Introduction
The formation of distant metastasis is the main
cause of morbidity and mortality in patients with cancer (1). Once a solid tumor spreads to other
tissues and organs, curative intervention with currently available
cancer drugs, surgical operation and radiotherapy are ineffective.
The principal characteristic of prostate cancer (PCa) is its
inclination to metastasize to the bones, which occurs in as many as
90% of the patients with advanced PCa (2). It appears that the bone
microenvironment has a pivotal role in this process (3). Intratumoral hypoxia is a common
pathophysiologic feature of solid tumors, from the smallest tumor
of a few millimeters in diameter to the largest tumor (4). The hypoxic tumor environment results
in aggressive and metastatic cancer phenotypes that are associated
with resistance to radiation therapy, chemotherapy and a poor
treatment outcome (5,6). Hypoxia-inducible factor-1 (HIF-1) is
the key factor in this process, regulating transcription of more
than 70 hypoxia-activated genes, that range in function from those
that promote anaerobic metabolism to those that initiate tumor
metastasis (7). HIF-1 is a
heterodimeric transcription factor composed of HIF-1α and
constitutively expressed HIF-1β subunits. Its biological activity
depends on the amount of HIF-1α, which is tightly regulated by
oxygen tension (8). Overexpression
of HIF-1α has been demonstrated in human cancers as compared with
the respective normal tissues, making it a potential therapeutic
target in oncologic drug discovery (9). Inhibition of HIF-1α or its genetic
disruption can not only block tumor cell growth, but also inhibit
tumor metastasis, so that these tumor cells would be unable to
respond to the hypoxic stimulus and progress (10,11).
Emerging evidence indicates that CSCs may be the
critical drivers of tumor progression and metastasis (12,13).
Somatic tumors, including PCa, contain a small subset of stem-like
cells, called cancer stem cells, with capacities for self-renewal,
differentiation and initiation of new tumors. Cancer stem cells
preferentially reside in specific hypoxic microenvironment-niches,
often existing inside tumors (14,15).
Meanwhile, stem-like cells are present in several established PCa
cell lines, such as the PC-3 cell line (16) and under hypoxia possess greater
stem-like properties (17).
Hypoxia, a negative prognostic factor for successful
treatment, is a potent driver of a multitude of molecular signal
pathways that allows cells to survive and thrive in the hostile
tumor microenvironment and induces epithelial-mesenchymal
transition (EMT) (18). EMT can be
activated by hypoxia-induced HIF-1 signaling (19), followed by a cellular switch from
epithelial to mesenchymal properties (20). EMT plays a key role in tumor cell
metastasis (21) and has also been
identified as an important step in bone metastasis of PCa (22,23).
Hypoxia also provides tumor cells with cues for maintenance of a
stem-like state and may help driving the linkage between EMT and
CSCs (18).
Pristimerin is a quinonemethide triterpenoid with
the potential of a promising anticancer agent and induces apoptosis
in PCa cells (24), yet its
activity in PCa under hypoxia has not been investigated. In light
of these findings, we hypothesized that pristimerin affects the
hypoxia-induced HIF-1α expression and impair hypoxia-stimulated
metastasis by inhibition of stem cell characteristics and EMT of
PC-3 cells. To test this hypothesis, we first identified the
anticancer efficacy of pristimerin in PC-3 cells under a hypoxic
environment. Then, we evaluated the inhibitory effect of
pristimerin on hypoxia-induced invasiveness of cancer cells.
Furthermore, we investigated the reduction of the expression of
HIF-1α protein by pristimerin. We detected the stem cell
characteristics of PC-3 cells by sphere formation and colony
formation assay, and KLF4, OCT4 and AGO2 protein expression. In
addition, EMT was evaluated by detecting N-cadherin, fibronectin,
vimentin, ZEB1 protein expression. Finally, our results supported
that pristimerin exerted its repressive effect on the metastasis,
at least in part, via restraining stem cell characteristics and EMT
of PC-3 PCa cells. These findings provide further evidence that
pristimerin can be a promising chemopreventive and anti-cancer
agent in human cancer by inhibiting tumor metastasis under hypoxic
stress.
Materials and methods
Reagents and antibodies
Pristimerin was purchased from Sigma-Aldrich Inc.
(St. Louis, MO, USA). Pristimerin was solubilized in 100% dimethyl
sulfoxide (DMSO) at 100 μg/ml concentration and frozen at −20°C in
small aliquots until needed. The antibody, anti-HIF-1α was
purchased from BD Biosciences; CD44 and β-actin were obtained from
Santa Cruz Biotechnology; and KLF4, OCT4, AGO2, N-cadherin,
fibronectin, vimentin and ZEB1 were purchased from Cell Signaling
Technology.
Cell lines and hypoxic treatment
Human PCa PC-3 cell lines were purchased from the
American Type Culture Collection (ATCC; USA) and maintained in our
laboratory for the present study. For conventional cell culture,
cells were seeded in culture flasks with F-12 culture medium
(HyClone) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 mg/ml streptomycin. All cells were cultured at
37°C in a humidified atmosphere and 5% CO2 in air. For
hypoxic exposure, the cells were placed in a sealed modular
incubator chamber flushed with 1% O2, 5% CO2
and 94% N2.
Cell proliferation assay
One day before the assay, cells were seeded under
hypoxic or normoxic conditions for 48 h, and were then displaced in
96-well plates in a volume of 100 μl for 36 h and treated with
increasing concentrations of pristimerin. All samples, including
controls (normoxic), contained 0.1% DMSO. Viable cells were
determined using CCK-8. The absorbance at 450 nm was determined
using the ELx808 microplate reader (Bio-Tek, Winooski, VT, USA).
Five wells were assayed at each concentration and the mean
absorbance was determined. The data are expressed as mean ± SD from
three independent experiments.
Cell invasion assay
The migration and invasion assays were performed in
a Transwell Boyden Chamber (Corning) using a polycarbonate filter
with an 8-μm pore size in a 24-well plate and the filter membranes
were coated with Matrigel (BD Biosciences) as previously described
(25). One day before the assay,
cells were seeded under hypoxic or normoxic conditions for 48 h,
and then cells were added to the inner chamber of the insert in 200
μl of serum-free medium and 500 μl of 10% FBS medium to determine
the effect of pristimerin on cell invasion. Indicated
concentrations of pristimerin were added to the lower chamber and
all samples, including controls (normoxic), contained 0.1% DMSO.
After incubation under normoxia for 48 h at 37°C, the cells were
displated to the inner chamber of the insert in 200 μl of
serum-free medium for 24 h, and then remained on the upper surface
where they were gently removed using a cotton-tipped swab. The
cells on the opposite surface of the filter membrane were stained
with 0.1% crystal violet and counts were obtained from five
randomly selected fields (x100 magnification) using a computer
imaging system.
Spheroid formation assay
Spheroid formation assay was performed as previously
described (26). To investigate the
effect of pristimerin treatment in preventing hypoxia-induced
spheroid formation, we maintained PC-3 cells under normoxic or
hypoxic conditions for 48 h, and then the cells were plated at 400
cells/well onto 6-well poly-HEMA (Sigma)-coated plates and they
were grown in F12 medium (HyClone) with increasing concentrations
of pristimerin for 14 days supplemented with B27 (Invitrogen), 20
ng/ml EGF (Sigma) and 20 ng/ml basic FGF (Invitrogen). After 14
days, the number of prostaspheres (tight, spherical, non-adherent
masses >100 μm in diameter) were counted, and images of the
prostaspheres were captured under an inverse microscope. Sphere
formation efficiency = colonies/input cells × 100%.
Colony formation assay
A colony formation assay was performed as previously
described (26). To investigate the
effect of pristimerin treatment in preventing hypoxia-induced
colony formation, we treated PC-3 cells under normoxic or hypoxic
conditions for 48 h and then treated them with indicative
concentrations of pristimerin for 24 h. After that the cells were
plated at 300 cells as single cells onto a 65-mm Petri dish with
increasing concentrations of pristimerin for 14 days and colonies
were stained with crystal violet. Plating efficiency = number of
colonies (≥50 cells/colony)/input cells × 100%. To determine
different colony morphologies, the different colony morphologies
were scored under a light microscope.
Western blot analysis
PC-3 cells were maintained under normoxic or hypoxic
conditions for 48 h, and were then treated by the indication
concentrations of pristimerin for 24 h and cells were lysed with
buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl,
0.5% Triton X-100, 10 mM sodium fluoride, 20 mM β-mercaptoethanol,
250 μM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and
complete protease inhibitor cocktail (Sigma), and were incubated at
4°C for 30 min. The lysates were ultrasonicated and centrifuged at
14,000 × g for 15 min. The proteins were separated on a 8–10%
gradient SDS-PAGE and transferred onto nitrocellulose membranes
(Hybond ECL; Amersham Pharmacia, Piscataway, NJ, USA). After being
blocked with skimmed milk in 5% TBST, the membranes were incubated
overnight at 4°C with primary antibodies against HIF-1α, CD44,
KLF4, OCT4, AGO2, N-cadherin, fibronectin, vimentin, ZEB1 or
β-actin. After being washed for three times with TBST for 5 min,
the membranes were incubated with secondary anti-rabbit or
anti-mouse antibodies for 1 h at room temperature and were then
developed by ECL.
Statistical analysis
Data are shown as the means ± SD. Multiple group
comparison was performed by a one-way ANOVA for comparison of
means. Comparisons between 2 groups were analyzed by the unpaired
Student’s t-test. P-values of <0.05 were considered to indicate
a statistically significant result.
Results
Pristimerin inhibits the hypoxia-induced
proliferation of PC-3 cells
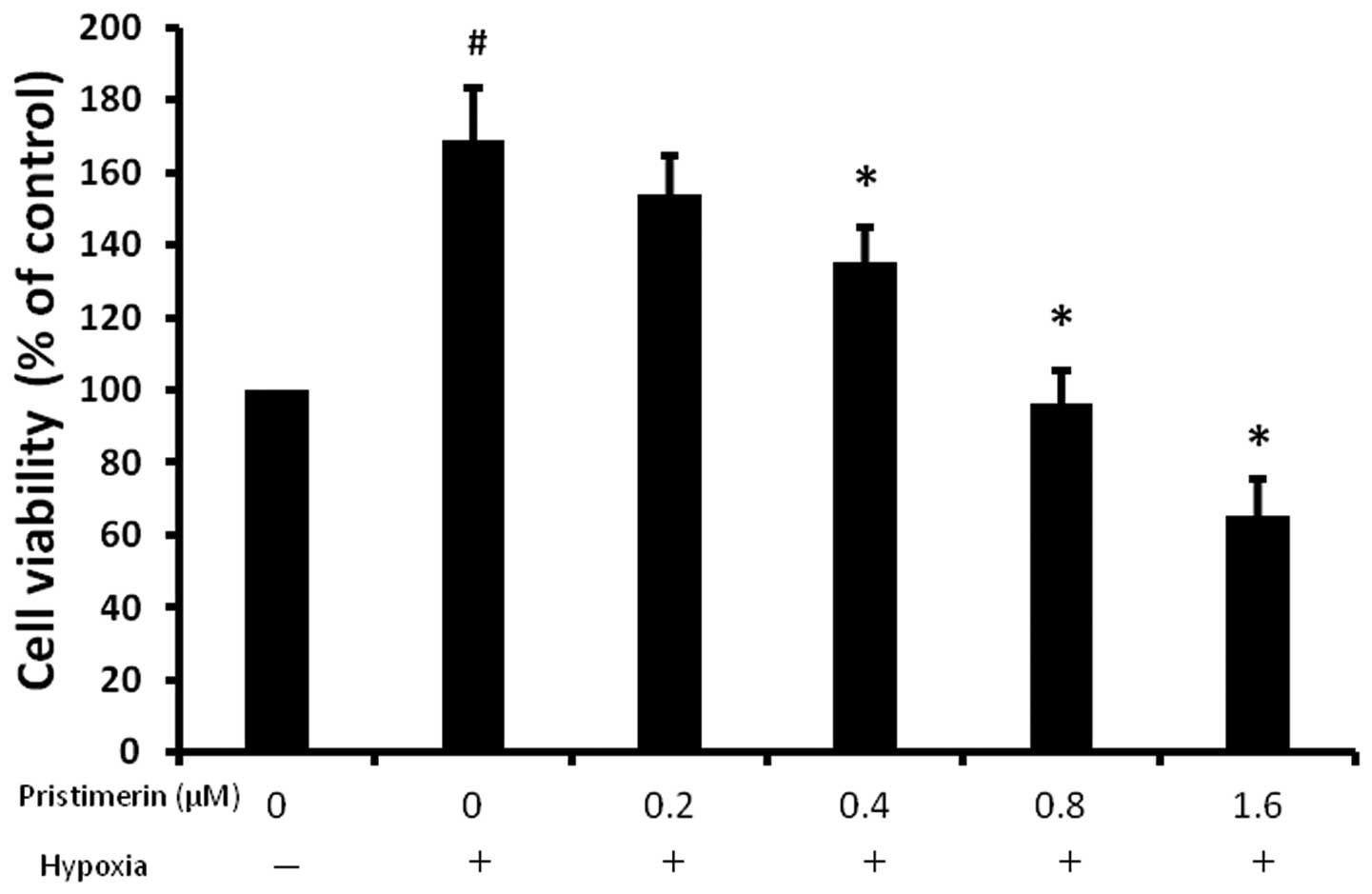
Uncontrolled proliferation is one of the most
distinguished traits of cancer cells. To determine the direct
effect of pristimerin on hypoxic-induced cancer cell proliferation,
we performed cell death assays on PC-3 cells treated with
pristimerin (Fig. 1). Pristimerin
treatment at a concentration of 0.4 μM for 36 h led to inhibition
of proliferation in the PC-3 cells and the effect was more marked
at 0.8 μM. Our findings suggest that pristimerin inhibits the
hypoxia-induced proliferation of cancer cells in a dose-dependent
manner.
Pristimerin prevents hypoxia-induced
invasion of PC-3 cells
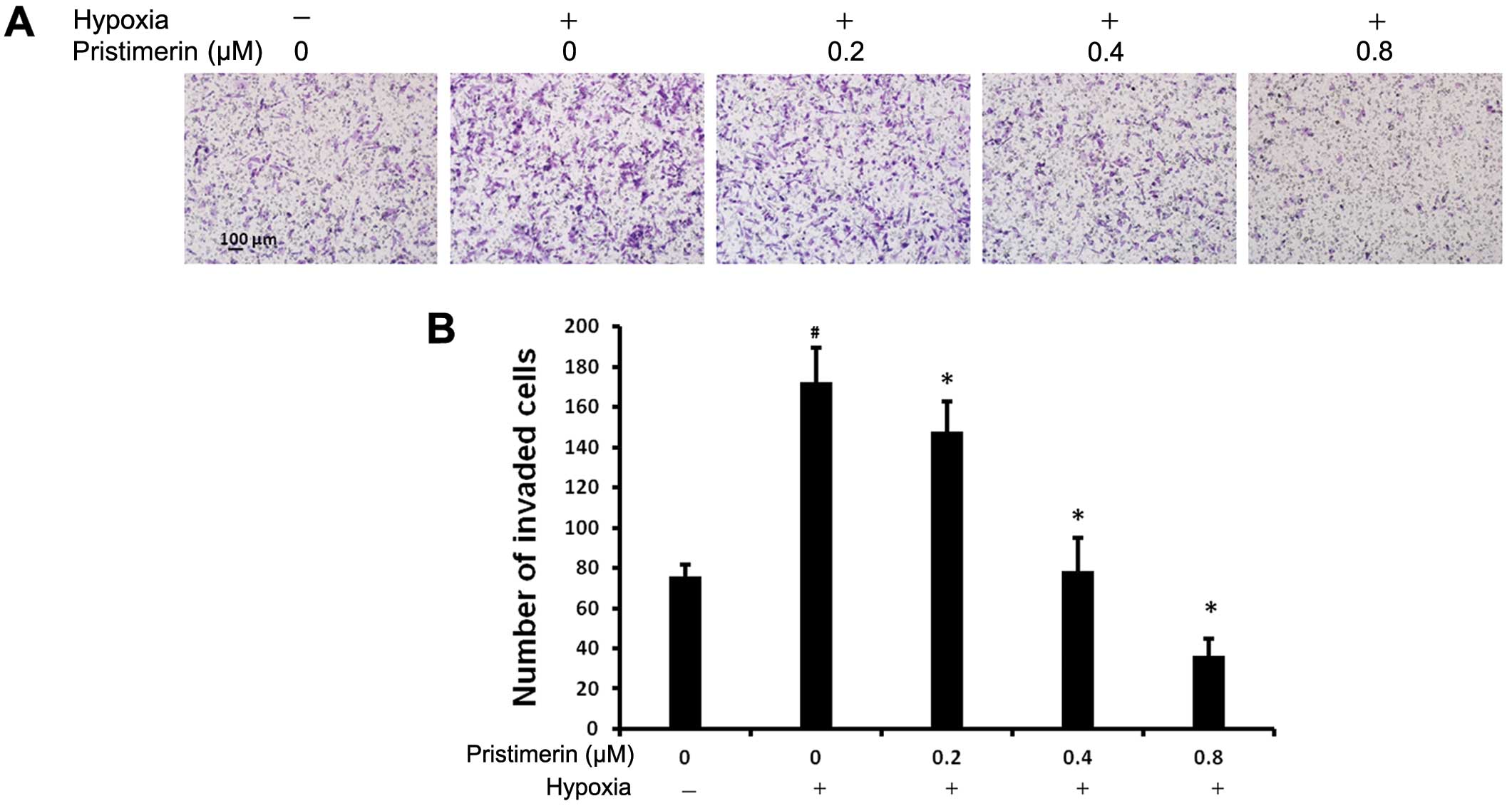
Hypoxia represents a physiological stimulus for
tumor cell invasion and metastasis. The progression of tumors to an
invasive phenotype and ultimately the formation of metastasis is
associated with increased mortality (27). We examined whether pristimerin
prevents the hypoxic-induced invasiveness of cancer cells by the
Matrigel-coated Transwell assay. As shown in Fig. 2A and B, an increase in the baseline
invasiveness of the cancer cells was observed under hypoxic
conditions as compared to normoxic conditions. The number of PC-3
cells that invaded through the Matrigel membrane increased from
normoxia to hypoxia. The stimulatory effect of hypoxia on the
invasiveness of cancer cells was reduced by pristimerin in a
dose-dependent manner. The number of invaded cells under hypoxia
decreased with the addition of 0.2 μM pristimerin. These results
indicate that pristimerin suppresses the hypoxia-stimulated
invasion ability of cancer cells.
Pristimerin prevents hypoxia-induced
spheroid formation of PC-3 cells
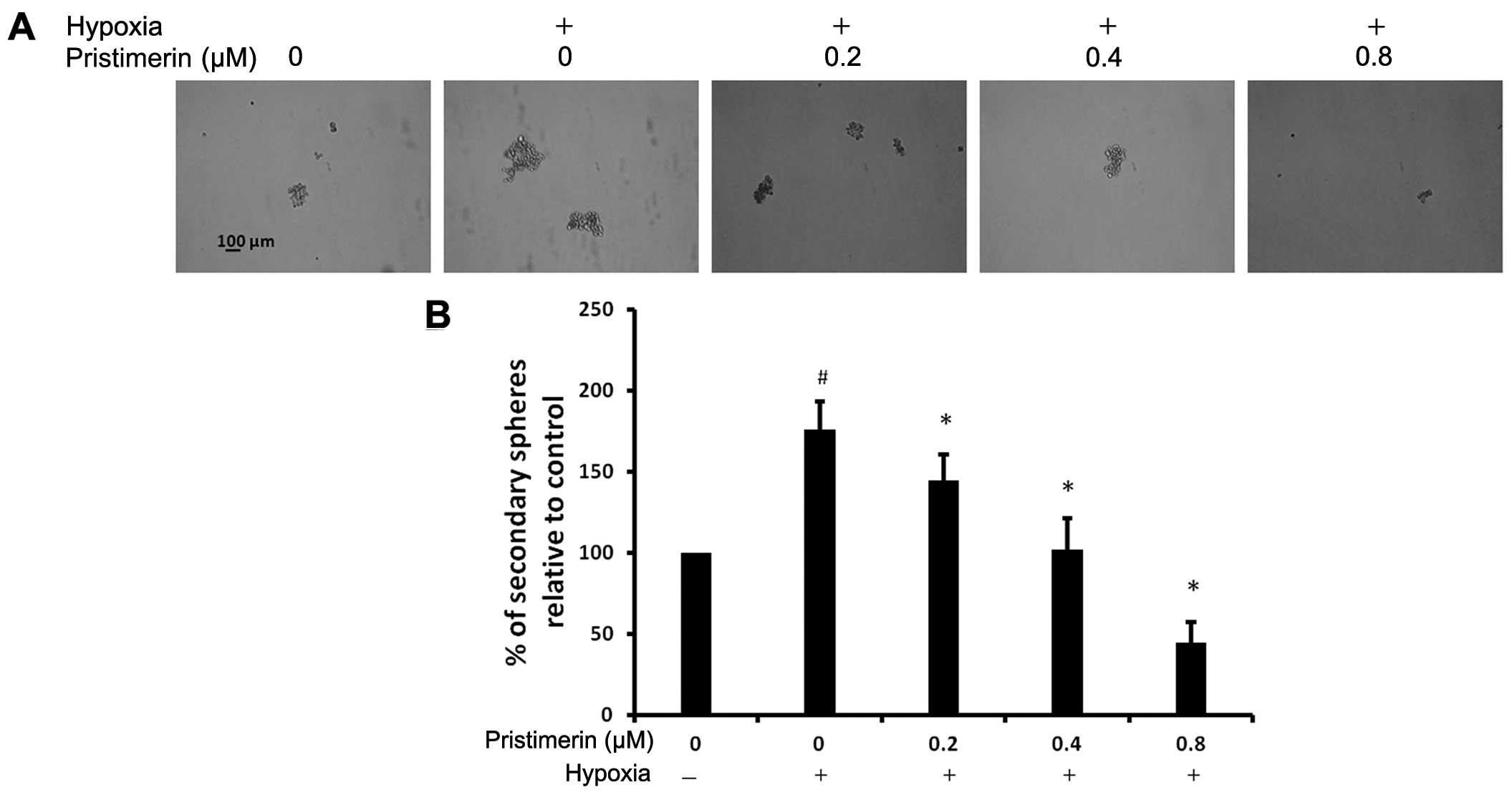
The ability to grow as non-adherent spheroids in
sphere medium has been widely used to assess the self-renewal
capability of CSCs and is one of the characteristics of prostate
CSCs (28,29). To investigate the effect of
pristimerin treatment in preventing hypoxia-induced spheroid
formation, we exposed PC-3 cells to normoxic or hypoxic conditions
for 48 h, and then the cells were pretreated with indicated
concentrations of pristimerin for 24 h. Next, the PC-3 cells were
plated at 400 cells/well onto 6-well polyHEMA-coated plates and
they were grown in F12 medium (HyClone). As shown in Fig. 3A and B, an increase in spheroid
formation was observed under hypoxic conditions as compared to
normoxic conditions. The stimulatory effect of hypoxia on the
spheroid formation of cancer cells was reduced by pristimerin in a
dose-dependent manner. The number of hypoxic invaded spheroids was
decreased with the addition of 0.2 μM pristimerin. These results
indicate that pristimerin could suppress the hypoxia-stimulated
spheroid formation ability of cancer cells.
Pristimerin prevents hypoxia-induced
colony formation of PC-3 cells
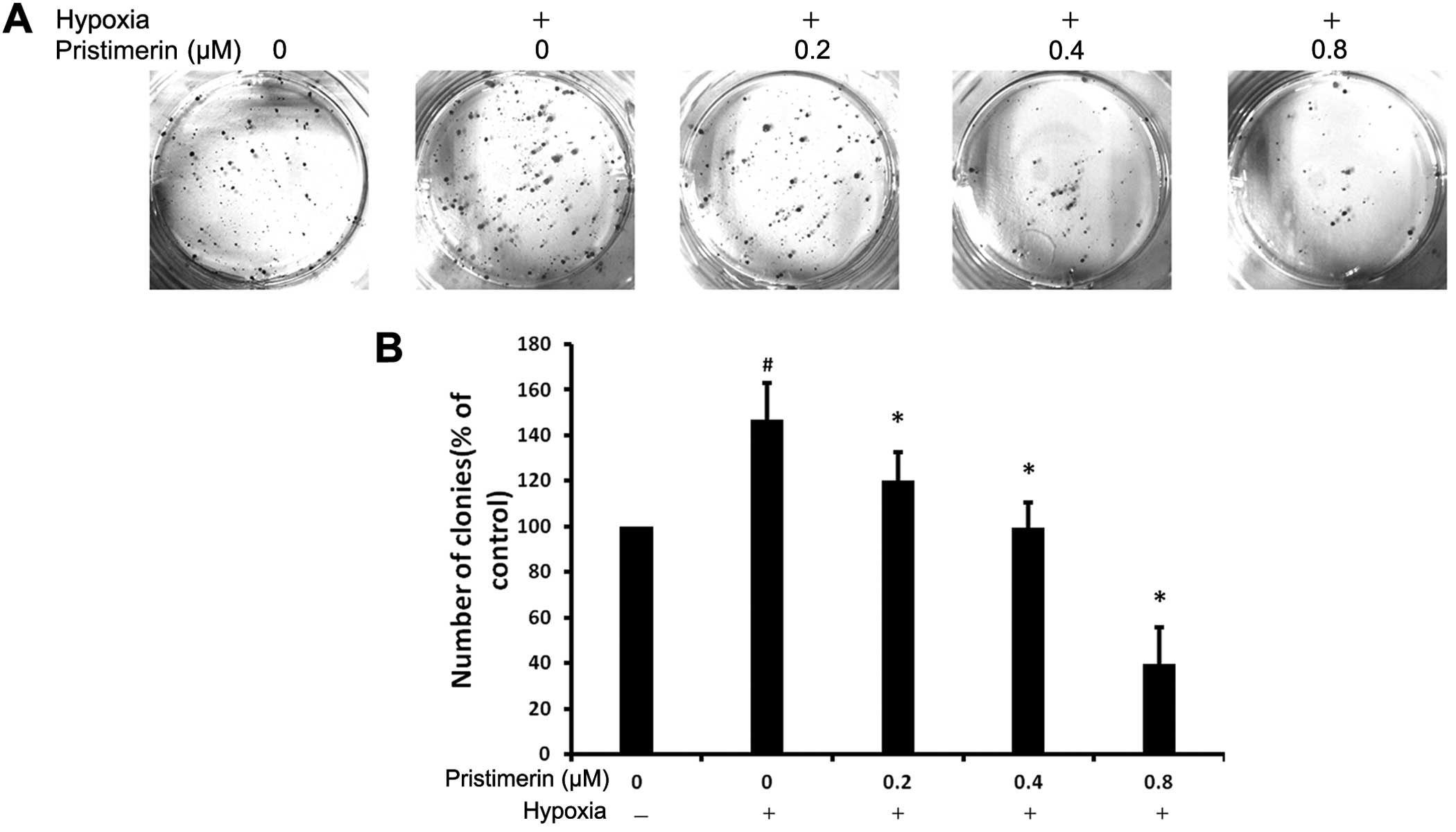
To investigate the effect of pristimerin treatment
in preventing hypoxia-induced colony formation, we subjected the
PC-3 cells to normoxic or hypoxic conditions for 48 h, and then the
cells were pretreated with the indicated concentrations of
pristimerin for 24 h. Next, they were plated at 300 cells as single
cells onto a 6-well plate with increasing concentrations of
pristimerin for 14 days. As shown in Fig. 4A and B, an increase in colony
formation was observed under hypoxic conditions as compared to
normoxic conditions. The stimulatory effect of hypoxia on the
colony formation of cancer cells was reduced by pristimerin in a
dose-dependent manner. The number of hypoxic-induced colonies was
decreased with the addition of 0.2 μM pristimerin. These results
indicate that pristimerin could suppress the hypoxia-stimulated
colony formation ability of cancer cells.
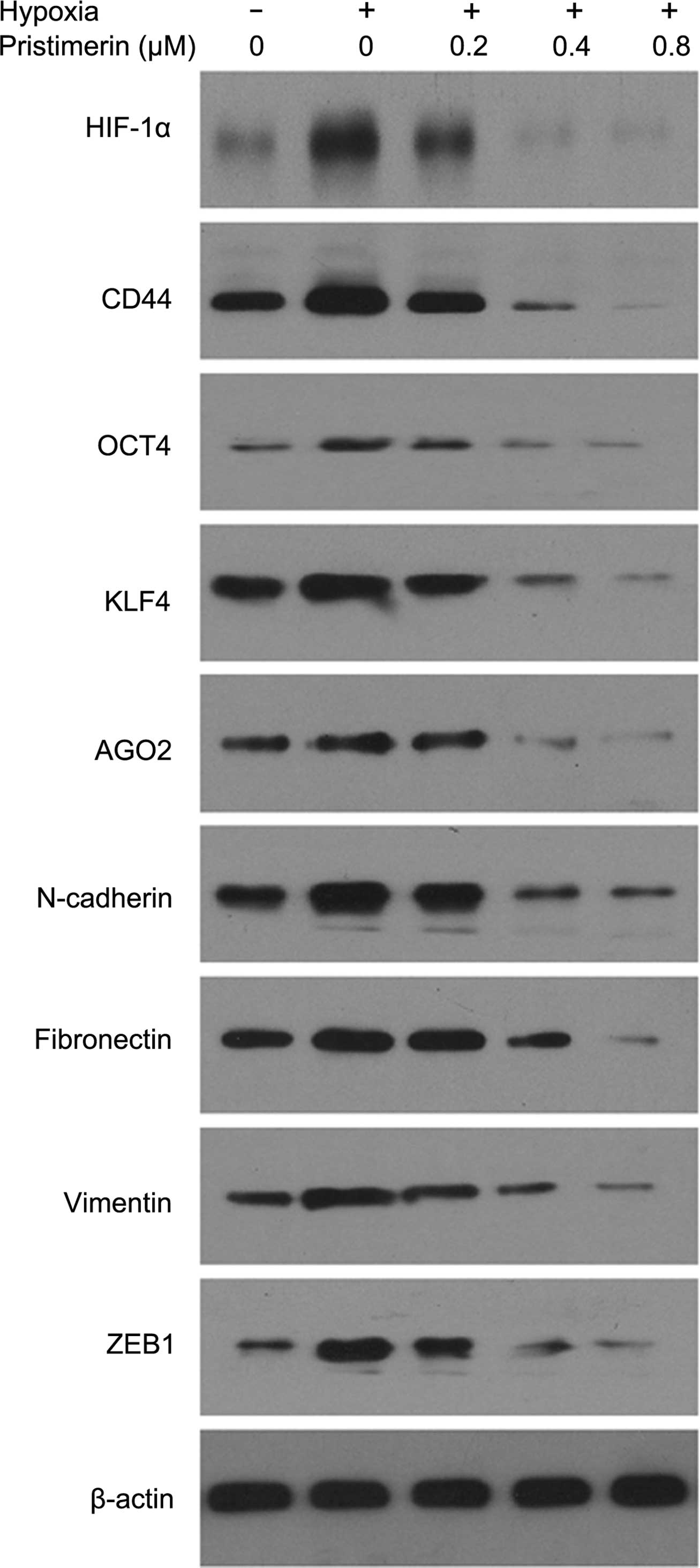
Pristimerin prevents hypoxia-induced stem
cell characteristics and EMT protein expression in PC-3 cells
To investigate the effect of pristimerin treatment
in preventing hypoxia-induced stem cells, we subjected PC-3 cells
to normoxic or hypoxic conditions for 48 h, and then the cells were
pretreated with the indicated concentrations of pristimerin for 24
h and CD44, KLF4, OCT4, AGO2, N-cadherin, fibronectin, vimentin and
ZEB1 levels were tested by western blotting. As shown in Fig. 5, an increase in CD44, KLF4, OCT4,
AGO2, N-cadherin, fibronectin, vimentin and ZEB1 expresssion was
observed under hypoxic conditions as compared to normoxic
conditions. The stimulatory effect of hypoxia on the expression of
these proteins was reduced by pristimerin at a very low
concentration (0.2 μM). Hence, we showed that pristimerin treatment
decreased CD44, KLF4, OCT4, AGO2, N-cadherin, fibronectin, vimentin
and ZEB1 levels in a dose-dependent manner under hypoxia.
Discussion
In the present study, we found that pristimerin
suppressed HIF-1α expression and hypoxia-induced metastasis via
blocking the proliferation, as well as the invasion ability of
cancer cells. Furthermore, pristimerin repressed hypoxia-induced
sphere formation, colony formation, expression of CSC markers and
stemness factors including CD44, KLF4, OCT4, AGO2 and expression of
EMT markers including N-cadherin, fibronectin, vimentin, ZEB1 in
PC-3 cells.
These findings demonstrate that pristimerin can
inhibit bone metastasis, hypoxia-induced stem cell characteristics
and EMT of tumor cells. More importantly, CSCs and EMT may be the
critical drivers of tumor progression and metastasis. Thus, our
results suggest that the ability of pristimerin to inhibit bone
metastasis is mediated partly by targeting hypoxia-induced stem
cell characteristics and EMT of tumor cells.
Intratumoral hypoxia is a common characteristic
feature of solid tumors and it is the driving force for tumor
metastasis. Activation of hypoxia-inducible factor-1 (HIF-1)
signaling is observed in a broad range of human cancers due to
tumor hypoxia and promotes physiological changes associated with
therapeutic resistance. It also includes the inhibition of
apoptosis and senescence, as well as the activation of drug efflux
and cellular metabolism, while approaches to inhibit HIF-1
signaling are primarily focused on reducing HIF-1α protein levels
(30). As a result, targeting HIF-1
represents an attractive strategy to enhance the efficacy of
current therapies, as well as reduce resistance to chemotherapy in
tumors (30). In the present study,
pristimerin inhibited hypoxia-induced HIF-1α protein levels.
Therefore, our findings suggested that pristimerin may possess a
similar function to hypoxia-induced progression and metastasis.
CSCs cells have been proposed to play a critical
role in cancer metastasis as demonstrated in several human
malignancies (31). With the
affirmation of the CSC concept, cancer biology and cancer drug
discovery have attained a new avenue to target cancer. CSCs are
considered to be responsible for tumor initiation, metastasis and
resistance to conventional radiotherapy and chemotherapy.
Therefore, different approaches to targeting these tumorigenic and
rare cells are urgently needed in order to improve the efficacy of
anticancer therapy (32). In the
present study, pristimerin inhibited hypoxia-induced stem cell
characteristics and pristimerin directly inhibited the
proliferation, sphere formation and colony formation of hypoxic
PC-3 cells and impaired hypoxia-induced expression of CD44, KLF4,
OCT4, AGO2 in hypoxic cancer cells. Therefore, our findings
suggested that pristimerin may possess an inhibitory function
against progression and metastasis by inhibited hypoxia-induced
stem cell characteristics of PC-3 cells.
Increasing evidence emphasizes a critical role of
EMT during PCa progression and malignant transformation, endowing
the incipient cancer cells with invasive and metastatic properties
(33–35). Thus, EMT could be a very promising
therapeutic target, and the inhibition of EMT may prevent or
restrain the invasion and metastasis of PCa. We found that
pristimerin could diminish the hypoxia-induced expression of
N-cadherin, fibronectin, vimentin, ZEB1 which showed that
pristimerin changes the predominant mesenchymal phenotype.
Therefore, our findings suggested that pristimerin may possess an
inhibitory function against progression and metastasis by
inhibition of EMT in PC-3 cells.
Due to the complexity of a tumor model, a single
drug targeting a particular oncogene is unlikely to be entirely
effective for cancer therapy. In this respect, pristimerin which
contains various phytochemicals targeting multiple dysregulated
pathways in cancer cells, may provide an alternative/complementary
way to treat cancer. The utility of antitumor strategies for cancer
control is strongly compromised by hypoxia-driven phenotypic
changes, which make cancer cells more invasive and more prone to
give rise to metastasis. In the present study, we showed that
pristimerin decreased the expression of HIF-1α, CD44, KLF4, OCT4,
AGO2, N-cadherin, fibronectin, vimentin, ZEB1 in a dose-dependent
manner under pre-treatment hypoxic conditions. Thus, it is evident
that concurrent measures for halting hypoxia-induced phenotypic
changes may be required if pristimerin therapy is ever to achieve
its goal of providing long-term cancer control and improved
survival.
In conclusion, we demonstrated that several
activities of pristimerin may account for its suppressive effect on
cancer metastasis under hypoxia. Firstly, pristimerin markedly
inhibited the stimulatory effect of hypoxia on the proliferation
and invasion ability of cancer cells. Secondly, pristimerin could
suppress stem cell characteristics, which has been shown to
regulate multiple steps in the complex process of metastasis.
Thirdly, pristimerin decreased levels of the predominant
mesenchymal phenotype, which is overexpressed in a variety of tumor
types and associated with increased metastases and poor
prognosis.
To the best of our knowledge, this is the first
documentation that pristimerin exerts its broad spectrum of
antimetastatic effects through its potent inhibition of stem cell
characteristics and EMT, in the context of tumors under hypoxia, a
common feature of most solid cancers. These results provide new
insight into the mechanisms of the antitumor action of
pristimerin.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81272938).
Abbreviations:
|
PCa
|
prostate cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CSCs
|
cancer stem cells
|
|
Ago2
|
Argonaute 2
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
References
|
1
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar
|
|
2
|
Carlin BI and Andriole GL: The natural
history, skeletal complications, and management of bone metastases
in patients with prostate carcinoma. Cancer. 88(Suppl 12):
S2989–S2994. 2000. View Article : Google Scholar
|
|
3
|
Papachristou DJ, Basdra EK and
Papavassiliou AG: Bone metastases: molecular mechanisms and novel
therapeutic interventions. Med Res Rev. 32:611–636. 2012.
View Article : Google Scholar
|
|
4
|
Höckel M and Vaupel P: Biological
consequences of tumor hypoxia. Semin Oncol. 28(Suppl 8): S36–S41.
2001. View Article : Google Scholar
|
|
5
|
Greco O, Marples B, Joiner MC and Scott
SD: How to overcome (and exploit) tumor hypoxia for targeted gene
therapy. J Cell Physiol. 197:312–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brizel DM, Scully SP, Harrelson JM, et al:
Tumor oxygenation predicts for the likelihood of distant metastases
in human soft tissue sarcoma. Cancer Res. 56:941–943.
1996.PubMed/NCBI
|
|
7
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1α in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
10
|
Blagosklonny MV: Hypoxia-inducible factor:
Achilles’ heel of antiangiogenic cancer therapy (Review). Int J
Oncol. 19:257–262. 2001.PubMed/NCBI
|
|
11
|
Maxwell PH, Dachs GU, Gleadle JM, et al:
Hypoxia-inducible factor-1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marignol L, Coffey M, Lawler M and
Hollywood D: Hypoxia in prostate cancer: a powerful shield against
tumour destruction. Cancer Treat Rev. 34:313–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharifi N, Kawasaki BT, Hurt EM and Farrar
WL: Stem cells in prostate cancer: resolving the castrate-resistant
conundrum and implications for hormonal therapy. Cancer Biol Ther.
5:901–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfeiffer MJ and Schalken JA: Stem cell
characteristics in prostate cancer cell lines. Eur Urol.
57:246–254. 2010. View Article : Google Scholar
|
|
17
|
Ma Y, Liang D, Liu J, et al: Prostate
cancer cell lines under hypoxia exhibit greater stem-like
properties. PLoS One. 6:e291702011. View Article : Google Scholar
|
|
18
|
Marie-Egyptienne DT, Lohse I and Hill RP:
Cancer stem cells, the epithelial to mesenchymal transition (EMT)
and radioresistance: potential role of hypoxia. Cancer Lett.
341:63–72. 2013. View Article : Google Scholar
|
|
19
|
Higgins DF, Kimura K, Bernhardt WM, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesenchymal transition. J Clin Invest. 117:3810–3820.
2007.PubMed/NCBI
|
|
20
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sethi S, Macoska J, Chen W and Sarkar FH:
Molecular signature of epithelial-mesenchymal transition (EMT) in
human prostate cancer bone metastasis. Am J Transl Res. 3:90–99.
2010.PubMed/NCBI
|
|
24
|
Liu YB, Gao X, Deeb D, Arbab AS and Gautam
SC: Pristimerin induces apoptosis in prostate cancer cells by
down-regulating Bcl-2 through ROS-dependent ubiquitin-proteasomal
degradation pathway. J Carcinog Mutagen. (Suppl 6): 0052013.
|
|
25
|
Peng X, Guo W, Liu T, et al:
Identification of miRs-143 and -145 that is associated with bone
metastasis of prostate cancer and involved in the regulation of
EMT. PLoS One. 6:e203412011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang S, Guo W, Tang Y, Ren D, Zou X and
Peng X: miR-143 and miR-145 inhibit stem cell characteristics of
PC-3 prostate cancer cells. Oncol Rep. 28:1831–1837.
2012.PubMed/NCBI
|
|
27
|
Liao D, Corle C, Seagroves TN and Johnson
RS: Hypoxia-inducible factor-1α is a key regulator of metastasis in
a transgenic model of cancer initiation and progression. Cancer
Res. 67:563–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Warfel NA and El-Deiry WS: HIF-1 HIF-1
signaling in drug resistance to chemotherapy. Curr Med Chem.
21:3021–3028. 2014. View Article : Google Scholar
|
|
31
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duggal R, Minev B, Geissinger U, et al:
Biotherapeutic approaches to target cancer stem cells. J Stem
Cells. 8:135–149. 2013.
|
|
33
|
Xu J, Wang R, Xie ZH, et al: Prostate
cancer metastasis: role of the host microenvironment in promoting
epithelial to mesenchymal transition and increased bone and adrenal
gland metastasis. Prostate. 66:1664–1673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whitbread AK, Veveris-Lowe TL, Lawrence
MG, Nicol DL and Clements JA: The role of kallikrein-related
peptidases in prostate cancer: potential involvement in an
epithelial to mesenchymal transition. Biol Chem. 387:707–714. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kasper S and Cookson MS: Mechanisms
leading to the development of hormone-resistant prostate cancer.
Urol Clin North Am. 33:201–210. vii2006. View Article : Google Scholar : PubMed/NCBI
|