cells to chemotherapy and increased apoptotic cell
death.
Inhibition of autophagy promotes genomic
instability, interferes with cellular differentiation, perturbs
cellular metabolism, and prevents resistance to chemotherapy or
radiotherapy (46). The present
study demonstrated that inhibition of autophagy, through knockdown
of Atg5 and Beclin 1, may promote cisplatin-induced apoptotic cell
death. However, the expression and clinical application of
modulation of Atg5 and Beclin 1 in patients with lung cancer should
be further confirmed by large sample cohort studies. Furthermore,
in vivo studies investigating the functions and downstream
effectors of Atg5 and Beclin 1 are required, as are further studies
investigating the association between Atg5, Beclin 1 and genomic
instability, cellular differentiation, mitochondrial energy
homeostasis, and efficacy of chemotherapy and radiotherapy.
In conclusion, the present study demonstrated that
cisplatin can induce apoptosis and autophagy in human lung cancer
cells in vitro. Inhibition of autophagy via the knockdown of
Atg5 and Beclin 1 promoted cisplatin-induced apoptosis. The results
of the present study suggested that targeting autophagy-associated
pathways may be considered a therapeutic strategy to overcome
cisplatin resistance by inducing apoptosis. The present study
suggested a rationale for modulating autophagy to treat lung
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81401631).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JC and LZ conceived and designed the experiments;
HZ, WW and YL performed the experiments and contributed to
molecular analysis; HY and H-hY analyzed the data; LZ wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Awala H, Gilson JP, Retoux R, Boullay P,
Goupil JM, Valtchev V and Mintova S: Template-free nanosized
faujasite-type zeolites. Nat Mater. 14:447–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao S, Tortola L, Perlot T, Wirnsberger G,
Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic
V, et al: A dual role for autophagy in a murine model of lung
cancer. Nat Commun. 5:30562014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang
RG, Huang LL, Zhu F and Wu G: Acquired cisplatin resistance in
human lung adenocarcinoma cells is associated with enhanced
autophagy. Cancer Biother Radiopharm. 25:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimizu S, Yoshida T, Tsujioka M and
Arakawa S: Autophagic cell death and cancer. Int J Mol Sci.
15:3145–3153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng X and Kinsella TJ: Impact of
autophagy on chemotherapy and radiotherapy mediated tumor
cytotoxicity: ‘To Live or not to Live’. Front Oncol. 1:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang Y, Yan W, He X, Zhang L, Li C, Huang
H, Nace G, Geller DA, Lin J and Tsung A: miR-375 inhibits autophagy
and reduces viability of hepatocellular carcinoma cells under
hypoxic conditions. Gastroenterology. 143:177–187.e8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Cardinal JS, Pan P, Rosborough
BR, Chang Y, Yan W, Huang H, Billiar TR, Rosengart MR and Tsung A:
Splenocyte apoptosis and autophagy is mediated by interferon
regulatory factor 1 during murine endotoxemia. Shock. 37:511–517.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Cardinal JS, Bahar R, Evankovich
J, Huang H, Nace G, Billiar TR, Rosengart MR, Pan P and Tsung A:
Interferon regulatory factor-1 regulates the autophagic response in
LPS-stimulated macrophages through nitric oxide. Mol Med.
18:201–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitazumi I and Tsukahara M: Regulation of
DNA fragmentation: The role of caspases and phosphorylation. FEBS
J. 278:427–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu K, Dunner K Jr and McConkey DJ:
Proteasome inhibitors activate autophagy as a cytoprotective
response in human prostate cancer cells. Oncogene. 29:451–462.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Castria TB, da Silva EM, Gois AF and
Riera R: Cisplatin versus carboplatin in combination with
third-generation drugs for advanced non-small cell lung cancer.
Cochrane Database Syst Rev: CD009256. 2013. View Article : Google Scholar
|
|
26
|
Claerhout S, Verschooten L, Van Kelst S,
De Vos R, Proby C, Agostinis P and Garmyn M: Concomitant inhibition
of AKT and autophagy is required for efficient cisplatin-induced
apoptosis of metastatic skin carcinoma. Int J Cancer.
127:2790–2803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaminskyy VO, Piskunova T, Zborovskaya IB,
Tchevkina EM and Zhivotovsky B: Suppression of basal autophagy
reduces lung cancer cell proliferation and enhances
caspase-dependent and -independent apoptosis by stimulating ROS
formation. Autophagy. 8:1032–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou Y, Ling YH, Sironi J, Schwartz EL,
Perez-Soler R and Piperdi B: The autophagy inhibitor chloroquine
overcomes the innate resistance of wild-type EGFR non-small-cell
lung cancer cells to erlotinib. J Thorac Oncol. 8:693–702. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu F, Liu D, Yang Y and Zhao S: Effect of
autophagy inhibition on chemotherapy-induced apoptosis in A549 lung
cancer cells. Oncol Lett. 5:1261–1265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang YH, Wu YL, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species contribute to oridonin-induced
apoptosis and autophagy in human cervical carcinoma HeLa cells.
Acta Pharmacol Sin. 32:1266–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Viola G, Bortolozzi R, Hamel E, Moro S,
Brun P, Castagliuolo I, Ferlin MG and Basso G: MG-2477, a new
tubulin inhibitor, induces autophagy through inhibition of the
Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem
Pharmacol. 83:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Driscoll JJ and Chowdhury RD: Molecular
crosstalk between the proteasome, aggresomes and autophagy:
Translational potential and clinical implications. Cancer Lett.
325:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu HM, Jiang ZF, Ding PS, Shao LJ and Liu
RY: Hypoxia-induced autophagy mediates cisplatin resistance in lung
cancer cells. Sci Rep. 5:122912015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livesey KM, Tang D, Zeh HJ and Lotze MT:
Autophagy inhibition in combination cancer treatment. Curr Opin
Investig Drugs. 10:1269–1279. 2009.PubMed/NCBI
|
|
38
|
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J,
Ge W, Feng L, Lin X, Wang X, et al: EGFR tyrosine kinase inhibitors
activate autophagy as a cytoprotective response in human lung
cancer cells. PLoS One. 6:e186912011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang ZF, Shao LJ, Wang WM, Yan XB and Liu
RY: Decreased expression of Beclin-1 and LC3 in human lung cancer.
Mol Biol Rep. 39:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Z, Li Y, Zhang C, Yi H, Wu C, Wang J,
Liu Y, Tan J and Wen J: Downregulation of Beclin 1 and impairment
of autophagy in a small population of colorectal cancer. Dig Dis
Sci. 58:2887–2894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen Y, Li DD, Wang LL, Deng R and Zhu XF:
Decreased expression of autophagy-related proteins in malignant
epithelial ovarian cancer. Autophagy. 4:1067–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou WH, Tang F, Xu J, Wu X, Yang SB, Feng
ZY, Ding YG, Wan XB, Guan Z, Li HG, et al: Low expression of Beclin
1, associated with high Bcl-xL, predicts a malignant phenotype and
poor prognosis of gastric cancer. Autophagy. 8:389–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Du Z, Li L, Shi M and Yu Y: Beclin
1 and p62 expression in non-small cell lung cancer: Relation with
malignant behaviors and clinical outcome. Int J Clin Exp Pathol.
8:10644–10652. 2015.PubMed/NCBI
|
|
44
|
Scarlatti F, Maffei R, Beau I, Codogno P
and Ghidoni R: Role of non-canonical Beclin 1-independent autophagy
in cell death induced by resveratrol in human breast cancer cells.
Cell Death Differ. 15:1318–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang SH, Minai-Tehrani A, Shin JY, Park
S, Kim JE, Yu KN, Hong SH, Hong CM, Lee KH, Beck GR Jr and Cho MH:
Beclin1-induced autophagy abrogates radioresistance of lung cancer
cells by suppressing osteopontin. J Radiat Res. 53:422–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michaud M, Martins I, Sukkurwala AQ,
Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot
G, et al: Autophagy-dependent anticancer immune responses induced
by chemotherapeutic agents in mice. Science. 334:1573–1577. 2011.
View Article : Google Scholar : PubMed/NCBI
|