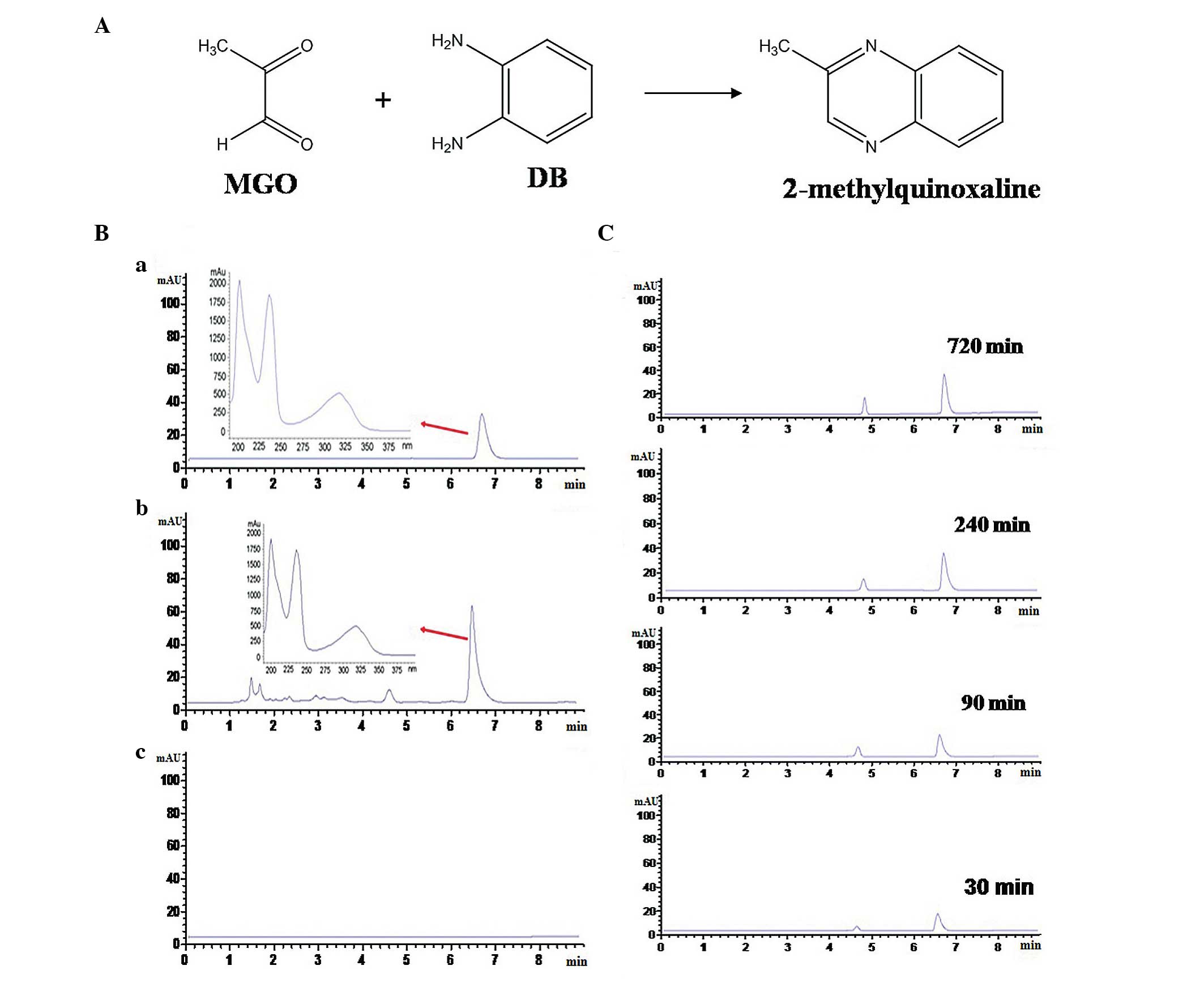
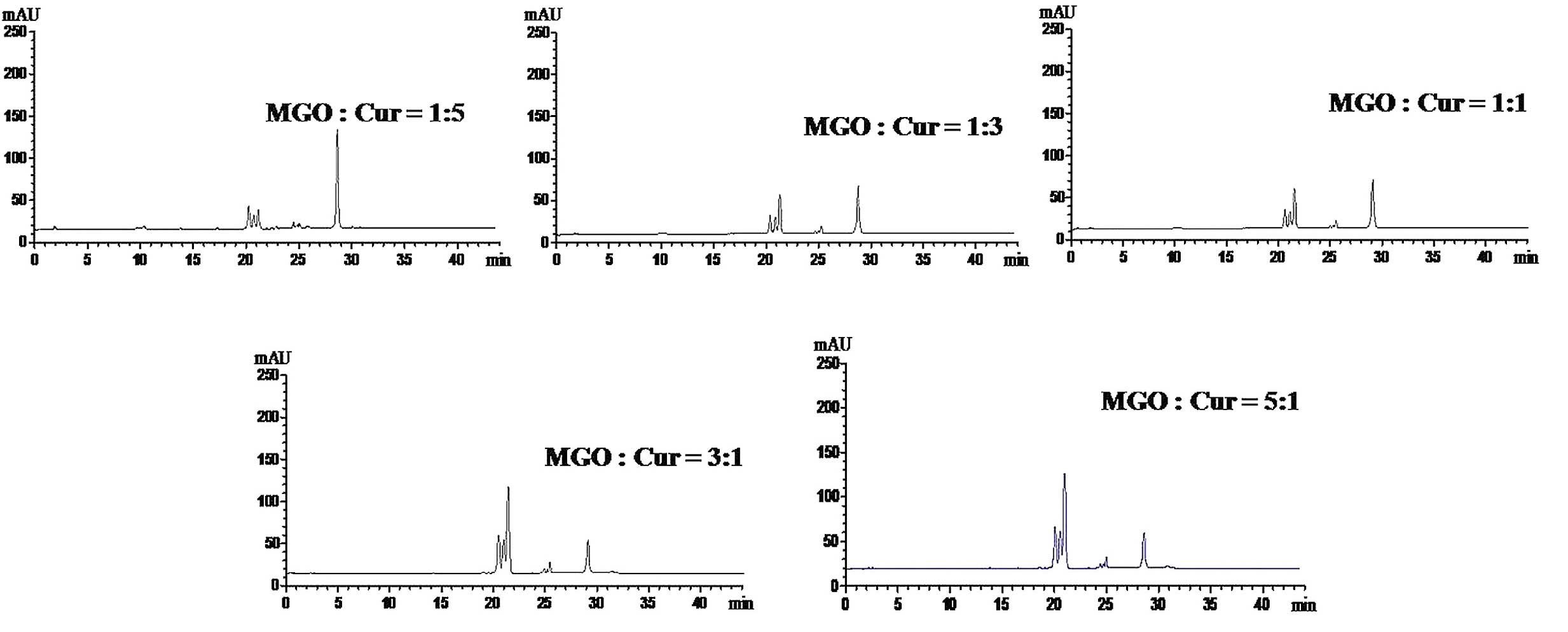
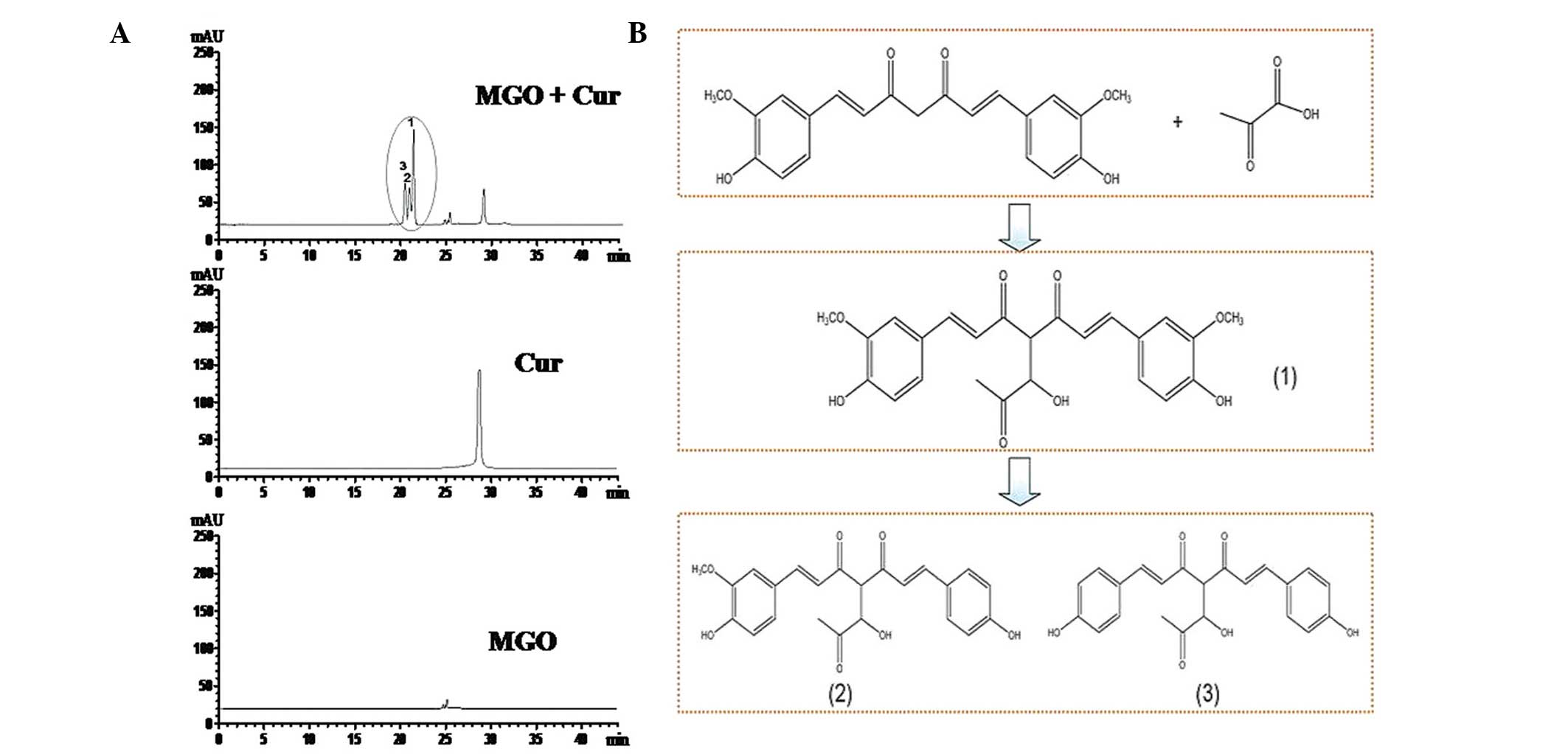
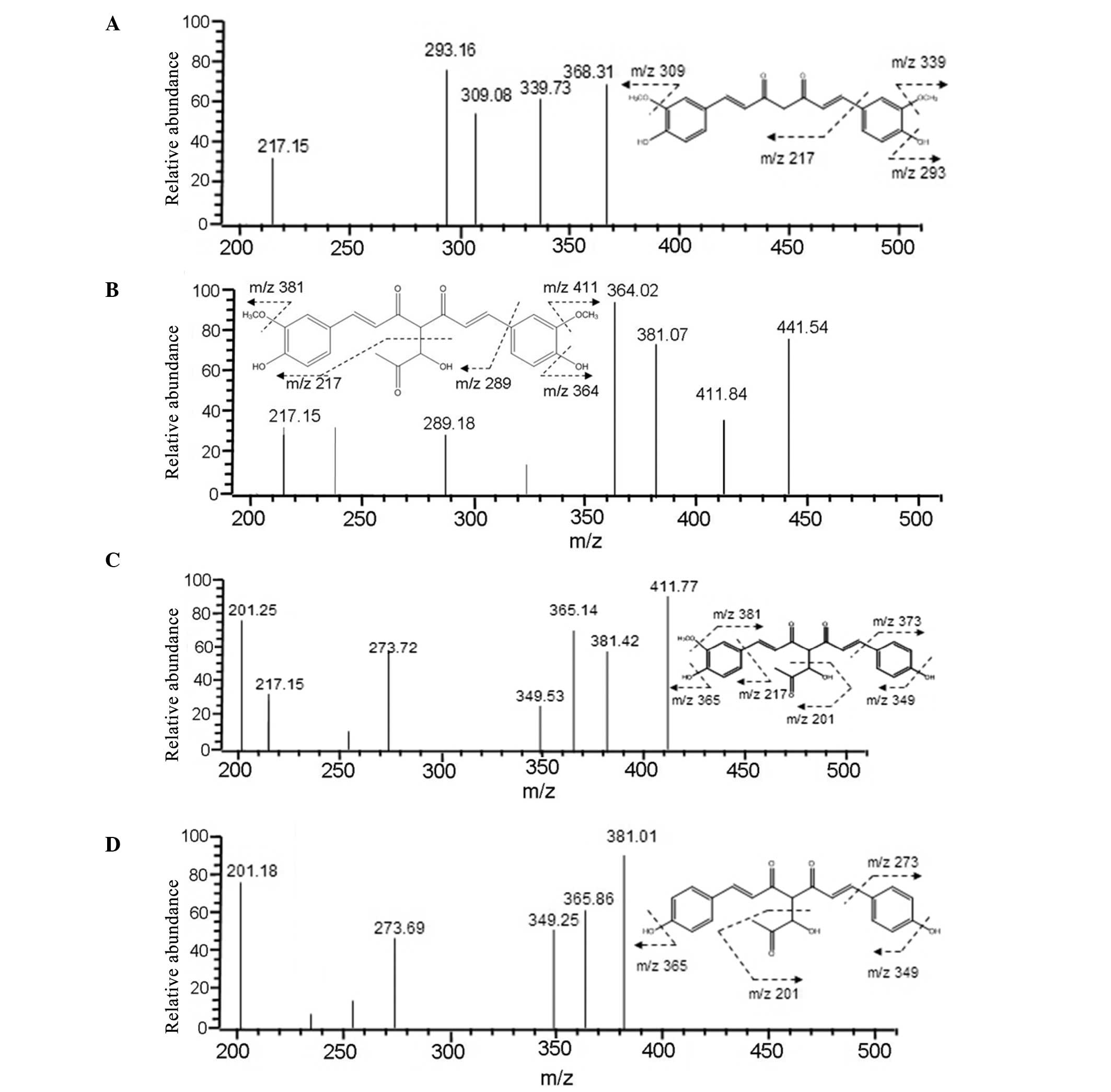
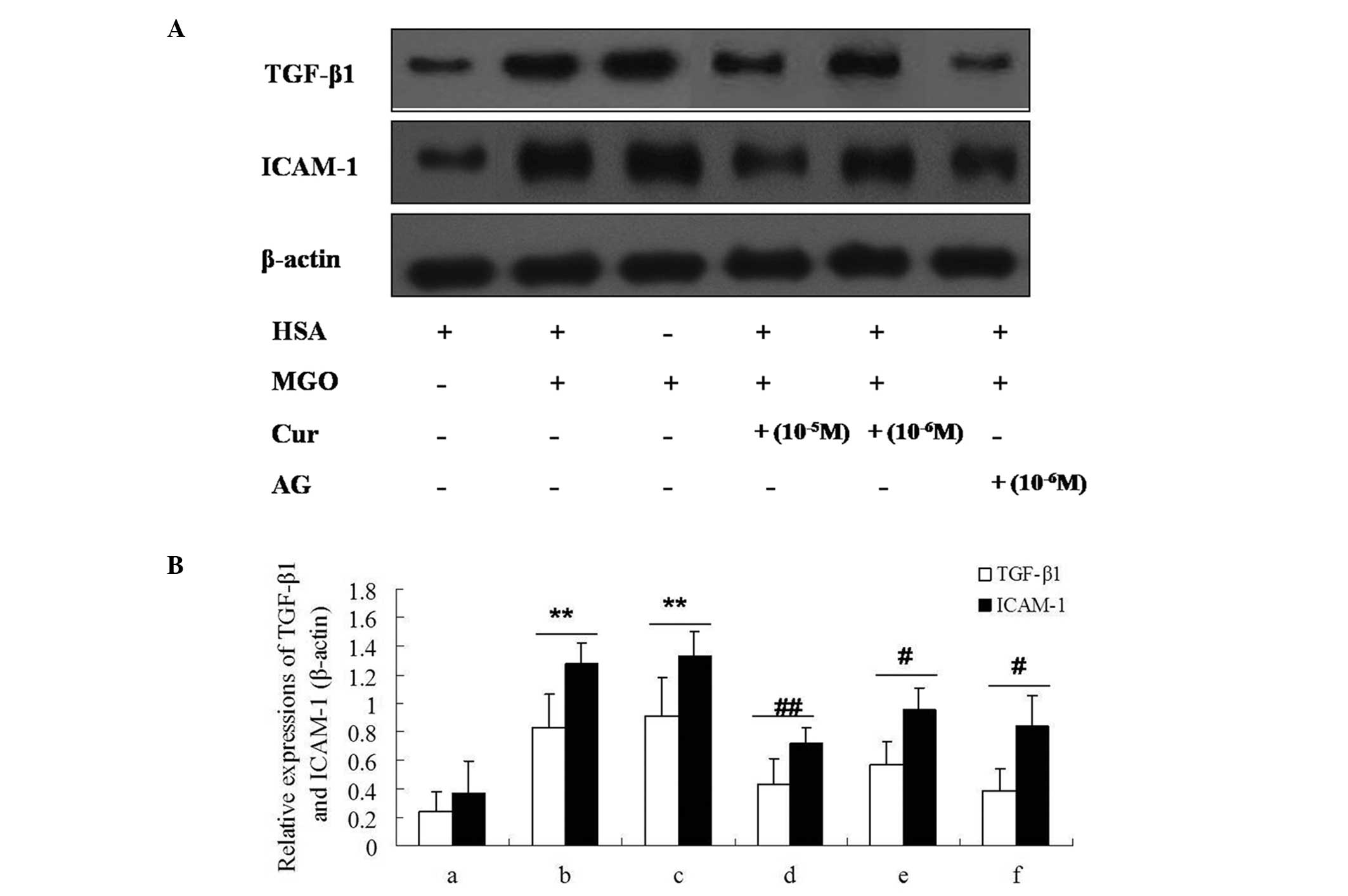
|
1
|
Sabayan B, Westendorp RG, Grond J, Stott
DJ, Sattar N, van Osch MJ, van Buchem MA and de Craen AJ: Markers
of endothelial dysfunction and cerebral blood flow in older adults.
Neurobiol Aging. 35:373–377. 2014. View Article : Google Scholar
|
|
2
|
Žižek B, Žižek D, Bedenčič K, Jerin A and
Poredoš P: Effect of metabolic abnormalities on endothelial
dysfunction in normotensive offspring of subject with hypertension.
Int Angiol. 32:386–393. 2013.PubMed/NCBI
|
|
3
|
Lo CY, Li S, Tan D, Pan MH, Sang S and Ho
CT: Trapping reactions of reactive carbonyl species with tea
polyphenols in simulated physiological conditions. Mol Nutr Food
Res. 50:1118–1128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Eupen MG, Schram MT, Colhoun HM,
Hanssen NM, Niessen HW, Tarnow L, Parving HH, Rossing P, Stehouwer
CD and Schalkwijk CG: The methylglyoxal-derived AGE
tetrahy-dropyrimidine is increased in plasma of individuals with
type 1 diabetes mellitus and in atherosclerotic lesions and is
associated with sVCAM-1. Diabetologia. 56:1845–1855. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akhand AA, Hossain K, Mitsui H, Kato M,
Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, et al:
Glyoxal and methylglyoxal trigger distinct signals for map family
kinases and caspase activation in human endothelial cells. Free
Radic Biol Med. 31:20–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santel T, Pflug G, Hemdan NY, Schäfer A,
Hollenbach M, Buchold M, Hintersdorf A, Lindner I, Otto A, Bigl M,
et al: Curcumin inhibits glyoxalase 1: A possible link to its
anti-inflammatory and anti-tumor activity. PLoS One. 3:e35082008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu JP, Feng L, Zhu MM, Wang RS, Zhang MH,
Hu SY, Jia XB and Wu JJ: The in vitro protective effects of
curcumin and demethoxycurcumin in Curcuma longa extract on advanced
glycation end products-induced mesangial cell apoptosis and
oxidative stress. Planta Med. 78:1757–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu TY, Liu CL, Chen JY and Hu ML: Curcumin
ameliorates methylglyoxal-induced alterations of cellular
morphology and hyperpermeability in human umbilical vein
endothelial cells. J Funct Foods. 5:745–754. 2013. View Article : Google Scholar
|
|
9
|
Lip H, Yang K, MacAllister SL and O'Brien
PJ: Glyoxal and methylglyoxal: Autoxidation from dihydroxyacetone
and polyphenol cytoprotective antioxidant mechanisms. Chem Biol
Interact. 202:267–274. 2013. View Article : Google Scholar
|
|
10
|
Hu TY, Liu CL, Chyau CC and Hu ML:
Trapping of methylglyoxal by curcumin in cell-free systems and in
human umbilical vein endothelial cells. J Agric Food Chem.
60:8190–8196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv L, Shao X, Chen H, Ho CT and Sang S:
Genistein inhibits advanced glycation end product formation by
trapping methylglyoxal. Chem Res Toxicol. 24:579–586. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt A, Bigl K, Meiners I and Schmitt
J: Induction of reactive oxygen species and cell survival in the
presence of advanced glycation end products and similar structures.
Biochim Biophys Acta. 1763:927–936. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng L, Zhu M, Zhang M, Gu J, Jia X, Tan
X, Gao C and Zhu Q: The protection of
4,4′-diphenylmethane-bis(methyl) carbamate from Cortex Mori on
advanced glycation end product-induced endothelial dysfunction: Via
inhibiting AGE formation or blocking AGEs-RAGE axis? Fitoterapia.
89:239–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Gu JF, Zou X, Wu J, Zhang MH, Jiang
J, Qin D, Zhou JY, Liu BX, Zhu YT, et al: The anti-lung cancer
activities of steroidal saponins of P polyphylla Smith var
chinensis (Franch) Hara through enhanced immunostimulation in
experimental Lewis tumor-bearing C57BL/6 mice and induction of
apoptosis in the A549 cell line. Molecules. 18:12916–12936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng L, Zhu M, Zhang M, Jia X, Cheng X,
Ding S and Zhu Q: Amelioration of compound
4,4′-diphenylmethane-bis(methyl) carbamate on high mobility group
box1-mediated inflammation and oxidant stress responses in human
umbilical vein endothelial cells via RAGE/ERK1/2/NF-κB pathway. Int
Immunopharmacol. 15:206–216. 2013. View Article : Google Scholar
|
|
17
|
Li W, Maloney RE, Circu ML, Alexander JS
and Aw TY: Acute carbonyl stress induces occludin glycation and
brain microvascular endothelial barrier dysfunction: Role for
gluta-thione-dependent metabolism of methylglyoxal. Free Radic Biol
Med. 54:51–61. 2013. View Article : Google Scholar :
|
|
18
|
Singh R, Kristensen S and Tønnesen HH:
Influence of vehicle properties and excipients on hydrolytic and
photochemical stability of curcumin in preparations containing
Pluronics: Studies of curcumin and curcuminoids XLVIII. Pharmazie.
68:160–169. 2013.PubMed/NCBI
|
|
19
|
Deshpande SS and Maru GB: Effects of
curcumin on the formation of benzo[a]pyrene derived DNA adducts in
vitro. Cancer Lett. 96:71–80. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okouchi M, Okayama N and Aw TY:
Preservation of cellular glutathione status and mitochondrial
membrane potential by N-acetylcysteine and insulin sensitizers
prevent carbonyl stress-induced human brain endothelial cell
apoptosis. Curr Neurovasc Res. 6:267–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciolino HP and Levine RL: Modification of
proteins in endothelial cell death during oxidative stress. Free
Radic Biol Med. 22:1277–1282. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tatsunami R, Oba T, Takahashi K and Tampo
Y: Methylglyoxal causes dysfunction of thioredoxin and thioredoxin
reductase in endothelial cells. J Pharmacol Sci. 111:426–432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazawa N, Abe M, Souma T, Tanemoto M,
Abe T, Nakayama M and Ito S: Methylglyoxal augments intracellular
oxidative stress in human aortic endothelial cells. Free Radic Res.
44:101–107. 2010. View Article : Google Scholar
|
|
24
|
Szarka A, Rigó J Jr, Lázár L, Beko G and
Molvarec A: Circulating cytokines, chemokines and adhesion
molecules in normal pregnancy and preeclampsia determined by
multiplex suspension array. BMC Immunol. 11:592010. View Article : Google Scholar : PubMed/NCBI
|