Introduction
According to the investigation conducted in 2015,
approximately 429,000 new cancers cases and 281,4000 cancer deaths
would occur in China (1). Breast
cancer ranks as the first leading cause of cancer-related death
among women in less developed countries (2,3). For
most breast cancer patients, surgical removal of the tumor is the
first step of treatment, adjuvant therapies are recommended to
follow, including systemic treatment with chemotherapy, endocrine
therapy, targeted therapy and postoperative radiation therapy.
Substantial advances have been made in the prognosis and treatment
of breast cancers. However, the treatment progress and prevention
effects were minimal. The need towards deeper understanding the
pathogenesis of breast cancer is highlighted and will advance the
development of novel strategies for effective control of this
disease.
Member of Rab family (4), RAB1A is anchored on the membranes of
endoplasmic reticulum (ER) and Golgi by prenylation and functions
as controller of vesicle trafficking from ER to Golgi apparatus
(5,6). The aberrant expression of RAB1A
induces many diseases, such as Parkinson's disease (7), aspirin-exacerbated respiratory disease
(8), cardiomyopathy (9) and cancer (10,11).
mTORC1 as a complicated pathway is demonstrated to play various
effects on cell survival, cell growth, cell metabolism, cell cycle
and is sensitive to rapamycin (12). Mounting studies conducted recently
found RAB1A is involved in the regulation of mTORC1 signaling, in
colorectal, prostate and hepatocellular cancer (13–15).
In 2014, Thomas et al revealed that Rab1A is an mTORC1
activator, and can stimulate oncogenic growth via mTORC1 pathway
with the presence of amino acid (AA) in colorectal cancer (13). Gulhati et al reported a study
on the effect of mTORC1 in regulating epithelial-mesenchymal
transition (EMT) (16).
To our knowledge, characterization of RAB1A is less
well developed. Thus, one goal of this study was to determine the
function of RAB1A in breast cancer and its relationship with mTOR
pathway. Our results may have an effect on the treatment and
prognosis of breast cancer.
Materials and methods
Cell culture
The breast cancer cell lines MDA-MB-231 and BT-549
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). MDA-MB-231 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), penicillin
(100 U/ml) and streptomycin (100 µg/ml) (Enpromise, Hangzhou,
China). BT-549 cells were grown in RPMI-1640 medium supplemented
with 10% FBS (Gibco), penicillin (100 U/ml) and streptomycin (100
µg/ml) (both from Enpromise). The cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2.
RAB1A small interfering RNA (siRNA) and negative
control siRNA (NC siRNA) oligonucleotides were synthesized by
GenePharma (Shanghai, China). The sequence of RAB1A siRNAs was
sense, 5′-CAGCAUGAAUCCCGAAUAUTT-3′ and antisense,
5′-AUAUUCGGGAUUCAUGCUGTT-3′; the sequence of NC siRNA was sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell transfection
For transfection, MDA-MB-231 and BT-549 cells were
seeded into 6-well plates with a starting cell number of
12×104 and cultured with serum and antibiotic free DMEM
or RPMI-1640 medium, respectively. Cells were transfected using
Lipofectamine 2000 transfection reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the instructions
provided by the manufacturer when cells density reached 50–60%. The
medium was replaced by complete DMEM or RPMI-1640 medium after 6 h
of incubation. The cells were used for future analysis after 48 h
transfection.
RNA isolation, reverse transcription
and real-time quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen Life Technologies) was
used to isolate total RNA according to the manufacturer's
instructions after 48 h transfection. RNA was reverse-transcribed
with PrimeScript RT-PCR kit (Takara Bio Inc., Tokyo, Japan),
according to the manufacturer's instructions. Conditions of the
reverse transcription (RT) reaction were 37°C for 15 min, then 85°C
for 5 sec. The SYBR-Green PCR master mix (Takara Bio Inc.) was used
for RT-qPCR, which was followed by detection with a 7900HT fast
RT-PCR instrument (Applied Biosystems, Singapore). GAPDH was used
as an internal standard.
RAB1A mRNA expression was assessed with the
following primers: 5′-TTGCCTTCTTCTTAGGTTTGC-3′ (forward), and
5′-GCTTGATTGTTTTCCCGTCT-3′ (reverse). RT-qPCR parameters for
quantification were as follows: 2 min at 95°C, followed by 40
cycles of 15 sec at 95°C and 30 sec at 60°C. The relative
expression was calculated using the relative quantification
equation (RQ) = 2−ΔΔCt. Each sample was performed in
triplicate.
Cell proliferation assay
Following 24 h transfection, the cells were seeded
at 2×103 cells/well in 96-well plates. Cell growth was
monitored every day for a period of 5 days. Then 20 µl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) solution was added in each well
and further incubated for 4 h. Then 150 µl dimethyl sulfoxide
(DMSO; Sigma-Aldrich) was added in each well and shaken for 10 min
gently to dissolve the MTT formazan crystals after removing the
supernatant. Absorbance was recorded at 490 nm with a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA).
Plate colony formation assay
MDA-MB-231 and BT-549 cells were seeded in a 6-well
plate at 500 cells/well after 24 h of transfection, incubated for 1
week at 37°C in humidified 5% CO2 conditions. Cells were
washed with PBS to remove the debris and fixed by 95% ethanol for
10 min, dried and stained with 0.1% crystal violet solution for 20
min. The number of colonies with diameters of more than 1.5 mm was
counted after washing with tap water 3 times.
Cell invasion assay
Transwell chambers (Corning Inc., Lowell, MA, USA)
with a pore size of 8 µm were used for invasion assay and were
pre-coated with Matrigel. Cells were harvested after transfected by
RAB1A siRNA or NC siRNA and resuspended with DMEM or RPMI-1640
medium without FBS. Medium (200 µl) containing 5×104
cells were added into the upper chamber with 0.1% BSA solution and
added to the 24-well plate. Complete DMEM or RPMI-1640 medium was
added into the bottom chamber, serving as a chemoattractant. After
16 h incubation at 37°C in 5% CO2, cells on the upper
surface were carefully removed with a cotton swab. Cells penetrated
to the lower surface of the membrane were fixed with 10% formalin,
stained with crystal violet and counted under a microscope. Results
from 1 of 3 representative experiments are shown.
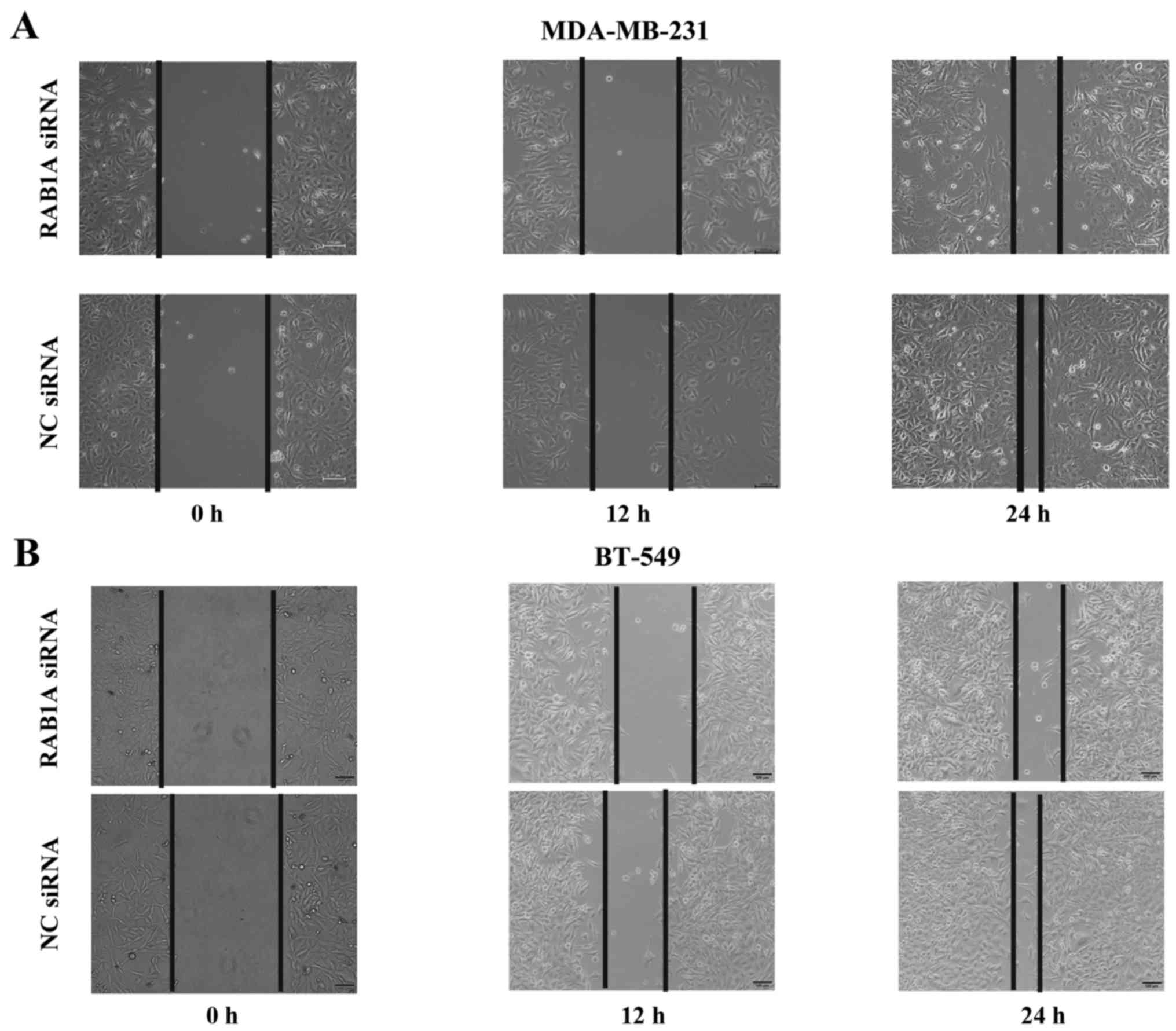
Wound healing assay
To evaluate the cell mobility, wound healing assay
was conducted. MDA-MB-231 and BT-549 cells were transfect. Six-well
plates were used with 15×104 cells/well. The plates were
washed 3 times with PBS, when the confluence of cells reached ~90%,
a scratch was made using a sterile pipette tip. The process of
wound healing was observed at 0, 12, 24 and 48 h after incubating
at 37°C in 5% CO2. Representative migration images are
presented. Each treatment was performed in triplicate.
Protein extraction and western
blotting
Total cell protein content was extracted after 48–72
h transfection by using radio immunoprecipitation assay (RIPA)
lysis buffer (80 µl/well; Beyotime Institute of Biotechnology,
Jiangsu, China). The supernatants were collected and centrifuged at
4°C, then protein concentrations were qualified by BCA protein
assay kit (Beyotime Institute of Biotechnology). Subsequently,
protein samples were denatured with 6X sodium dodecyl sulfate (SDS)
loading buffer at 95°C for 5 min. Protein lysates were resolved by
10 or 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then
transferred onto a 0.45 µm nitrocellulose membrane (both from
Beyotime Institute of Biotechnology). Later, the membranes were
blocked with 5% skim milk for 1 h and then incubated overnight at
4°C with primary polyclonal or monoclonal antibodies as follows:
anti-RAB1A (rabbit, 1:500; Proteintech, Chicago, IL, USA),
anti-β-actin (mouse, 1:1,000), anti-E-cadherin (mouse, 1:750),
anti-N-cadherin (mouse, 1:750), anti-vimentin (mouse, 1:750),
anti-ERK (mouse, 1:1,000), anti-phospho-ERK (mouse, 1:1,000),
anti-AKT (mouse, 1:1,000), anti-phospho-AKT (mouse, 1:1,000) and
anti-pS6K (mouse, 1:1,000) (all from Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-phospho-pS6K (Ser-418, mouse,
1:1,000; Ruiying Biological, Jiangsu, China). Next, the membranes
were washed three times with PBST for 10 min each time, and
incubated with anti-mouse or anti-rabbit secondary antibody
(1:1,000; Epitomics, Burlingame, CA, USA) for 1 h at room
temperature. Finally, after 3 times wash with PBST, the target
proteins were detected with an Odyssey Scanning system (LI-COR
Biosciences, Lincoln, NE, USA). The expression levels of the target
protein were normalized to those of β-actin or tubulin. Each
treatment was performed in triplicate.
Statistical analysis
The data were analyzed using the SPSS, version 20.0
(IBM Corp., Somers, NY, USA) or GraphPad Prism, version 6.0
(GraphPad Software, San Diego, CA, USA). The data were expressed as
the mean ± standard error of the mean (SEM) for at least 3 repeated
individual experiments for each group. Statistical analyses were
performed using an unpaired two-tailed Student's t-test. P-values
<0.05 were considered statistically significant.
Results
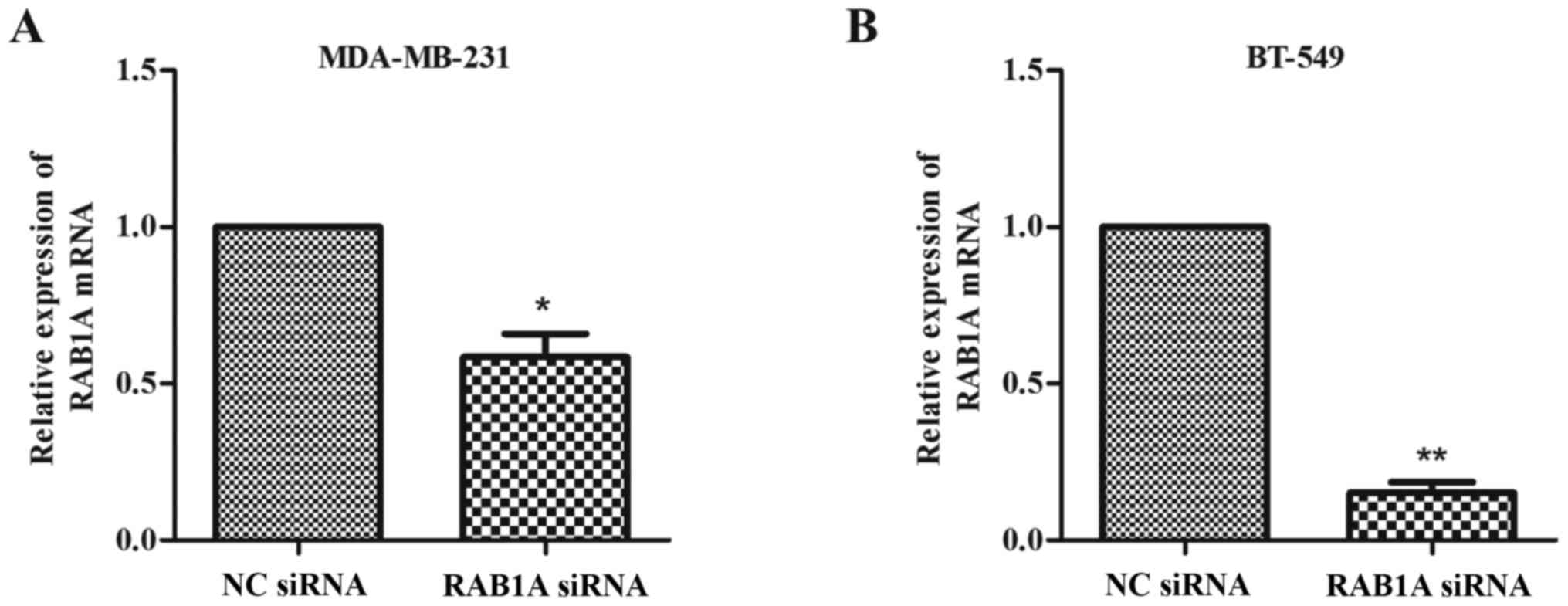
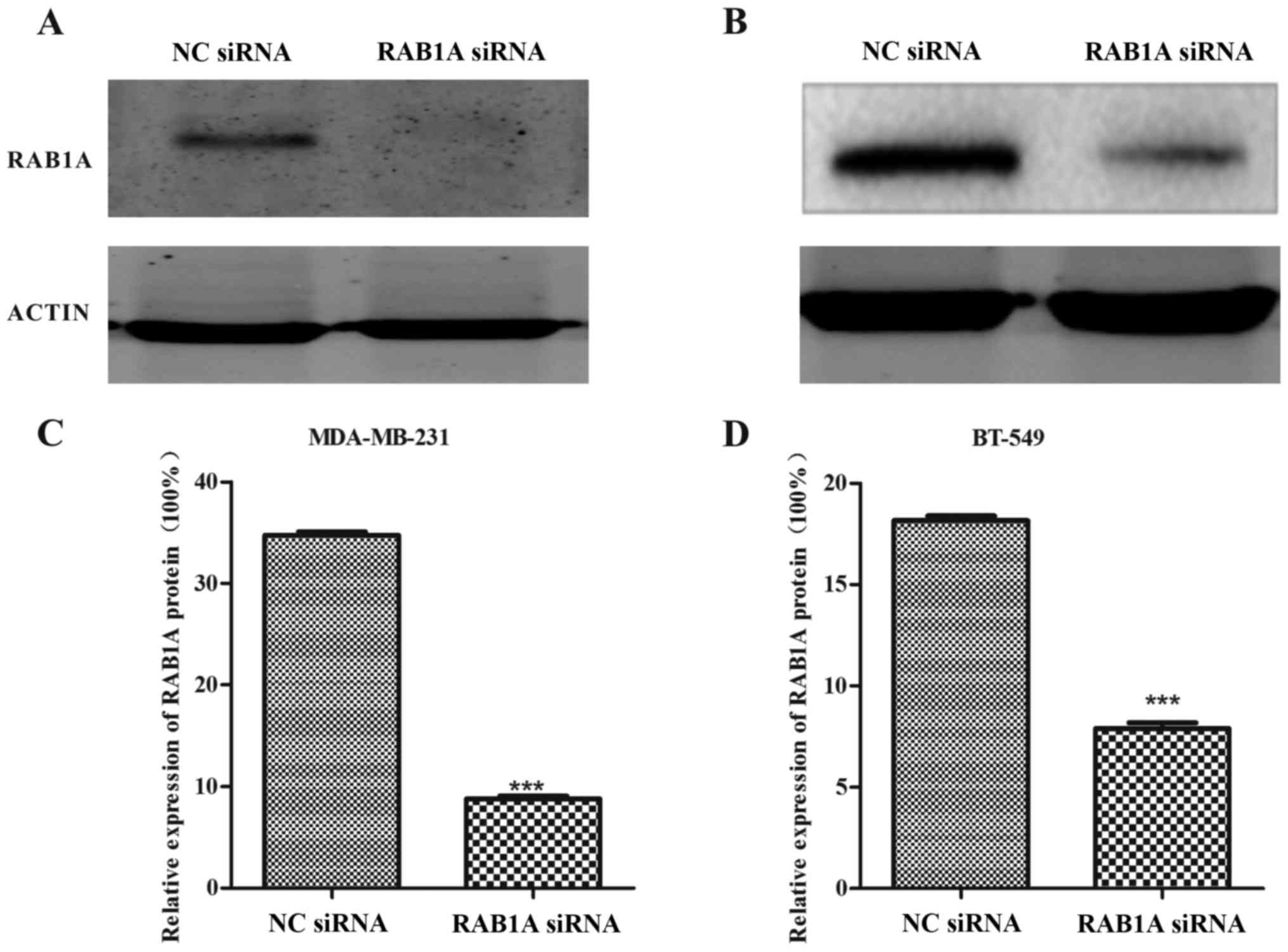
Expression of RAB1A is downregulated
by siRAB1A in breast cancer cells
The RT-qPCR and western blotting were conducted to
analyze the expression of RAB1A at the protein level and mRNA
level, respectively. As shown in Fig.
1A and B, MDA-MB-231 and BT-549 cells were successfully
transfected with siRAB1A, leading to the significant suppression of
RAB1A mRNA expression. Furthermore, the expression of target
proteins were effectively inhibited in siRNA transfected breast
cancer cells compared with NC cells (Fig. 2; P<0.001). According to the
experiments above, the suppression effects of siRAB1A on RAB1A
expression were verified.
RAB1A siRNA inhibits the expression of
mTORC1 effector p-P70S6K
To verify the relationship between RAB1A and S6K.
RAB1A expression was suppressed by RAB1A siRNA and the expression
of S6K was detected by western blotting. According to Fig. 3A and B, following the downregulation
of RAB1A in protein level, protein expression of p-P70S6K was
decreased in breast cancer cells, while the expression of p-ERK or
p-AKT had no change. All the results above indicate the vital role
of RAB1A in regulation of activation of S6K. The integrated density
values of bands were as shown in Fig.
3C and D (P<0.05, P<0.01).
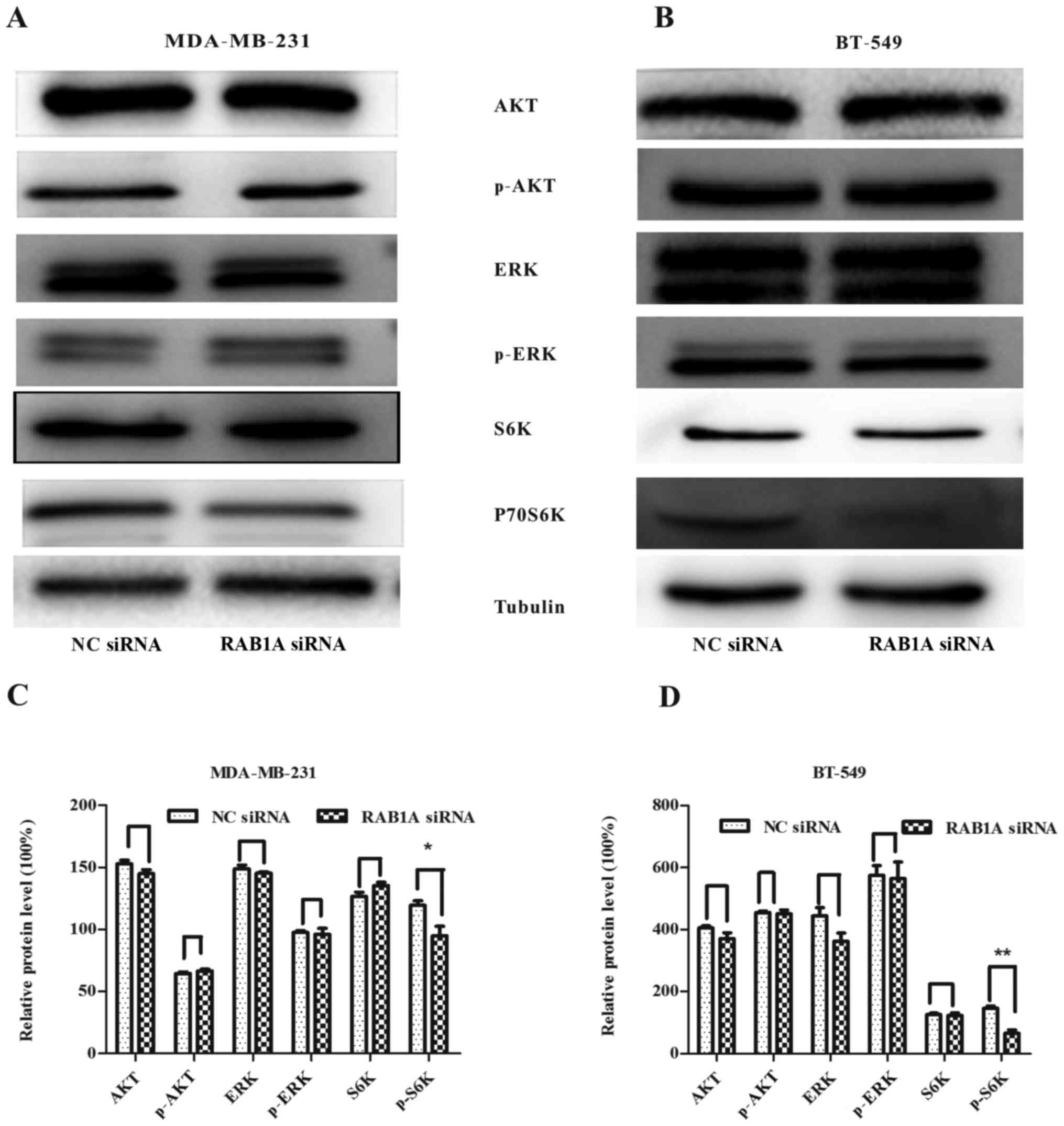 | Figure 3.RAB1A siRNA is involved in restraining
the mTORC1 pathway. In (A and C) MDA-MB-231 and (B and D) BT-549
cells, RAB1A knockdown did not decrease AKT, p-AKT, ERK, p-ERK,
P70S6K protein levels, while inhibited the expression of p-P70S6K,
the effector of mTORC1. The graph shows the mean ± SEM of AKT,
p-AKT, ERK, p-ERK, P70S6K, p-P70S6K protein levels related to their
loading control. Quantitative analysis was conducted by measuring
the integrated density value of bands. Blots are representative of
results from 3 experiments. *P<0.05, **P<0.01 (two-tailed
t-test). |
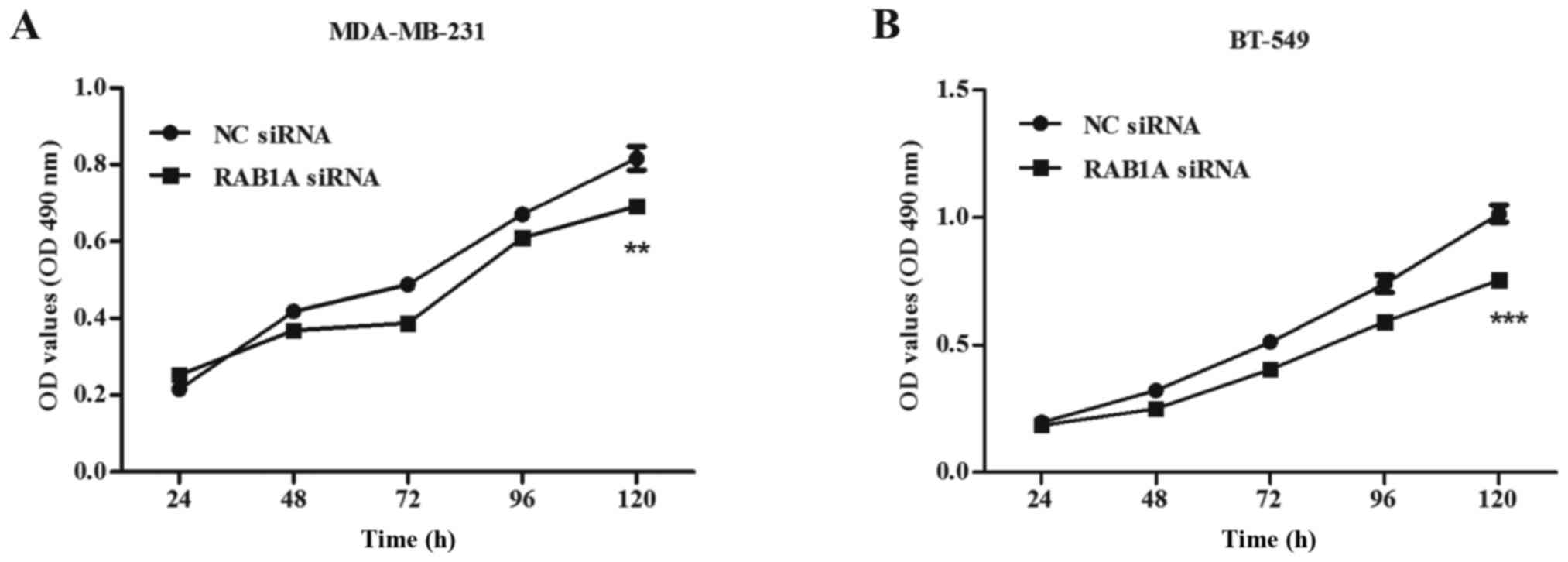
Proliferation ability of breast cancer
cells is suppressed by the RAB1A siRNA
To investigate the impact of siRAB1A on the
proliferation ability of breast cancer cells, both MDA-MB-231 and
BT-549 cells transfected with RAB1A siRNA or non-specific NC were
used in the MTT assay. Transfections were performed as described
above. Absorbance of the two groups was recorded at 24, 48, 72, 96
and 120 h. Briefly, inhibition rate was calculated as: inhibition
rate (%) = (OD value of the control group - OD value of
experimental group)/OD value of the control group × 100%. A great
suppression of cell viability at 120 h in RAB1A siRNA transfected
cells was detected compared with the control group, both in
MDA-MB-231 cells (inhibition rate = 14.78±6.54%, Fig. 4A; P<0.01) and BT-549 cells
(inhibition rate = 25.06±9.20%, Fig.
4B; P<0.001). These results indicated RAB1A depletion
suppressed cellular proliferation.
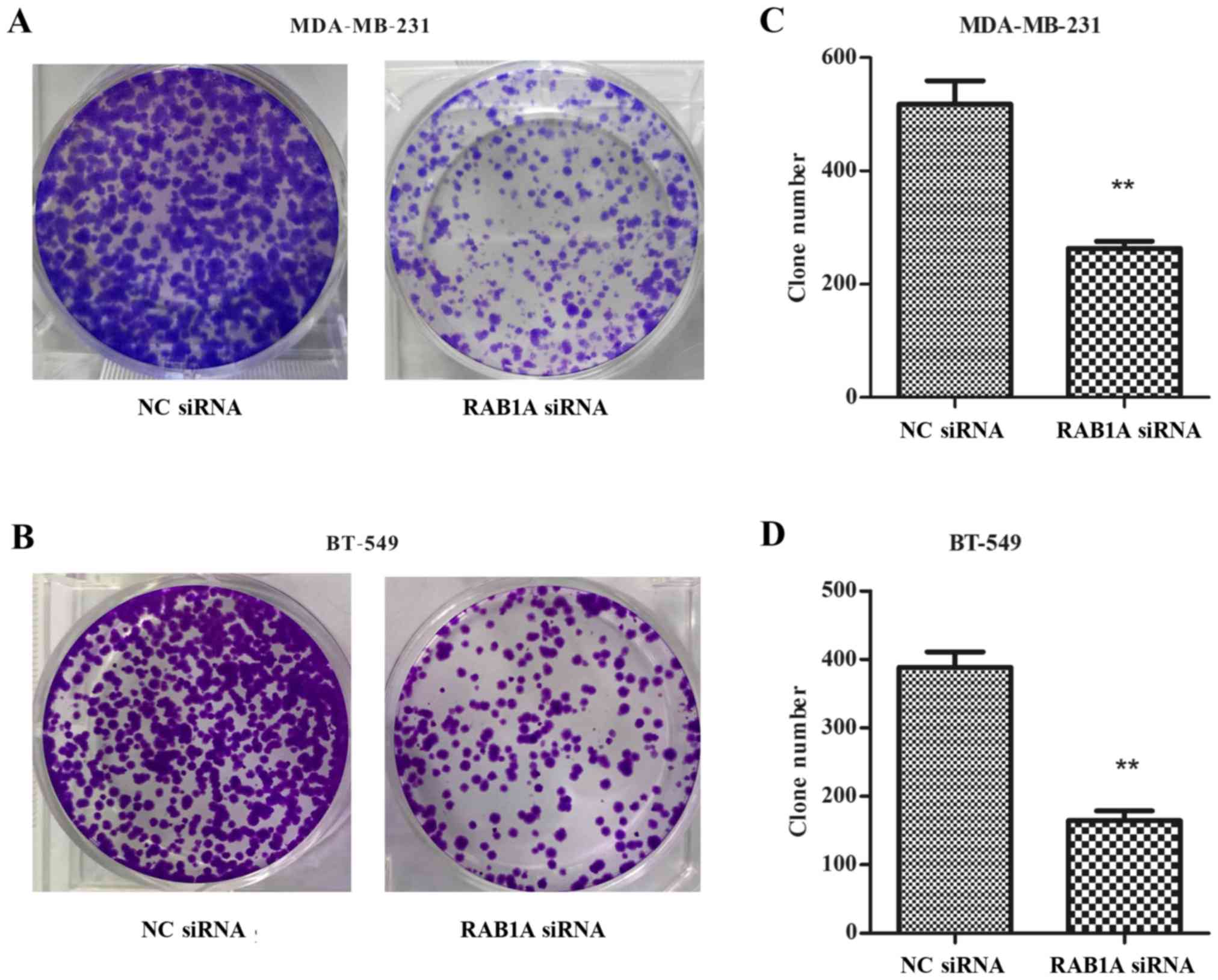
Colony formation was inhibited by the
RAB1A siRNA in breast cancer cells
As shown in Fig. 5A and
B, RAB1A siRNA transfected cells exhibited fewer colonies than
NC transfected cells in colony formation assays. The colony
formation rate in RAB1A siRNA-transfected MDA-MB-231 was
significantly decreased compared with the NC transfected cells
(Fig. 5C; P<0.01). The same
tendency was shown in BT-549 cells (Fig. 5D; P<0.01). Taken together, these
data indicated that depletion of RAB1A inhibited clonogenesis of
breast cancer cells.
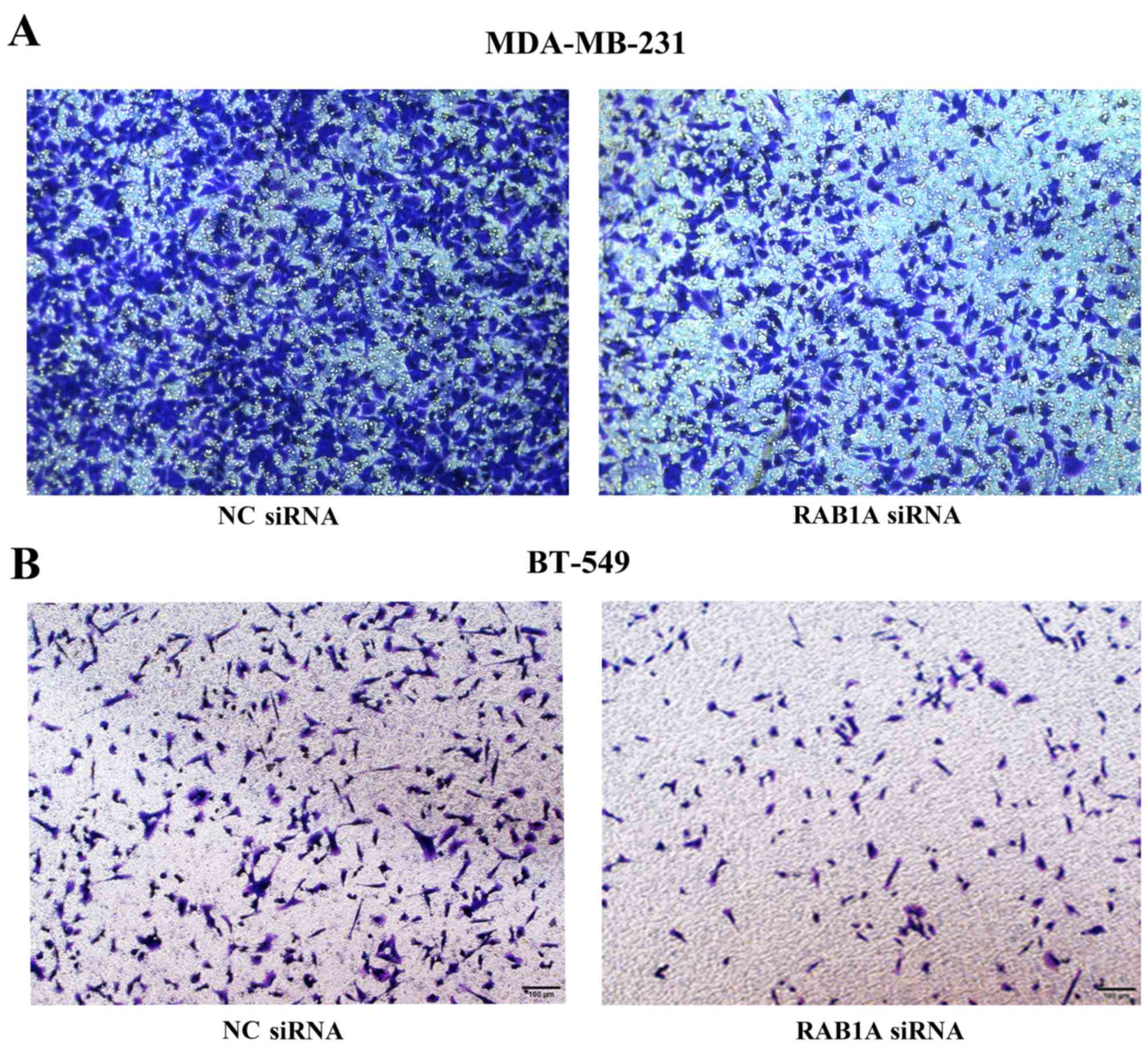
RAB1A siRNA reduces cell invasion and
metastasis in vitro
To evaluate the effect of RAB1A siRNA on breast
cancer metastasis, two aggressive breast cancer cell lines
MDA-MB-231 and BT-549 were transiently transfected with RAB1A siRNA
or NC control. The invasion and migration of cells were measured by
Transwell assays and wound healing assays, respectively. All the
results revealed that the number of MDA-MB-231 or BT-549 cells
penetrated to the lower membrane was significantly decreased at 16
h after RAB1A siRNA transfection compared to the control group
(Fig. 6). Notably, as shown in
Fig. 7, RAB1A siRNA but not NC
siRNA markedly decrease the migratory property of these breast
cancer cells.
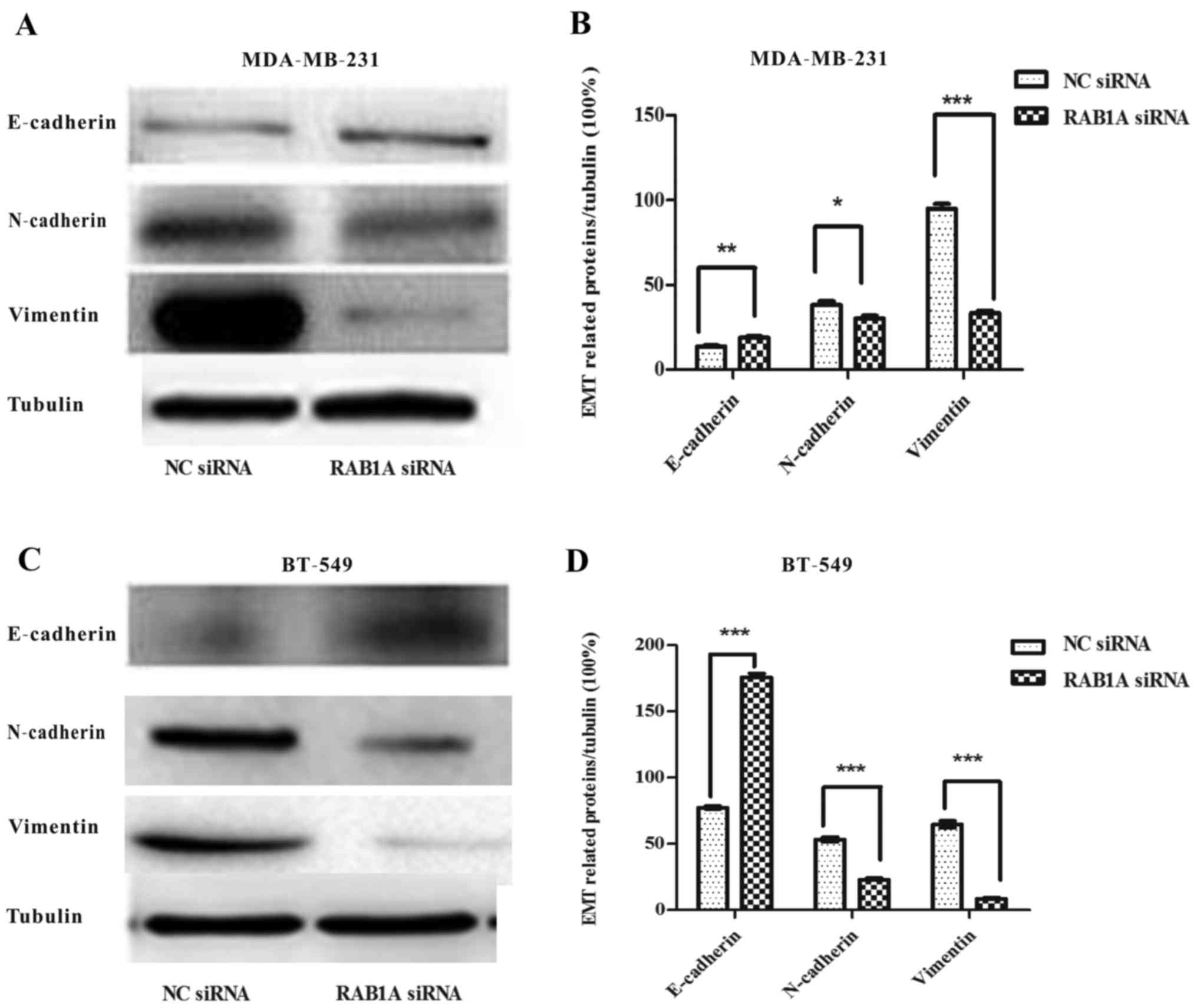
RAB1A depletion suppresses EMT in
breast cancer cells
Next, we explored the role of RAB1A siRNA in breast
cancer EMT. Western blotting were carried out. Fig. 8A and C shows, the expression of
E-cadherin protein, an epithelial cell marker, was increased in
RAB1A siRNA transfected MDA-MB-231 and BT-549 cells while the
mesenchymal markers N-cadherin and vimentin were decreased. The
integrated density values of bands were measured and shown
(Fig. 8B and D; P<0.05,
P<0.01, P<0.001). Taken together, these results indicated
that RAB1A depletion in breast cancer cells may be responsible for
the EMT suppression and cell migration suppression.
Discussion
As the leading cause of cancer-related death among
women worldwide, breast cancer have a severe influence on the
living standard of the patients. During the investigation of the
potential role in breast cancer, RAB1A siRNA was transfected in the
MDA-MB-231 and BT-549 cells. Cell invasion and proliferation
abilities were significantly abolished by depletion of RAB1A
according to the Transwell and MTT assay, respectively. Moreover,
our data showed that the downregulated expression of RAB1A
increased the epithelial cell marker E-cadherin in breast cancer
cells, while the mesenchymal cell markers, N-cadherin and vimentin,
were decreased (17). The loss of
E-cadherin mediated adherens junction was the first step in EMT and
plays a vital role in cells invasiveness and distance metastasis
(18). All the results indicate EMT
suppression mediated by exogenous silencing of RAB1A contributing
to the inhibition of breast cancer metastasis.
mTOR pathway as an important regulator in malignant
tumor process consists of two complexes, mTORC1 and mTORC2. As an
important effector of mTORC1, p-P70S6K plays a vital role in
regulating cell growth, invasion, and lymph node metastasis in
colorectal cancer (19). In this
experiment, downregulated expression of RAB1A by transfection with
RAB1A siRNA suppressed the expression of p-P70S6K, rather than the
p-AKT and p-ERK, which revealed a close connection between RAB1A
and p-P70S6K and the reasons for the suppression of proliferation
and metastasis by exogenous silencing of RAB1A gene in breast
cancer.
Several groups have already investigated the
function of RAB1A in colorectal cancer and what we found in this
study was in accordance with previous reports that RAB1A depletion
was involved in the suppression of mTORC1 pathway and it acts in
oncogenesis of breast cancer proliferation, growth and metastasis.
Our results may help the finding of new strategies for the
treatment of breast cancer.
A number of limitations should be noted in this
study. Clinical specimens were not used. The relationship between
RAB1A expression and patient survival was not identified.
Therefore, further studies are needed.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China for the project 81272240.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
RT-qPCR
|
real-time quantitative polymerase
chain reaction
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyl tetrazolium bromide
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schöppner P, Csaba G, Braun T, Daake M,
Richter B, Lange OF, Zacharias M, Zimmer R and Haslbeck M:
Regulatory implications of non-trivial splicing: isoform 3 of Rab1A
shows enhanced basal activity and is not controlled by accessory
proteins. J Mol Biol. 428:1544–1557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allan BB, Moyer BD and Balch WE: Rab1
recruitment of p115 into a cis-SNARE complex: programming budding
COPII vesicles for fusion. Science. 289:444–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satoh A, Wang Y, Malsam J, Beard MB and
Warren G: Golgin-84 is a rab1 binding partner involved in Golgi
structure. Traffic. 4:153–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coune PG, Bensadoun JC, Aebischer P and
Schneider BL: Rab1A over-expression prevents Golgi apparatus
fragmentation and partially corrects motor deficits in an
alpha-synuclein based rat model of Parkinson's disease. J
Parkinsons Dis. 1:373–387. 2011.PubMed/NCBI
|
|
8
|
Park JS, Heo JS, Chang HS, Choi IS, Kim
MK, Lee JU, Park BL, Shin HD and Park CS: Association analysis of
member RAS oncogene family gene polymorphisms with aspirin
intolerance in asthmatic patients. DNA Cell Biol. 33:155–161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu G, Yussman MG, Barrett TJ, Hahn HS,
Osinska H, Hilliard GM, Wang X, Toyokawa T, Yatani A, Lynch RA, et
al: Increased myocardial Rab GTPase expression: a consequence and
cause of cardiomyopathy. Circ Res. 89:1130–1137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Hou N, Wang X, Wang L, Chang S, He
K, Zhao Z, Zhao X, Song T and Huang C: miR-15b-5p induces
endoplasmic reticulum stress and apoptosis in human hepatocellular
carcinoma, both in vitro and in vivo, by suppressing Rab1A.
Oncotarget. 6:16227–16238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Chu Y, Wang W and Yuan W: mTORC
signaling in hematopoiesis. Int J Hematol. 103:510–518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun T, Wang X, He HH, Sweeney CJ, Liu SX,
Brown M, Balk S, Lee GS and Kantoff PW: miR-221 promotes the
development of androgen independence in prostate cancer cells via
downregulation of HECTD2 and RAB1A. Oncogene. 33:2790–2800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL,
Wang HY and Zheng XF: Aberrant amino acid signaling promotes growth
and metastasis of hepatocellular carcinomas through Rab1A-dependent
activation of mTORC1 by Rab1A. Oncotarget. 6:20813–20828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, et al:
mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makki J, Myint O, Wynn AA, Samsudin AT and
John DV: Expression distribution of cancer stem cells, epithelial
to mesenchymal transition, and telomerase activity in breast cancer
and their association with clinicopathologic characteristics. Clin
Med Insights Pathol. 8:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M,
Shao Z, Zhang F, Luo Y, Shen Z, et al: Epigenetic silencing of
miR-490-3p promotes development of an aggressive colorectal cancer
phenotype through activation of the Wnt/β-catenin signaling
pathway. Cancer Lett. 376:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q, Wang J, Yu G, Guo T, Hu C and Ren P:
Expression and clinical significance of mammalian target of
rapamycin/P70 ribosomal protein S6 kinase signaling pathway in
human colorectal carcinoma tissue. Oncol Lett. 10:277–282.
2015.PubMed/NCBI
|