Introduction
Aging is defined as the accumulation of diverse
deleterious changes in cells and tissues (1). It is commonly associated with a
reduction in physiological functions, and is closely related to
apoptosis. In biology, the state or process of aging is known as
senescence. It occurs at the level of the organism (organismal
senescence), as well as at the level of individual cells (cellular
senescence). At the individual cell level there are two types of
senescence, replicative and stress-induced premature senescence
(SIPS). Genetic and environmental manipulations have revealed that
aging is regulated by specific pathways, involved in hormonal
signaling, nutrient sensing and signaling, mitochondria and ROS
signaling and genome surveillance (2). However, the question of whether all
these signaling pathways exert their effects separately, or involve
one or more junction co-regulators among these signaling pathways
in the aging process remains to be determined.
Chinese herbs have been widely applied in the field
of medicine as anti-aging drugs because of their few side-effects
(3,4). The plant species Lycium
barbarum (L. barbarum) belongs to the family of
Solanaceae, and its fruit or extract has been regarded in
Chinese pharmacopoeia as a superior-grade medicine for the
modulation of body immunity (5–7). The
effects of L. barbarum are attributed to the polysaccharides
(LBPs) it contains, which can upregulate both innate and adaptive
immune responses (8,9). It has been shown that LBPs can
protect neurons from β-amyloid peptide neurotoxicity (10,11),
and from neuronal death, glial activation and oxidative stress in a
murine retinal ischemia/reperfusion model (12,13).
The tumor suppressor gene p53 has been known
to play an important role in the induction of cell cycle arrest and
apoptosis (14). In a recent
study, LBPs were found to stimulate the proliferation of MCF-7
cells via the ERK pathway, which may be associated with the p53
pathway (15). Although findings
of previous studies have demonstrated the beneficial effects of
LBPs on the health of humans and animals (16,17),
to the best of our knowledge, there have been scarce studies that
systematically investigate the signaling pathway(s) via which LBPs
exert these beneficial effects, especially in zebrafish, an
excellent model for studying angiogenesis, senescence, and toxicity
responses (18–21). In this study, we examined
senescence during the early development stages of zebrafish, cell
apoptosis, and senescence-associated gene expression upon treatment
with LBPs from the Chinese herb L. barbarum in the zebrafish
model, in order to investigate the effects of this herb and
identify the related signaling pathway(s) potentially involved in
anti-aging processes.
Materials and methods
Materials and animals
Purified L. barbarum polysaccharides (LBPs)
were purchased from Shanxi Undersun Biomedtech Co., Ltd. (Xi’an,
Shaanxi, China) and an extract was prepared using the method of Luo
et al (22). Zebrafish were
maintained in standard fish facility conditions with a 14:10 h
light/dark cycle and fed with living brine shrimp twice per day.
The water temperature was maintained at 28°C. The study was
approved by the ethics committee of Ocean University of China
(Qingdao, China).
Embryo treatment and image
collection
Embryos at 8 hours post-fertilization (hpf) were
placed into a 24-well microplate (Millipore Co., Bedford, MA, USA).
Thirty embryos were continuously exposed to varying concentrations
of LBPs (0, 1, 2, 3 and 4 mg/ml) for 3 days (d). LBPs were renewed
daily. Each treatment with an LBP concentration was repeated three
times and the standard deviation was calculated. To collect images
of embryo phenotypes, treated and non-treated embryos were
anesthetized with 0.2 mg/ml tricaine (3-aminobenzoic acid
ethylester; Sigma-Aldrich, St. Louis, MO, USA) and embedded in
methylcellulose.
Senescence associated-β-galactosidase
(SA-β-gal) assay
Embryos of 72 hpf were fixed in 4% paraformaldehyde
in phosphate-buffered saline, at 4°C overnight. Staining of
SA-β-gal and quantifications were performed according to the method
of Kishi et al (23).
In vivo detection of cell death
For the in vivo detection of cell death, 2
day-old embryos were incubated in 5 mg/ml acridine orange stain
(AO; Sigma-Aldrich) in zebrafish embryo medium [NaCl, 5.03 mM; KCl,
0.17 mM; CaCl2·2H2O, 0.03 mM;
MgSO4·7H2O, 0.03 mM; methylene blue, 0.1%
(w/v)] and kept in the dark for 15–30 min at 28°C, then rinsed
thoroughly 8 times with egg water. Stained embryos were
anesthetized with MESAB (0.5 mM 3-aminobenzoic acid ethyl ester, 2
mM Na2HPO4) and mounted in a depression slide
for observation using methylcellulose. They were then visualized
using a fluorescence microscope (AZ100, Nikon, Tokyo, Japan) and
images were captured for <60 sec (the signal is quenched after
60 sec of exposure to fluorescence). Embryos that were not stained
with AO were used to determine baseline fluorescence.
Semi-quantitative RT-PCR
Total RNA was extracted from 72 hpf embryos using
the TRIzol reagent. Semi-quantitative PCR was performed with an
initial incubation for 5 min at 94°C, followed by 22 cycles of
incubation at 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30
sec. The primers for amplification of p53 and p21
were the same as in (24):
p53 forward/reverse: CTCTCCCACCAACATCCACT/ACGTCCACC
ACCATTTGAAC; p21 forward/reverse: CGGAATAAA
CGGTGTCGTCT/CGCAAACAGACCAACATCAC. The primers for amplification of
Bax, Mdm2, TERT and β-actin were:
Bax forward/reverse: GGAGATGAGCTGGAT
GGAAAT/ATGACGTGCTCCTGAATGTAG; Mdm2 forward/reverse:
GACTACTGGAAGTGTCCCAAAT/GTCCACTCCATCATCTGTTTCT; TERT
forward/reverse: GTGTGTGTGTCCTGGGTAAA/CAGCCTGAGGTCTAA GAAGATG;
β-actin forward/reverse: CCCAGACATCAG
GGAGTGAT/TCTCTGTTGGCTTTGGATT. Subsequently, 10 μl PCR products were
loaded into gels for agarose gel electrophoresis.
Statistical analysis
Data are presented as mean ± SD (n=30). Differences
between groups were assessed by analysis of variance (ANOVA) and
Student’s t-tests. Statistical analyses was carried out using SPSS
for Windows, Version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Zebrafish embryo bioassay for assessing
the toxicity of LBPs
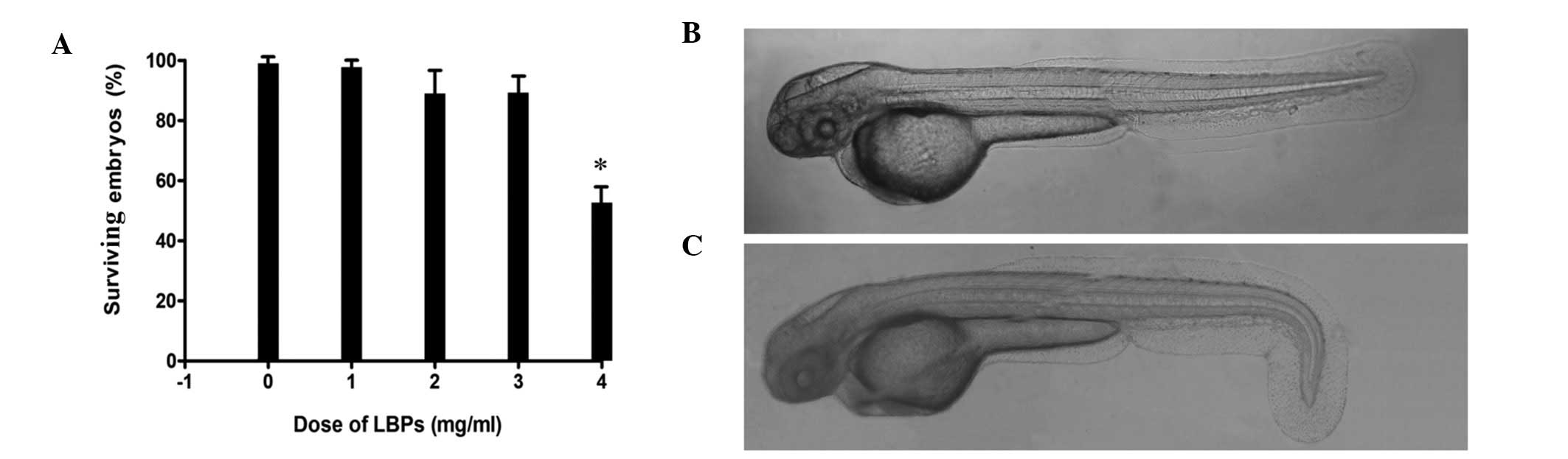
The dose of LBPs that was lethal for embryos was
determined by incubating embryos in different concentrations of
LBPs. The results indicated that the growth and development of
embryos were significantly inhibited at the concentration of 4.0
mg/ml (Fig. 1A). The number of
surviving embryos was not significantly different among the lower
concentrations (0, 1, 2 and 3 mg/ml). Half of the embryos died in
this experiment, and the embryos showed an abnormal phenotype
(Fig. 1B and C) when treated with
the highest concentration of LBPs tested in the present study (4
mg/ml).
Assessing senescence in zebrafish
embryos
SA-β-gal staining
Cytochemically and histochemically detectable
SA-β-gal at pH 6.0 has been shown to increase during the
replicative senescence of cells in vitro and in tissue
samples (25), and has
subsequently been widely used as an in vivo and in
vitro marker of cellular senescence in a number of vertebrate
animal systems (26–28). To assess the effect of LBPs on
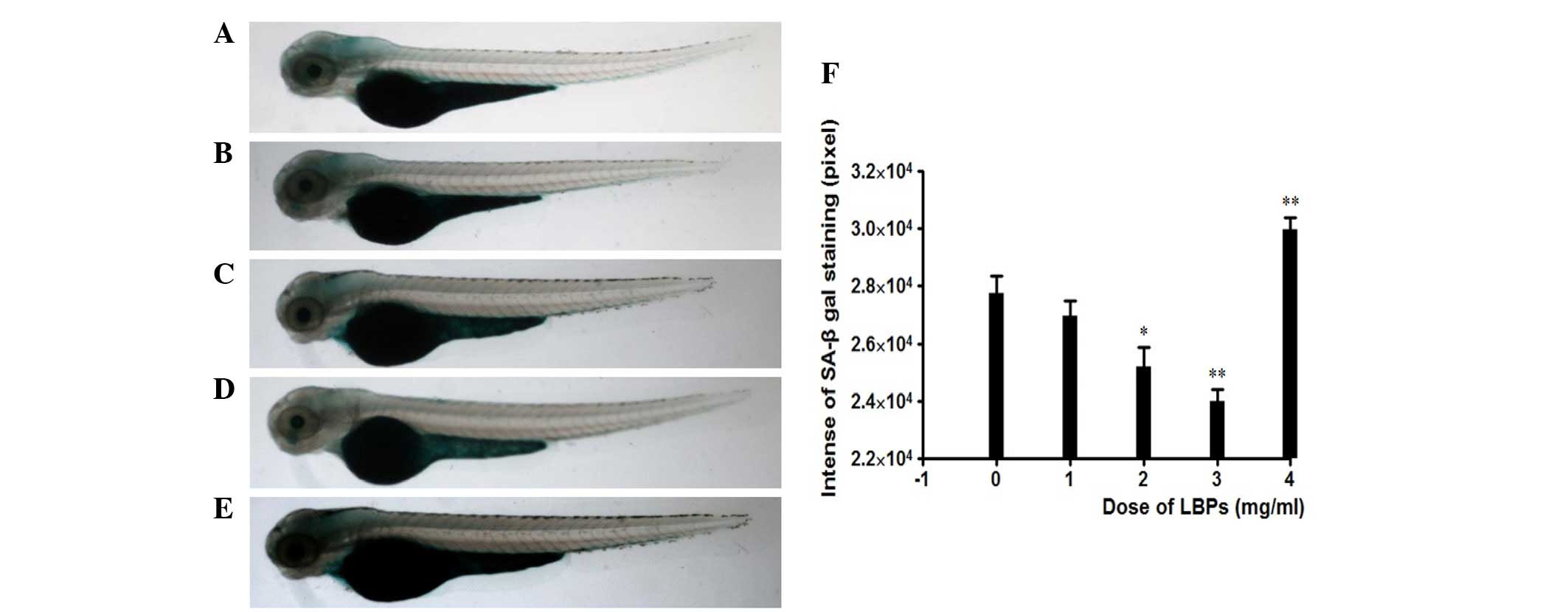
replicative senescence, the SA-β-gal activity was histochemically
detected at pH 6.0 in the 72 hpf embryos. The untreated embryos
exhibited low background staining, especially in the head (Fig. 2A), while embryos treated with LBPs
showed faint background staining, which was reduced with treatment
with increased concentrations of LBPs (Fig. 2B–D). Only at the 4 mg/ml
concentration were embryos more strongly stained compared to
untreated ones and to those treated with 1–3 mg/ml LBPs (Fig. 2E). Quantification of SA-β-gal
staining was performed using high-resolution digital imagery.
SA-β-gal staining at 1, 2 and 3 mg/ml LBPs was estimated to
correspond to 88.3, 81.7 and 68.3% of the staining observed in the
control (treated with 0 mg/ml LBPs), while it was 112.7% of the
control for the 4 mg/ml LBPs, as shown in Fig. 2F. The less amount of staining was
equivalent to that of the less senescent mass.
Acridine orange (AO) staining
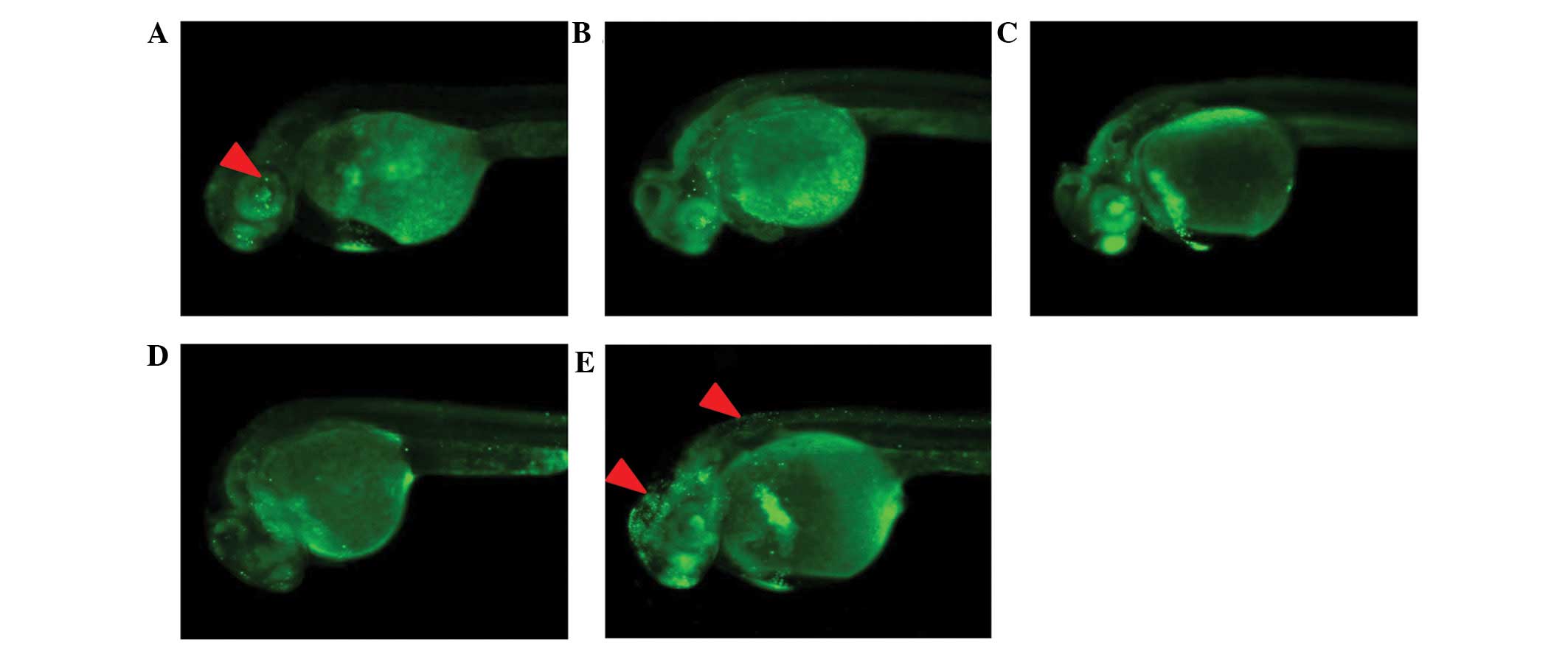
To explore the effect of LBPs on apoptosis, AO
staining was performed. Treatment with 1–3 mg/ml LBPs reduced cell
apoptosis in a dose-dependent manner (Fig. 3A–D), while at the 4 mg/ml LBPs
concentration, cell apoptosis was induced, as observed from the
comparison to control animals and to those treated with lower LBPs
concentrations (Fig. 3E). Embryos
treated with 4 mg/ml LBPs showed high levels of AO staining (red
arrowheads) in the brain and the neural tube, compared to
non-treated embryos (Fig. 3A) at
24 hpf.
The expression levels of genes related
to the p53 signaling pathway after LBPs treatment
It was reported that in MCF-7 breast cancer cells,
LBPs can activate ERK, which may be associated with the p53
signaling pathway (15). There is
also the hypothesis that different types of intrinsic and extrinsic
stress signals likely converge on the activation of the p53
protein, the Rb protein, or both during senescence (29). In this context, we examined whether
zebrafish and mammals share similar mechanisms for the response to
LBPs treatment. To further investigate p53-dependent
transcriptional responses in the treated embryos, we examined the
expression of p53, along with that of response genes such as
p21, Mdm2 and Bax, using semi-quantitative
RT-PCR and the β-actin gene as a control of baseline expression.
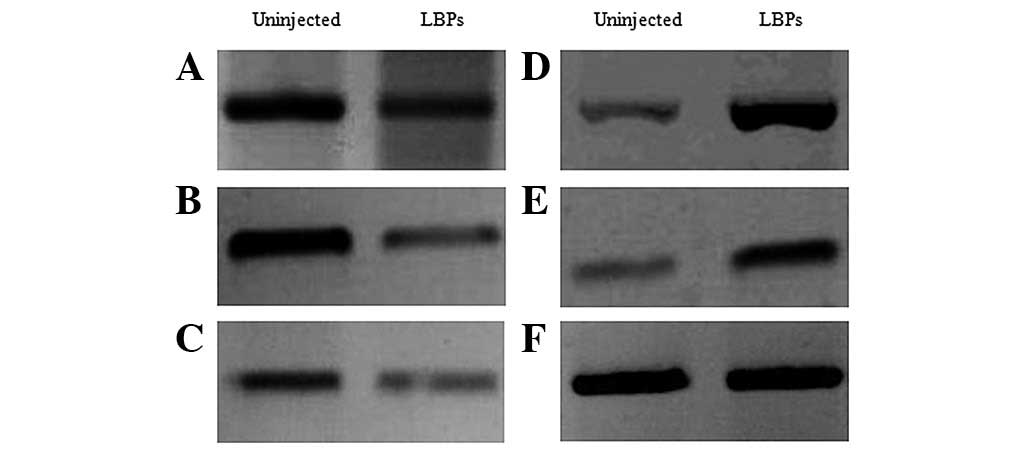
The results showed that the expression level of p53,
p21 and Bax were decreased in treated embryos
compared to non-treated ones (Fig.
4A–C), while the levels of Mdm2 and even, that of the
cellular senescence-related (30–32)
telomerase reverse transcriptase (TERT), which allows to
maintain the telomere ends, were increased (Fig. 4D and E).
Discussion
L. barbarum fruits (berries) or extract have
been widely used for centuries to balance the ‘Yin’ and the ‘Yang’
in the body (33). The major
ingredient of the liquid fraction of these berries, LBPs, has been
the object of research focus in studies aiming to identify
anti-aging remedies (34) or
agents alleviating cellular damage (12,35).
p53, as a central mediator of cellular responses
induced in various processes including apoptosis (36), was shown to exert similar effects
to ERK during stress induced by DNA damage (37). In MCF-7 cells, LBPs increased the
activity of ERK (15). p21 as a
regulator of cell cycle progression controlled by the tumor
suppressor protein p53, inhibited tumor progression, thus
preventing cell proliferation, while inducing cell apoptosis
(38). In our study, cell
apoptosis was inhibited following treatment with non-toxic doses of
LBPs (1–3 mg/ml), as shown by AO staining, the reduced expression
of p53 and the increased expression of its negative
regulator, Mdm2. A potential explanation for these findings
is that the cellular responses triggered by the activated p53
protein (cellular senescence) act as potent barriers against the
effects of LBPs.
In this study, we assessed the relevance of one
molecular mechanism potentially underlying the effect of LBPs on
cellular senescence in a zebrafish model by phenotypic and SA-β-gal
assays, in vivo detection of survival rates and expression
profiling of genes that related to the p53 signaling pathway during
senescence. Zebrafish senescence was alleviated by LBPs via
inhibition of cell death and apoptosis in the early development, a
reduction in the expression level of p53, p21 and
Bax genes and an increase in the expression of Mdm2
and TERT genes. In conclusion, our findings suggest that the
anti-aging effects of LBPs are mediated by the p53 signal pathway.
The specific targets of LBPs in this pathway may be identified in
future studies.
Acknowledgements
This study was financially supported by a grant from
the Jilin Provincial Science & Technology Department (no.
201215227).
References
|
1
|
Harman D: The free radical theory of
aging. Antioxid Redox Signal. 5:557–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greer EL and Brunet A: Signaling networks
in aging. J Cell Sci. 121:407–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bastianetto S and Quirion R: Natural
extracts as possible protective agents of brain aging. Neurobiol
Aging. 23:891–897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang IM: Anti-aging and health-promoting
constituents derived from traditional oriental herbal remedies:
information retrieval using the TradiMed 2000 DB. Ann NY Acad Sci.
928:281–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan L, Zhang SH, Liu Q and Xu HB: A
polysaccharide-protein complex from Lycium barbarum
upregulates cytokine expression in human peripheral blood
mononuclear cells. Eur J Pharmacol. 471:217–222. 2003.PubMed/NCBI
|
|
6
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative
stress in aged mice. J Ethnopharmacol. 111:504–511. 2007.
|
|
7
|
Zhang M, Chen H, Huang J, Li Z, Zhu C and
Zhang S: Effect of lycium barbarum polysaccharide on human
hepatoma QGY7703 cells: inhibition of proliferation and induction
of apoptosis. Life Sci. 76:2115–2124. 2005.
|
|
8
|
Du G, Liu L and Fang J: Experimental study
on the enhancement of murine splenic lymphocyte proliferation by
Lycium barbarum glycopeptide. J Huazhong Univ Sci Technolog
Med Sci. 24:518–520. 5272004.PubMed/NCBI
|
|
9
|
Vidal K, Benyacoub J, Sanchez-Garcia J, et
al: Intake of a milk-based wolfberry formulation enhances the
immune response of young-adult and aged mice. Rejuvenation Res.
13:47–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu MS, Lai CS, Ho YS, et al:
Characterization of the effects of anti-aging medicine Fructus
lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med.
20:261–268. 2007.PubMed/NCBI
|
|
11
|
Yu MS, Leung SK, Lai SW, et al:
Neuroprotective effects of anti-aging oriental medicine Lycium
barbarum against beta-amyloid peptide neurotoxicity. Exp
Gerontol. 40:716–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li SY, Yang D, Yeung CM, et al: Lycium
barbarum polysaccharides reduce neuronal damage, blood-retinal
barrier disruption and oxidative stress in retinal
ischemia/reperfusion injury. PLoS One. 6:e163802011. View Article : Google Scholar
|
|
13
|
Jin M, Huang Q, Zhao K and Shang P:
Biological activities and potential health benefit effects of
polysaccharides isolated from Lycium barbarum L. Int J Biol
Macromol. 54:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
May P and May E: Twenty years of p53
research: structural and functional aspects of the p53 protein.
Oncogene. 18:7621–7636. 1999.PubMed/NCBI
|
|
15
|
Shen L and Du G: Lycium barbarum
polysaccharide stimulates proliferation of MCF-7 cells by the ERK
pathway. Life Sci. 91:353–357. 2012. View Article : Google Scholar
|
|
16
|
Keller ET and Murtha JM: The use of mature
zebrafish (Danio rerio) as a model for human aging and
disease. Comp Biochem Physiol C Toxicol Pharmacol. 138:335–341.
2004.PubMed/NCBI
|
|
17
|
Tsai SB, Tucci V, Uchiyama J, et al:
Differential effects of genotoxic stress on both concurrent body
growth and gradual senescence in the adult zebrafish. Aging Cell.
6:209–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parng C, Seng WL, Semino C and McGrath P:
Zebrafish: a preclinical model for drug screening. Assay Drug Dev
Technol. 1:41–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerhard GS, Kauffman EJ, Wang X, et al:
Life spans and senescent phenotypes in two strains of zebrafish
(Danio rerio). Exp Gerontol. 37:1055–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herrera M and Jagadeeswaran P: Annual fish
as a genetic model for aging. J Gerontol A Biol Sci Med Sci.
59:101–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granato M and Nusslein-Volhard C: Fishing
for genes controlling development. Curr Opin Genet Dev. 6:461–468.
1996. View Article : Google Scholar
|
|
22
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kishi S, Bayliss PE, Uchiyama J, et al:
The identification of zebrafish mutants showing alterations in
senescence-associated biomarkers. PLoS Genet. 4:e10001522008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robu ME, Larson JD, Nasevicius A, et al:
p53 activation by knockdown technologies. PLoS Genet. 3:e782007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dimri GP, Lee X, Basile G, et al: A
biomarker that identifies senescent human cells in culture and in
aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao L, Li W, Kim S, Brodie SG and Deng CX:
Senescence, aging, and malignant transformation mediated by p53 in
mice lacking the Brca1 full-length isoform. Genes Dev. 17:201–213.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW
and Mills AA: p63 deficiency activates a program of cellular
senescence and leads to accelerated aging. Genes Dev. 19:1986–1999.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikegami R, Zhang J, Rivera-Bennetts AK and
Yager TD: Activation of the metaphase checkpoint and an apoptosis
programme in the early zebrafish embryo, by treatment with the
spindle-destabilising agent nocodazole. Zygote. 5:329–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ben-Porath I and Weinberg RA: When cells
get stressed: an integrative view of cellular senescence. J Clin
Invest. 113:8–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Concetti F, Lucarini N, Carpi FM, et al:
The functional VNTR MNS16A of the TERT gene is associated with
human longevity in a population of Central Italy. Exp Gerontol.
48:587–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaskelioff M, Muller FL, Paik JH, et al:
Telomerase reactivation reverses tissue degeneration in aged
telomerase-deficient mice. Nature. 469:102–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wyllie FS, Jones CJ, Skinner JW, et al:
Telomerase prevents the accelerated cell ageing of Werner syndrome
fibroblasts. Nat Genet. 24:16–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008.PubMed/NCBI
|
|
34
|
Potterat O: Goji (Lycium barbarum
and L. chinense): Phytochemistry, pharmacology and safety in
the perspective of traditional uses and recent popularity. Planta
Med. 76:7–19. 2010.
|
|
35
|
Yang D, Li SY, Yeung CM, et al: Lycium
barbarum extracts protect the brain from blood-brain barrier
disruption and cerebral edema in experimental stroke. PLoS One.
7:e335962012. View Article : Google Scholar
|
|
36
|
Koivusalo R, Krausz E, Ruotsalainen P,
Helenius H and Hietanen S: Chemoradiation of cervical cancer cells:
targeting human papillomavirus E6 and p53 leads to either augmented
or attenuated apoptosis depending on the platinum carrier ligand.
Cancer Res. 62:7364–7371. 2002.
|
|
37
|
Singh S, Upadhyay AK, Ajay AK and Bhat MK:
p53 regulates ERK activation in carboplatin induced apoptosis in
cervical carcinoma: a novel target of p53 in apoptosis. FEBS Lett.
581:289–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gomez-Manzano C, Fueyo J, Kyritsis AP, et
al: Characterization of p53 and p21 functional interactions in
glioma cells en route to apoptosis. J Natl Cancer Inst.
89:1036–1044. 1997. View Article : Google Scholar : PubMed/NCBI
|