Introduction
Extracts from certain dietary foods or medical
plants may function as chemopreventive agents and may inhibit
tumorigenesis, including the initiation and promotion of several
types of human cancer (1,2). Butein
(3,4,2′,4′-tetrahydroxychalcone) is a bioactive polyphenol that is
isolated from a number of plants, including Semecarpus
anacardium, Dalbergia odorifera and Rhus
verniciflua Stokes (R. verniciflua)(3). In Korea, butein is used as a food
additive (3). Butein has been
shown to demonstrate multiple biological functions, including
anti-inflammatory, antioxidative and antimicrobial functions
(4,5). Butein abolishes the effects of
lipopolysaccharide-induced inflammation through the inhibition of
nuclear factor κB (NFκB) activities and c-jun N-terminal kinase
(JNK)-dependent pathways (5).
Butein has also been shown to exert free radical scavenging
activities and suppress H2O2-induced
cytotoxicity in RAW264.7 macrophage cell lines (4). Additionally, butein decreases phorbol
ester-induced skin cancer formation (6), ameliorates renal concentration
capacity in cisplatin-induced renal failure (7), attenuates diabetic complications
(8) and facilitates recovery in
carbon tetrachloride-induced liver fibrosis (9).
Previous reports have focused on the
anti-proliferative and anti-metastatic effects of butein. Jang
et al showed that flavonoids isolated from R.
verniciflua inhibited proliferation and triggered apoptosis in
human osteosarcoma cells (10).
These compounds, including butein, enhance p53 and Bax expression,
decrease Bcl2 levels and subsequently induce apoptosis in
osteosarcoma cells (11). Butein
inhibits the colony formation of UACC-812 human breast cancer cells
when it is co-cultured with fibroblast cells (11). Butein-treated colon adenocarcinoma
and HeLa cells showed a significant reduction in cell proliferation
(12,13). Iwashita et al showed that
butein also triggered melanoma cells to undergo apoptosis, as
evidenced by DNA condensation, DNA fragmentation and an increased
frequency of hypodiploid cells; the authors also demonstrated that
increased Bax and decreased Bcl-xL levels contribute to this
butein-induced apoptosis (14).
The treatment of U937 human leukemia cells with sublethal
concentrations of butein has been shown to sensitize the cells to
tumor necrosis factor-(TNF)-related apoptosis-inducing ligand
(TRAIL)-induced apoptosis through increasing caspase 3-dependent
pathways (14). Moreover, butein
suppresses the signal transduction and activation of transcription
3 (STAT3) activity and reduces STAT3 target gene expression in
multiple myeloma cells and human hepatocarcinoma cells (15,16).
Treatment with butein was shown to induce G2/M arrest,
by enhancing ataxia telangiectasia mutated (ATM), Chk1 and Chk2
activities in hepatoma cells (15). Butein has also been found to
inhibit the invasion and angiogenesis of prostate cancer through
the downregulation of matrix metalloproteinase (MMP)-9 and vascular
endothelial growth factor expression (17).
Due to the high prevalence and increasing drug
resistance of breast cancer, this disease has become the leading
cause of cancer-related mortality in women. Studies focusing on
natural compounds for the treatment of breast cancer have begun to
emerge (18). A polyphenol-rich
fraction purified from R. verniciflua containing fesetin,
sulfuretin and butein, has demonstrated anti-proliferative effects
both in gastric and breast cancer (19). Butein also diminishes the
testosterone-induced cell proliferation of breast cancer cells by
reducing aromatase activity (19).
Chua et al demonstrated that butein suppresses the migration
and invasion of breast cancer through the inhibition of NFκB
activity and subsequent decrease in CXC chemokine receptor 4
(CXCR4) expression (20).
Recently, butein has been shown to block phorbol 12-myristate
13-acetate (PMA)-elevated cyclooxygenase 2 (COX2) expression by
inhibiting extracellular signal-regulated kinase (ERK) activation
in cancerous and non-cancerous breast cells (21). However, the effects of butein on
the growth and proliferation of breast cancer cells remain unclear.
In this study, the molecular mechanisms of the effects of butein on
breast cancer cell proliferation are delineated for the first
time.
Materials and methods
Materials
All chemicals that were used, including butein,
isopropanol, dimethylsulfoxide (DMSO) and propidium iodine, were
purchased from Sigma Chemical Company (St. Louis, MA, USA). The
phospho-p38 antibody was purchased from Cell Signaling Technology
(Beverly, MA, USA). Antibodies against p38, phospho-ERK and ERK
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Anti-β actin and horseradish peroxidase (HRP)-conjugated secondary
antibodies were obtained from Sigma Chemical Company. Dulbecco’s
modified Eagle’s medium (DMEM), fetal bovine serum and
penicillin-streptomycin mixture were obtained from Gibco Laboratory
(Gaithersburg, MO, USA).
Cell culture
The human breast cancer cell line MDA-MB-231 was
maintained in DMEM supplemented with 10% fetal bovine serum, 100
units/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2.
3-(4,5 Dimethylthiazol-2-yl)-2,5
diphenyltetrazolium bromide (MTT) assay
Cells were seeded in 24-well plates at a density of
4×104 cells/ml and were treated with the indicated
concentrations of butein for 24 or 48 h. After removing the
supernatant, the cells were incubated with fresh medium containing
5.0 mg/l MTT at 37°C for an additional 3 h. After washing with
phosphate-buffered saline (PBS), the purple-blue formazan was
dissolved in 1 ml of isopropanol, and the absorbance was measured
at 563 nm.
Reactive oxygen species (ROS)
analysis
Cells treated with the indicated concentrations of
butein, with or without pre-treatment with 1 mM N-acetyl cysteine
(NAC) for 1 h, were loaded with 5 μM fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular
Probes Inc., Eugene, OR, USA) at 37°C for 1 h. The fluorescence
intensity was analyzed on BD biosciences FACscan system using
CellQuest™ Pro software.
Western blot analysis
MDA-MB-231 cells were treated with the indicated
concentrations of butein for 48 h and then cell lysate extraction
was performed. Protein concentration was detected by using a
Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA,
USA). Protein (20 μg) was separated by a 10% polyacrylamide gel and
electrotransferred to a nitrocellulose membrane. The membrane was
blocked by PBS containing 0.5% non-fat milk for 1 h at room
temperature. After being washed with PBS containing 0.1% Tween-20
(PBST), the membrane was probed with primary antibodies at 4°C
overnight. The following day, the membrane was washed with PBST and
then incubated with HRP-conjugated goat anti-mouse IgG antibody
(Santa Cruz Biotechnology; 1:5,000 dilution) at room temperature
for 1 h. The membrane was extensively washed with PBS, and the
reactive signal was detected using an enhanced chemiluminescence
kit (Amersham Pharmacia Biotech, UK). β-actin expression was used
as the loading control.
Statistical analysis
Reported data are the means ± standard deviation
(SD) of 3 independent experiments and were evaluated by the
Student’s t-test with SPSS. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Butein inhibits cell proliferation in
breast cancer cells
Previous reports have shown that butein is a potent
anti-proliferative agent for several types of cancer. The present
study investigated the cytotoxic effects of butein on the breast
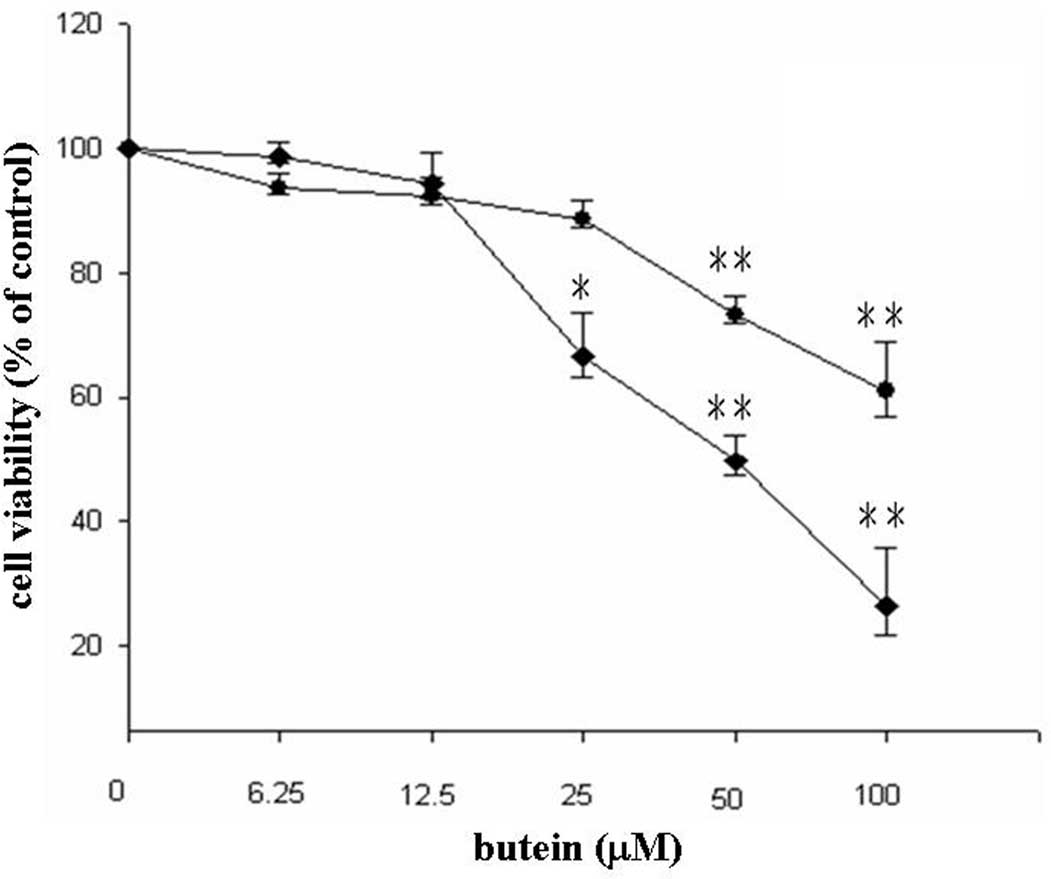
cancer cell line, MDA-MB-231. Low doses (6.25 and 12.5 μM) of
butein did not affect cell viability, whereas treatment with 25, 50
and 100 μM butein reduced cell viability to 88, 73 and 61% after 24
h and to 66, 49 and 26% after 48 h, respectively (Fig. 1). In addition, a significantly
increased sub-G1 population was found in the 50 and 100
μM butein-treated groups (Table
I). Taken together, our results suggest that butein induces
apoptosis in breast cancer cells.
 | Table ICell cycle distribution of butein-
and/or NAC-treated MDA-MB-231 cells. |
Table I
Cell cycle distribution of butein-
and/or NAC-treated MDA-MB-231 cells.
| Treatment
group | Sub-G1
(%) |
G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Control | 0.38±0.12 | 48.93±3.75 | 9.43±0.73 | 30.59±7.79 |
| Butein 6.25 μM | 0.35±0.1 | 47.93±5.38 | 9.57±1.68 | 32.47±5.69 |
| Butein 12.5 μM | 0.39±0.2 | 49.42±7.84 | 9.32±1.13 | 30.39±6.73 |
| Butein 25 μM | 0.67±0.37 | 47.33±8.78 | 9.54±1.58 | 44.08±7.04 |
| Butein 50 μM | 3.71±0.6a | 45.69±6.08 | 16.26±1.77 | 24.73±1.69 |
| Butein 100 μM | 11.22±2.62a | 50.42±11.44 | 8.53±4.19 | 24.01±2.71 |
| NAC alone | 0.51±0.34 | 57.82±7.45 | 9.56±1.23 | 16.64±4.39 |
| Butien 50 μM +
NAC | 1.57±0.33b | 51.77±8.82 | 11.43±1.81 | 24.16±1.98 |
| Butien 100 μM +
NAC | 3.61±0.79b | 52.09±4.82 | 11.63±3.37 | 24.01±2.71 |
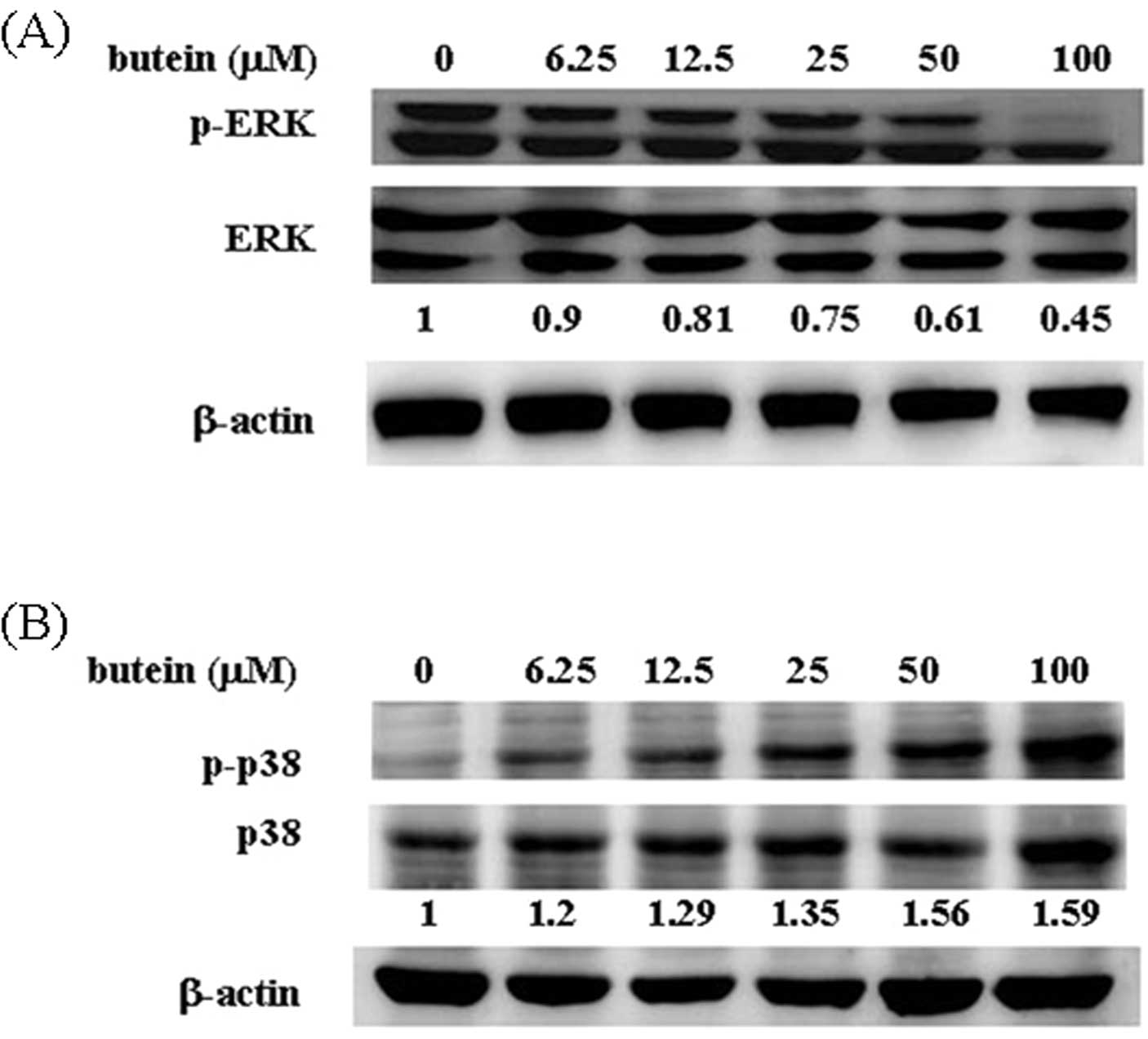
Butein modulates ERK and p38 activity in
breast cancer cells
In general, ERK is involved in cell proliferation,
whereas JNK and p38 participate in stress-induced apoptosis
(22). To detect whether MAPK
family proteins are involved in butein-induced apoptosis, the
phosphorylation of ERK and p38 was measured by western blot
analysis. As shown in Fig. 2A, ERK
phosphorylation was significantly decreased in the butein-treated
cells (90% at 6.25 μM, 80% at 12.5 μM, 75% at 25 μM, 61% at 50 μM
and 45% at 100 μM, compared with 100% in the control group). By
contrast, 100 μM butein increased the phosphorylation of p38 up to
1.59-fold compared with the vehicle-treated group (Fig. 2B). No overt alteration in JNK
phosphorylation was observed in the presence of butein (data not
shown).
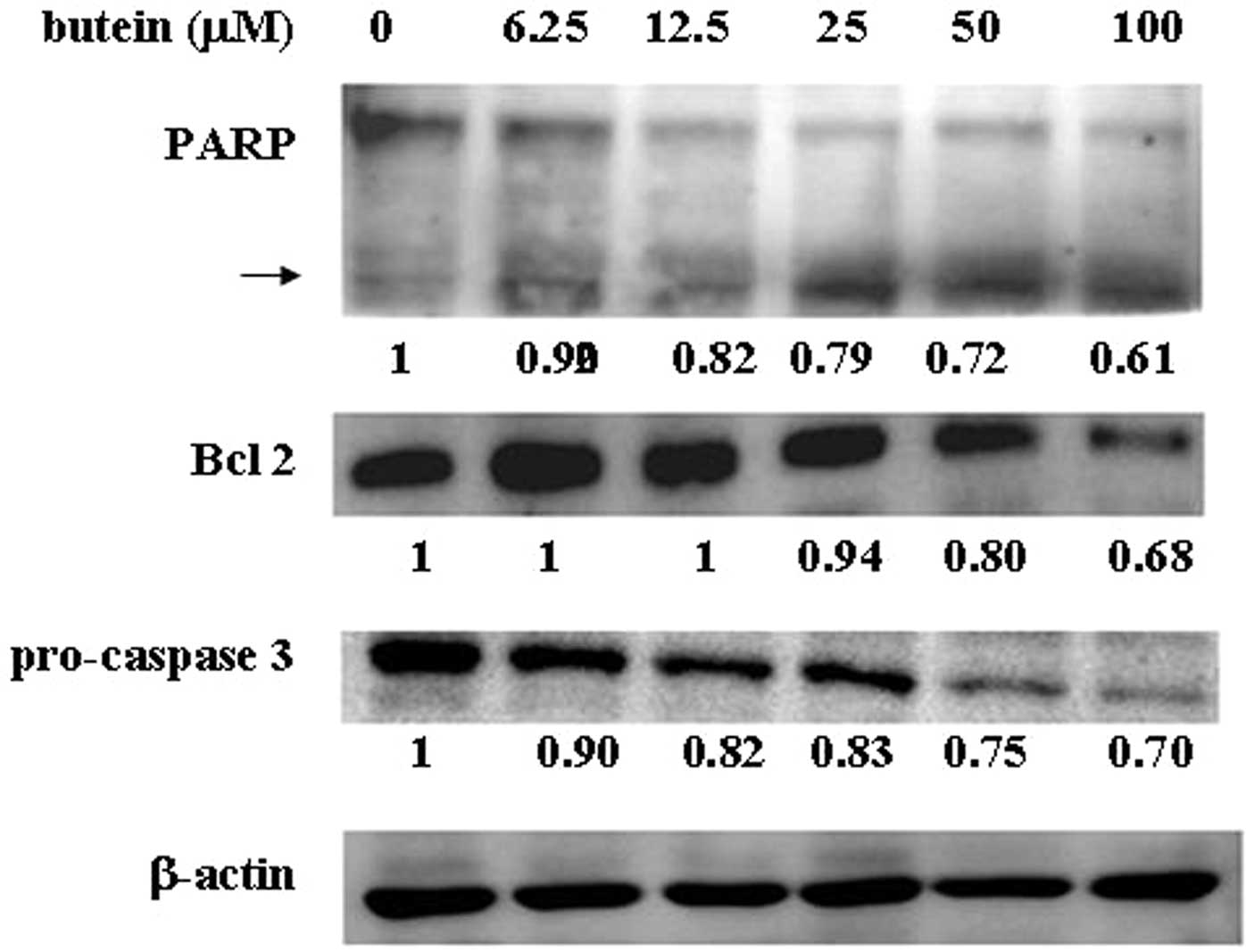
Effects of butein on apoptosis-related
protein expression
In order to determine whether butein affects
apoptosis-related protein expression, cell lysates derived from
butein-treated MB-MDA-231 cells were harvested, and the expression
of Bcl-2, caspase 3 and poly-(ADP-ribose)-polymerase (PARP) were
analyzed by western blot analysis. The expression of the
anti-apoptotic protein, Bcl-2, was dramatically decreased in a
dose-dependent manner based on the butein concentration. In
addition, butein also reduced pro-caspase 3 expression and
increased cleavage of PARP in a dose-dependent manner (Fig. 3). However, no significant
alteration in Bax expression was found in the presence of butein
(data not shown).
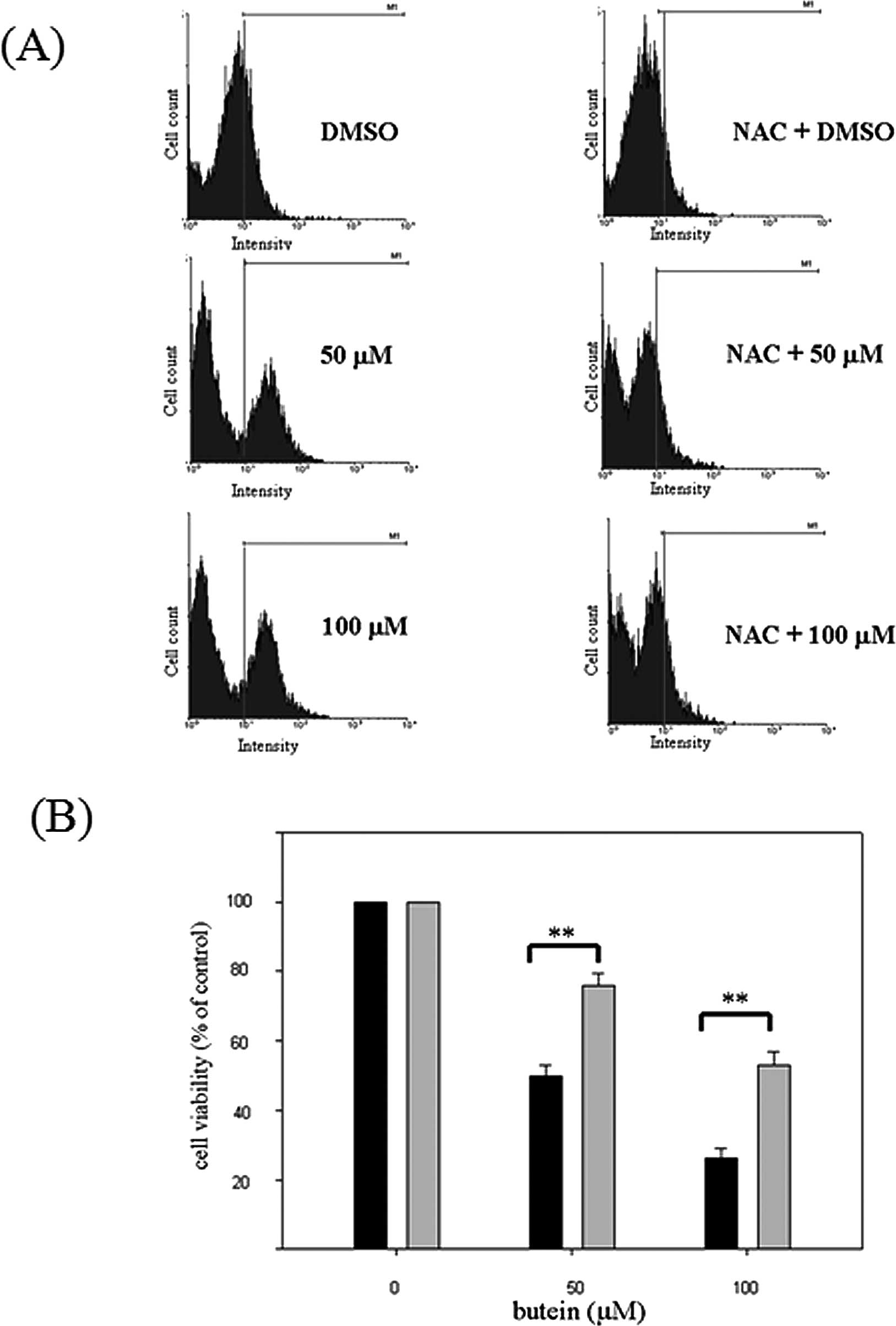
Butein triggers ROS generation
To examine whether butein triggers ROS production,
butein-treated MB-MDA-231 cells were stained with
2′-7′-dichlorofluorescin diacetate fluorescent dye and were
analyzed by flow cytometry. As shown in Fig. 4A, treatment with 50 and 100 μM
butein significantly increased ROS generation. Pre-treatment with
NAC significantly decreased ROS production.
Pre-treatment with the antioxidant agent,
NAC, prevents butein-induced protein expression and cell death
To determine whether the butein-induced apoptosis is
mediated by ROS generation, the cells were pre-treated with 1 mM
NAC and were subsequently co-treated with the indicated
concentrations of butein for an additional 48 h. Cell viability was
measured by MTT assay, and the apoptotic population was detected by
flow cytometry. Pre-treatment with NAC markedly increased the
viability of butein-treated cells as shown by MTT assays (Fig. 4B). Similarly, NAC pre-treatment
decreased the sub-G1 population from 3.71–1.57 and 11.22–3.61%, in
response to 50 and 100 μM butein treatment, respectively (Table I). Moreover, to address whether the
effects of butein on apoptosis-related protein expression are
affected by the abrogation of ROS generation, the phosphorylation
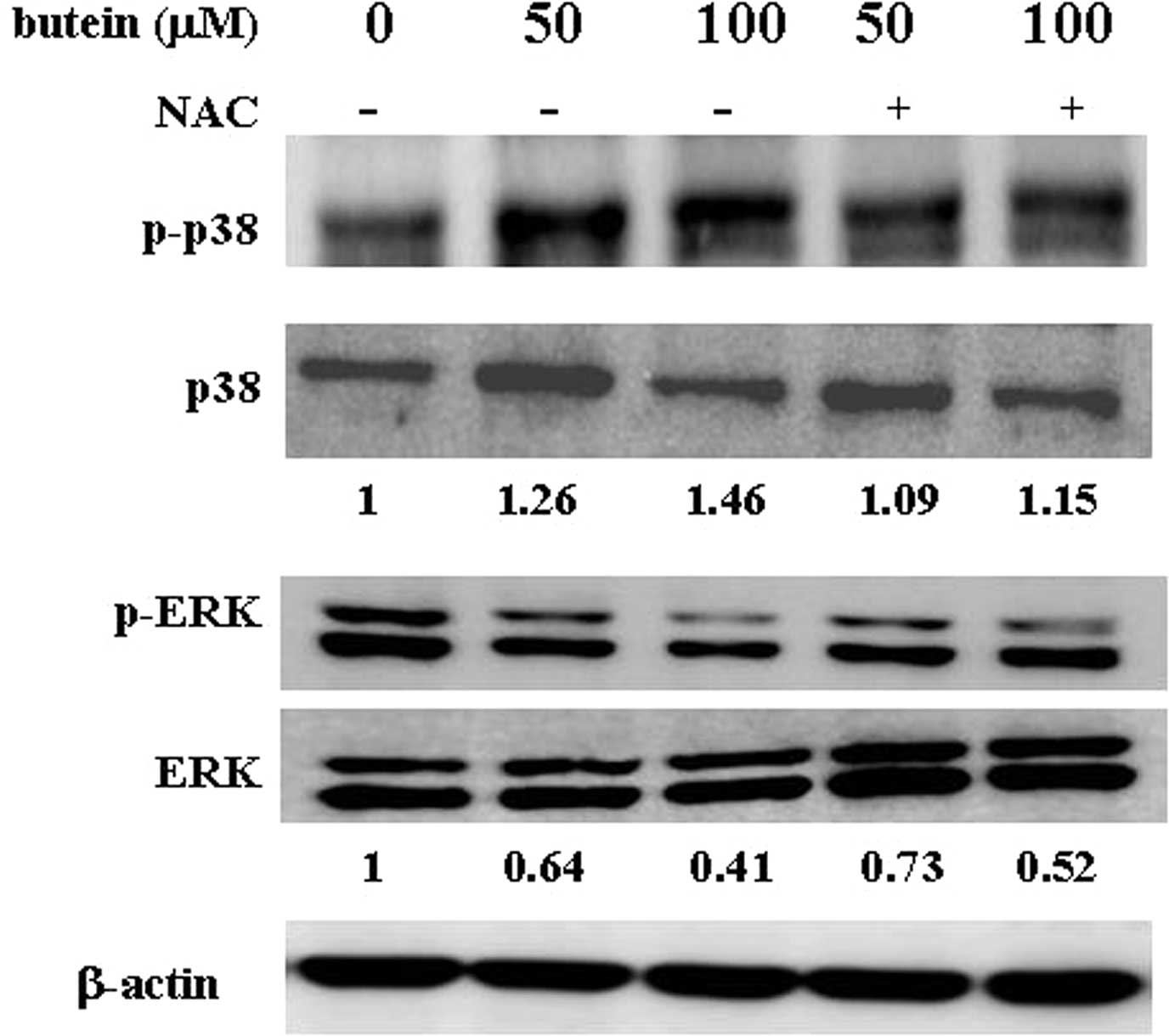
of ERK and p38 was measured in NAC-pre-treated cells. Notably, as
shown in Fig. 5, pre-treatment
with NAC significantly blocked the butein-induced phosphorylation
of p38. In addition, ERK phosphorylation was upregulated in the
presence of NAC. Our findings suggest that the generation of ROS,
which may modulate ERK and p38 activities, plays an important role
in butein-induced apoptosis.
Discussion
Flavonoids, which are compounds found in fruits and
vegetables, have received a great deal of attention for their
application as chemopreventive and chemotherapeutic agents
(23,24). Butein, one of the major
constituents of R. verniciflua, has been shown to exert a
wide range of biological functions. Butein has been demonstrated to
inhibit the proliferation of several human cancer cell lines,
including B16 melanoma 4A5 cells (14), lymphoma (3), breast carcinoma (19) and osteosarcoma cells (10). In this study, the molecular
mechanisms underlying the role of butein in the cell proliferation
of breast cancer cells were delineated for the first time. Our data
demonstrate that butein reduces cell viability in a dose- and
time-dependent manner. Butein induced cell apoptosis, as evidenced
by an increase in the sub-G1 cell population. Treatment
with butein elevated ROS generation, enhanced the proteolytic
activity of caspase 3, decreased the expression of Bcl-2 protein,
decreased the phosphorylation of ERK and stimulated p38
phosphorylation. Pre-treatment with the antioxidant, NAC,
significantly abrogated butein-induced apoptosis.
Mitogen-activated protein kinase (MAPK) family
proteins have been shown to regulate numerous cellular functions,
such as cell proliferation, cell growth and apoptosis, in response
to different extracellular stimuli (22,25).
In general, ERK-mediated growth factors enhance cell proliferation,
whereas JNK and p38 kinases transduce signals from stress and
inflammation to promote apoptosis (26). Flavonoid-triggered cancer cells
undergo apoptosis through the modulation of MAPK protein kinases
(27–29). Epigallocatechin-3-gallate (EGCG)
inhibits ERK activation and increases p38 kinases and JNK activity,
which subsequently enhances apoptosis in pancreatic cancer cells
(30). Treatment with quercetin
significantly reduces the phosphorylation of ERK and AKT, which is
accompanied by decreased cell viability in glioma and HepG2 cells
(31,32). In bladder cancer cells, treatment
with butein decreases the phosphorylation of ERK in a
time-dependent manner (33). Lau
et al showed that butein attenuated COX2 expression induced
by PMA, via the inhibition of ERK activities (21). In our study, treatment with butein
significantly abrogated ERK activities in MDA-MB-231 breast cancer
cells, consistent with previous observations. Unlike other
flavonoids that induce p38 activation, Lee et al showed that
treatment with butein significantly diminished TNF-α-mediated MMP-7
and interleukin 8 production by decreasing p38 activity in HT-29
cells (34). However, our findings
provide the first evidence that treatment with butein significantly
elevates p38 activity in a dose-dependent manner. Taken together,
our results indicate that treatment with butein attenuates survival
signals (ERK) and elevates death signals (p38), which leads to
apoptosis in breast cancer cells.
The Bcl-2 family proteins have both anti- and
pro-apoptotic functions. The ratio of pro-apoptotic (Bax) to
anti-apoptotic (Bcl-2) proteins determines whether a cell lives or
dies. An increased Bax/Bcl-2 ratio triggers apoptosis by releasing
cytochrome c from mitochondria, which in turn activates caspase 3
(35). As proof of principle,
butein induces apoptosis in HL60 leukemia cells through diminished
Bcl-2 and elevated Bax expression, which results in stimulated
caspase 3 activity (36).
Similarly, crude extracts of R. verniciflua, rich in butein,
fustin and fisetin have demonstrated apoptotic effects on human
osteosarcoma cells through the inhibition of Bcl-2 expression and
the activation of Bax expression (10). In the present study, decreased
Bcl-2 and pro-caspase 3 levels, accompanied by increased PARP
cleavage, were found in butein-treated breast cancer cells.
The production of ROS, which damages DNA, proteins
and lipids, has been associated with a number of human diseases,
such as atherosclerosis and cancer (37,38).
Increased ROS concentrations help facilitate the chemotherapeutic
effects of flavonoids. Apigenin triggers prostate cancer cells to
undergo apoptosis through the generation of ROS and the activation
of the p53 pathway (40).
Similarly, kaempferol and catechins also induce apoptosis in
glioblastoma and malignant B cells, respectively, via the
production of ROS (40,41). Emerging reports have demonstrated
that elevated ROS levels trigger signal transduction pathways
involved in apoptosis. In hepatoma cells, butein triggers ROS
generation, modulates ATM, Chk1 and Chk2 activities, and
subsequently causes cell cycle arrest in the G2/M phase
(42). Very recently, it has also
been shown that butein elevates ROS levels and subsequently
triggers apoptosis in neuroblastoma cells (43). Pre-treatment with antioxidants,
such as NAC or glutathione, abrogates the effects of butein
(41). In our study, concurrent
with a previous report, treatment with 50 and 100 μM butein
markedly induced ROS generation in MDA-MB-231 breast cancer cells.
Pre-treatment with the antioxidant, NAC, counteracted the effects
of butein on cell viability and ROS generation. However,
G2/M phase arrest was not observed in butein-treated
breast cancer cells. Our data reveal that butein triggers
apoptosis, but not cell cycle arrest in breast cancer cells, via
the generation of ROS.
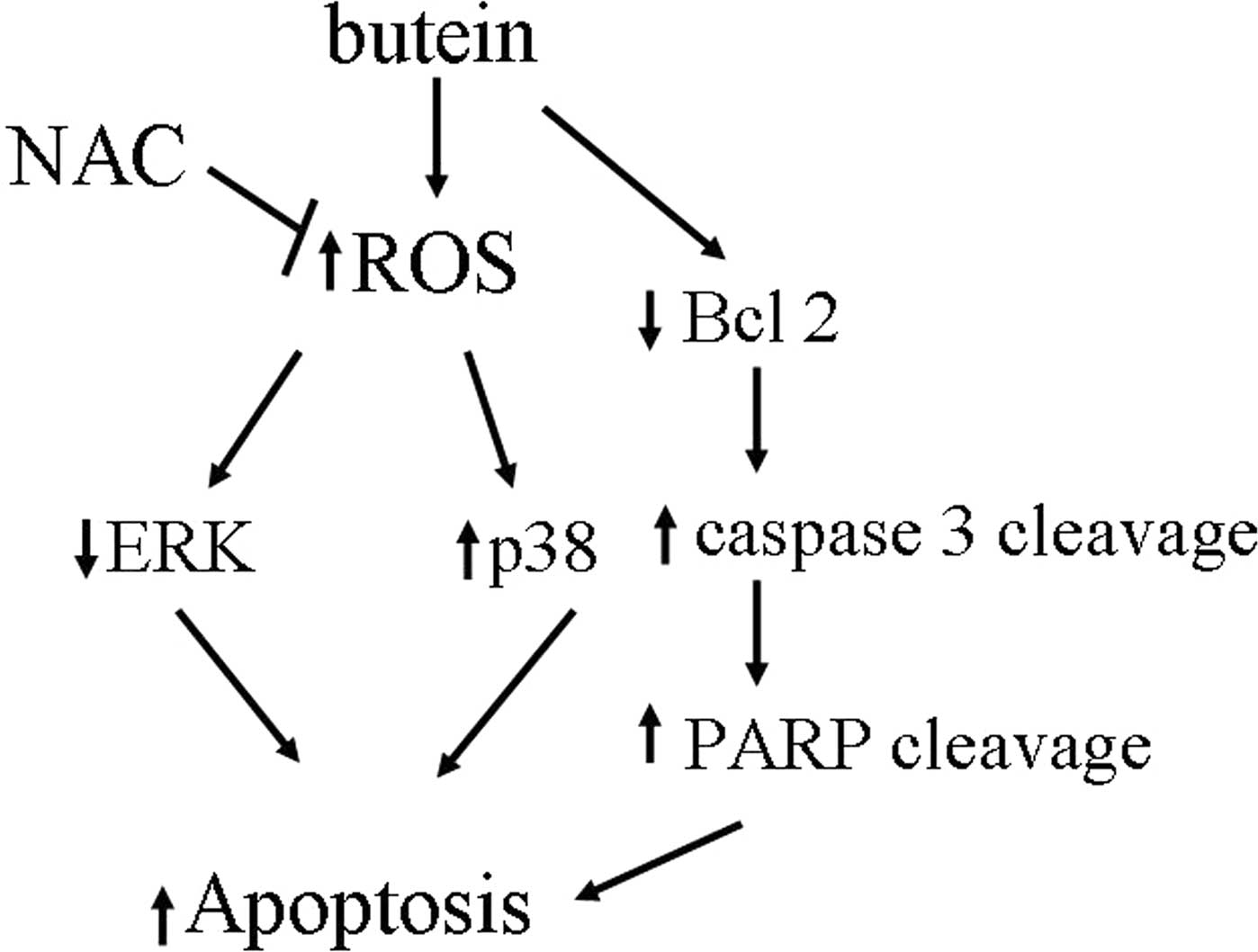
In conclusion, our results, to our knowledge,
provide the first evidence that butein triggers apoptosis in breast
cancer cells via multiple mechanisms and the generation of ROS,
inhibition of ERK, activation of p38, decreased Bcl-2 expression
levels and induced cleavage of caspase 3 and PARP, whereas
pre-treatment with the antioxidant, NAC, prevents these
butein-induced effects (Fig. 6).
In conclusion, our results suggest that butein has
anti-proliferative effects and induces apoptosis in breast cancer
cells.
Acknowledgements
The present study was supported by grants from the
National Science Council of Taiwan (NSC-94-2311-B-040-002 and
NSC-93-2311-B-040-009) and from the Chung Shan Medical University
(98-CCH-CSMU-05). The authors thank the Instrument Center of the
Chung Shan Medical University, supported by the National Science
Council of the Ministry of Education and the Chung Shan Medical
University for providing the equipment.
References
|
1
|
Kelloff GJ, Boone CW, Crowell JA, Steele
VE, Lubet R and Sigman CC: Chemopreventive drug development:
perspectives and progress. Cancer Epidemiol Biomarkers Prev.
3:85–98. 1994.PubMed/NCBI
|
|
2
|
Khan N, Afaq F and Mukhtar H: Cancer
chemoprevention through dietary antioxidants: progress and promise.
Antioxid Redox Signal. 10:475–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JC, Lee KY, Kim J, Na CS, et al:
Extract from Rhus verniciflua Stokes is capable of
inhibiting the growth of human lymphoma cells. Food Chem Toxicol.
42:1383–1388. 2004.
|
|
4
|
Jung CH, Jun CY, Lee S, Park CH, Cho K and
Ko SG: Rhus verniciflua stokes extract: radical scavenging
activities and protective effects on
H2O2-induced cytotoxicity in macrophage RAW
264.7 cell lines. Biol Pharm Bull. 29:1603–1607. 2006. View Article : Google Scholar
|
|
5
|
Jung CH, Kim JH, Hong MH, et al:
Phenolic-rich fraction from Rhus verniciflua Stokes (RVS)
suppress inflammatory response via NF-kappaB and JNK pathway in
lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol.
110:490–497. 2007.
|
|
6
|
Aizu E, Nakadate T, Yamamoto S and Kato R:
Inhibition of 12-O-tetradecanoylphorbol-13-acetate-mediated
epidermal ornithine decarboxylase induction and skin tumor
promotion by new lipoxygenase inhibitors lacking protein kinase C
inhibitory effects. Carcinogenesis. 7:1809–1812. 1986. View Article : Google Scholar
|
|
7
|
Kang DG, Lee AS, Mun YJ, et al: Butein
ameliorates renal concentrating ability in cisplatin-induced acute
renal failure in rats. Biol Pharm Bull. 27:366–370. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim SS, Jung SH, Ji J, Shin KH and Keum
SR: Synthesis of flavonoids and their effects on aldose reductase
and sorbitol accumulation in streptozotocin-induced diabetic rat
tissues. J Pharm Pharmacol. 53:653–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Nan JX, Zhao YZ, et al: The
chalcone butein from Rhus verniciflua shows antifibrogenic
activity. Planta Med. 69:990–994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang HS, Kook SH, Son YO, et al:
Flavonoids purified from Rhus verniciflua Stokes actively
inhibit cell growth and induce apoptosis in human osteosarcoma
cells. Biochim Biophys Acta. 1726:309–316. 2005.PubMed/NCBI
|
|
11
|
Kook SH, Son YO, Chung SW, et al:
Caspase-independent death of human osteosarcoma cells by flavonoids
is driven by p53-mediated mitochondrial stress and nuclear
translocation of AIF and endonuclease G. Apoptosis. 12:1289–1298.
2007. View Article : Google Scholar
|
|
12
|
Ramanathan R, Tan CH and Das NP: Cytotoxic
effect of plant polyphenols and fat-soluble vitamins on malignant
human cultured cells. Cancer Lett. 62:217–224. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yit CC and Das NP: Cytotoxic effect of
butein on human colon adenocarcinoma cell proliferation. Cancer
Lett. 82:65–72. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwashita K, Kobori M, Yamaki K and
Tsushida T: Flavonoids inhibit cell growth and induce apoptosis in
B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 64:1813–1820.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pandey MK, Sung B, Ahn KS and Aggarwal BB:
Butein suppresses constitutive and inducible signal transducer and
activator of transcription (STAT) 3 activation and STAT3-regulated
gene products through the induction of a protein tyrosine
phosphatase SHP-1. Mol Pharmacol. 75:525–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajendran P, Ong TH, Chen L, et al:
Suppression of signal transducer and activator of transcription 3
activation by butein inhibits growth of human hepatocellular
carcinoma in vivo. Clin Cancer Res. 17:1425–1439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon DO, Choi YH, Moon SK, Kim WJ and Kim
GY: Butein suppresses the expression of nuclear factor-kappa
B-mediated matrix metalloproteinase-9 and vascular endothelial
growth factor in prostate cancer cells. Toxicol In Vitro.
24:1927–1934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang CS, Yang GY, Landau JM, Kim S and
Liao J: Tea and tea polyphenols inhibit cell hyperproliferation,
lung tumorigenesis, and tumor progression. Exp Lung Res.
24:629–639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Jung CH, Jang BH, et al: Selective
cytotoxic effects on human cancer cell lines of phenolic-rich
ethyl-acetate fraction from Rhus verniciflua Stokes. Am J
Chin Med. 37:609–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chua AW, Hay HS, Rajendran P, et al:
Butein downregulates chemokine receptor CXCR4 expression and
function through suppression of NF-kappaB activation in breast and
pancreatic tumor cells. Biochem Pharmacol. 80:1553–1562. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lau GT, Huang H, Lin SM and Leung LK:
Butein downregulates phorbol 12-myristate 13-acetate-induced COX-2
transcriptional activity in cancerous and non-cancerous breast
cells. Eur J Pharmacol. 648:24–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shankar S, Ganapathy S and Srivastava RK:
Green tea polyphenols: biology and therapeutic implications in
cancer. Front Biosci. 12:4881–4899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoner GD and Mukhtar H: Polyphenols as
cancer chemopreventive agents. J Cell Biochem Suppl. 22:169–180.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar
|
|
28
|
Fresco P, Borges F, Diniz C and Marques
MP: New insights on the anticancer properties of dietary
polyphenols. Med Res Rev. 26:747–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarkar FH, Li Y, Wang Z and Kong D:
Cellular signaling perturbation by natural products. Cell Signal.
21:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shankar S, Suthakar G and Srivastava RK:
Epigallocatechin-3-gallate inhibits cell cycle and induces
apoptosis in pancreatic cancer. Front Biosci. 12:5039–5051. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Granado-Serrano AB, Martin MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
32
|
Kim EJ, Choi CH, Park JY, Kang SK and Kim
YK: Underlying mechanism of quercetin-induced cell death in human
glioma cells. Neurochem Res. 33:971–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Chen W and Li X: A novel
anticancer effect of butein: inhibition of invasion through the
ERK1/2 and NF-kappa B signaling pathways in bladder cancer cells.
FEBS Lett. 582:1821–1828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SH, Seo GS, Jin XY, Ko G and Sohn DH:
Butein blocks tumor necrosis factor alpha-induced interleukin 8 and
matrix metalloproteinase 7 production by inhibiting p38 kinase and
osteopontin mediated signaling events in HT-29 cells. Life Sci.
81:1535–1543. 2007. View Article : Google Scholar
|
|
35
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim NY, Pae HO, Oh GS, et al: Butein, a
plant polyphenol, induces apoptosis concomitant with increased
caspase-3 activity, decreased Bcl-2 expression and increased Bax
expression in HL-60 cells. Pharmacol Toxicol. 88:261–266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bechtel W and Bauer G: Modulation of
intercellular ROS signaling of human tumor cells. Anticancer Res.
29:4559–4570. 2009.PubMed/NCBI
|
|
38
|
Victor VM, Rocha M, Sola E, Banuls C,
Garcia-Malpartida K and Hernandez-Mijares A: Oxidative stress,
endothelial dysfunction and atherosclerosis. Curr Pharm Des.
15:2988–3002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shukla S and Gupta S: Apigenin-induced
prostate cancer cell death is initiated by reactive oxygen species
and p53 activation. Free Radic Biol Med. 44:1833–1845. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakazato T, Ito K, Ikeda Y and Kizaki M:
Green tea component, catechin, induces apoptosis of human malignant
B cells via production of reactive oxygen species. Clin Cancer Res.
11:6040–6049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma V, Joseph C, Ghosh S, Agarwal A,
Mishra MK and Sen E: Kaempferol induces apoptosis in glioblastoma
cells through oxidative stress. Mol Cancer Ther. 6:2544–2553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moon DO, Kim MO, Choi YH, Hyun JW, Chang
WY and Kim GY: Butein induces G(2)/M phase arrest and apoptosis in
human hepatoma cancer cells through ROS generation. Cancer Lett.
288:204–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen YH, Yeh CW, Lo HC, Su SL, Hseu YC and
Hsu LS: Generation of reactive oxygen species mediates
butein-induced apoptosis in neuroblastoma cells. Oncol Rep.
27:1233–1237. 2012.PubMed/NCBI
|