Introduction
In 1984, Zeldin et al (1) reported 10 cases of pulmonary
complications following pneumonectomy under one-lung ventilation
(OLV). Since then, it has been well established that the mechanisms
of OLV-induced acute lung injury (ALI) are similar to those of
ventilator-induced lung injury, in which inflammatory responses
serve a key role (2–4). Clinically, there is no specific
measure to prevent ALI induced by mechanical ventilation, apart
from certain protective ventilation strategies, including using
variable tidal volumes, tidal volume <8 ml/kg predicted body
weight, pressure control ventilation, and peak inspiratory pressure
(PIP) <35 cm H2O (4). However, animal studies have
demonstrated that, even with protective ventilation measures,
inflammatory responses still occurred (5). Therefore, it may be hypothesized that
inhibition of inflammation responses may alleviate lung injury
induced by OLV.
Phospholipase A2 (PLA2), a key
rate-limiting enzyme, catalyzes the hydrolysis of membrane
phospholipids to lysophospholipids, platelet-activation factor and
arachidonic acid (AA) (6). Group
IVA cytosolic c-PLA2 (GIVA c-PLA2; molecular
weight 85 kDa), which preferentially hydrolyzes AA at the
sn−2 position of phospholipids, is a member of the large
c-PLA2 family (7,8).
Currently, GIVA c-PLA2 is generally considered to be a
key enzyme mediating in the generation of multiple lipid mediators,
including the agonist-induced release of AA for eicosanoid
production, and is recognized as a potentially important
pharmacological target for the control of inflammatory diseases
(9). The downstream products of AA
and its metabolism possess the potential to increase vascular
permeability, which may lead to inflammatory responses (10,11).
The inhibition of cPLA2 may attenuate the OLV-induced
acute lung injury by reducing the downstream products in AA
metabolism, which is responsible for the increases in vascular
permeability, the recruitment of neutrophils and cell injury
(12).
Club cell secretory protein (CCSP) is secreted by
non-ciliated club cells (formerly referred to as Clara cells) into
the bronchial epithelium of the mammalian lung. As a biomarker of
club cells and lung health, CCSP has been used as a useful
diagnostic marker for toxicant exposure or airway epithelial
damages (13). Mice deficient in
CCSP developed more prominent lung injury and inflammatory
responses following exposure to oxidant treatment or virus
challenge, which indicated that CCSP acts as an endogenous
inhibitor of PLA2 (14,15).
Sevoflurane (SVF) is widely used in the induction
and maintenance of general anesthesia. A number of studies
demonstrated that SVF has anti-inflammation properties and it is
also involved in the protective mechanisms against
ischemia/reperfusion injury (16,17).
A previous study demonstrated that, in OLV-induced acute lung
injury model, SVF inhalation significantly reduced the lung injury
decreased c-PLA2 expression levels, lung wet-to-dry
(W/D) weight ratios and histological scores in a dose-dependent
manner. However, SVF inhalation exerted no significant effect on
the expression of CCSP (18).
These results suggested that the protective role of SVF against
OLV-induced acute lung injury may be associated with
c-PLA2 regulation, but not with CCSP. In the present
study, club cell-exfoliated rabbits were used to determine whether
SVF attenuates pulmonary inflammation via c-PLA2
downregulation, rather than CCSP regulation.
Materials and methods
Ethics approval
The present study complied with the Guidelines for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (19). All
experimental procedures were approved by the Animal Ethics
Committee and Research Ethics Committee of Yunnan Province, China
(license no. KM3476-821), and Kunming Medical University (Kunming,
China; license no. KY976222-m23).
Animal preparation
The present study strictly abided by the guidelines
of Kunming Medical University for the care and use of laboratory
animals. A total of 36 healthy Japanese white rabbits (weight,
2.2–2.5 kg) of either sex (1:1 male:female) were purchased from the
Experimental Animal Center of Kunming Medical University [Animal
Certificate of Conformity no. 0020946; animal license no. scxk
(Yunnan) 2011–0004]. The rabbits were randomly divided into six
groups (n=6 per group): Sham-operated group (group S), OLV group
(group O), OLV+SVF inhalation group (group OF), club cells
exfoliated + sham-operated group (group NA), club cells exfoliated
+ OLV group (group NAO) and club cells exfoliated + OLV +
sevoflurane group (group NAOF).
In group S, the trachea, left common carotid artery
and right external jugular vein were exposed, followed by placing
an endotracheal tube (inner diameter of 2.0 mm) via tracheotomy
between tracheal rings 2 and 3, without further surgery. OLV was
achieved by placing the tracheotomy tube into the right main
bronchus. Mechanical ventilation was performed by OLV for 2 h
followed by two-lung ventilation (TLV) for 1 h with an identical
ventilator (Aestiva/5 7900; Datex Ohmeda Inc.; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). The setting parameters were as
for groups that received OLV were as follows: Inspired oxygen
fraction, 1.0; tidal volume, 20 ml/kg; respiration rate, 30
breaths/min; and inspiratory:expiratory ratio, 1:2. SVF (2.5% vol)
was administered during mechanical ventilation by ventilator
(Aestiva/5 7900, as above; group OF and NAOF). For animals in the
group NA, exfoliating club cells were created by exposure to
naphthalene vapor at a concentration of 100 mg/l for 12 h.
Anesthesia and intraoperative
detections
The mean arterial pressure, end tidal CO2
and SVF concentrations were continuously monitored and maintained
within normal ranges. Ringer's solution (10 ml/kg/h) was
continuously infused through the right external jugular vein
catheter. The rabbits were anesthetized with pentobarbital sodium
(30 mg/kg intravenously; Shandong West Asia Chemical Industry Co.,
Ltd., Shandong, China) during the sham surgeries. Anesthesia was
maintained by continuous infusion of remifentanil (cat. no.
1110710; Yichang Humanwell Pharmaceutical Co., Ltd., Yichang,
China) at a rate of 1 µg/kg/min and intermittent administration of
vecuronium (0.1 mg/kg per 30 min; cat. no. 111004.2; Zhejiang
Xianju Pharmaceutical Co., Ltd., Zhejiang, China) during either OLV
or TLV.
Lung W/D ratio evaluation
The right lower lobe was excised and the wet weight
was recorded. The lobe was then desiccated at 80°C for 72 h to
measure the dry weight. The W/D ratio was calculated.
Lung histological score
The right upper lobe were fixed for 6–12 h in 4%
paraformaldehyde at 20–24°C, embedded in paraffin wax and then cut
into 5 µm sections using a microtome. The sections were stained
using a hematoxylin and eosin staining kit (cat. no. G1120; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
20–24°C according to the manufacturer's instructions. In brief,
sections were stained in hematoxylin (1 g/l) for 16 min and eosin
(1 g/100 ml) for 15 sec. Slides were viewed by a pathology
technician using light microscopy for histological evaluation of
the lung injury.
Quantification of CCSP and
c-PLA2 mRNA expression levels
Total RNA was extracted from the right middle lobes
using a TRIzol RNA Extraction kit (cat. no. 12183555; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The first-strand cDNA was
synthesized using Superscript IV Reverse Transcriptase system (cat.
no. 18091050; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, 50 µM Oligo(dT)20 and 10 mM dNTP
mix was added into 1.0 µg total RNA and annealed at 65°C for 5 min.
Following this, 200 U/µl Superscript IV reverse transcriptase and
100 mM DTT was added in the presence of SSIV buffer, and the RT
system was incubated for 10 min at 55°C, followed by 10 min at
80°C. Reverse transcription-quantitative polymerase chain reaction
was performed using a BioEasy SYBR Green I Real Time PCR kit (cat.
no. 04913850001; Roche Diagnostics GmbH) according to the
manufacturer's protocols. The reaction took place at 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec.
The experiment was replicated three times per sample. The results
were quantified using the 2−ΔΔCq method (20). The forward and reverse sequences
for the primers (5′-3′) used in this study were as follows:
β-actin, forward CATCCTGACGCTCAAGTA, reverse GTTGTAGAAGGTGTGGTG;
CCSP, forward CACCAAAGCCTCCAACCT, reverse GGCAGATGTCCGAAGAAG;
c-PLA2, forward CCTAATCATTGTTGGAAGATAA, reverse
ATGGTTGCTTGGAGAATA.
Western blotting
Freshly frozen samples of lung tissues from the
rabbits were homogenized in pre-cooled radioimmunoprecipitation
assay lysis buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology, Haimen, China) supplemented with 15 µl protease
inhibitor cocktail (cat. no. 5892970001; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The protein concentrations were determined by
using a bicinchoninic acid kit (cat. no. P0010; Beyotime Institute
of Biotechnology). Proteins (80 µg/lane) were separated by 10%
SDS-PAGE. The membrane was blocked with 5% bovine serum albumin
(cat. no. V900933; Sigma-Aldrich; Merck KGaA) at 20–24°C for 1.5 h.
GAPDH was used as the reference gene. For western blotting, the
nitrocellulose membranes were incubated overnight at 4°C with
primary antibodies against CCSP (2 µg/ml; cat. no. ab50711; Abcam,
Cambridge, UK), c-PLA2 (1 µg/ml; cat. no. ab73406;
Abcam) and GAPDH (1:2,000; cat. no. 20011; Abmart, Inc., Shanghai,
China), followed by incubation with the secondary antibody (cat.
no. M21003; Abmart) at room temperature for 2 h. The blots were
detected by enhanced chemiluminescence (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and the band intensities were quantified using
ImageJ software 1.44 (National Institutes of Health, Bethesda, MA,
USA).
ELISA
The content of AA in lung tissues was measured using
a commercially available sandwich ELISA kit (cat. no.
CSB-EQ027590RB; Wuhan Huamei Biotech Co., Ltd., Wuhan, China)
according to the manufacturer's protocols.
Statistical analysis
The quantitative data are presented as the mean ±
standard error of the mean. One-way analysis of variance (with
repeated measures) followed by Fisher's least significant
difference post hoc test was used as indicated for comparisons
between the groups. SPSS v18.0 (SPSS, Inc., Chicago, IL, USA) was
used to perform the statistical analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of sevoflurane on the
expression levels of c-PLA2 and CCSP
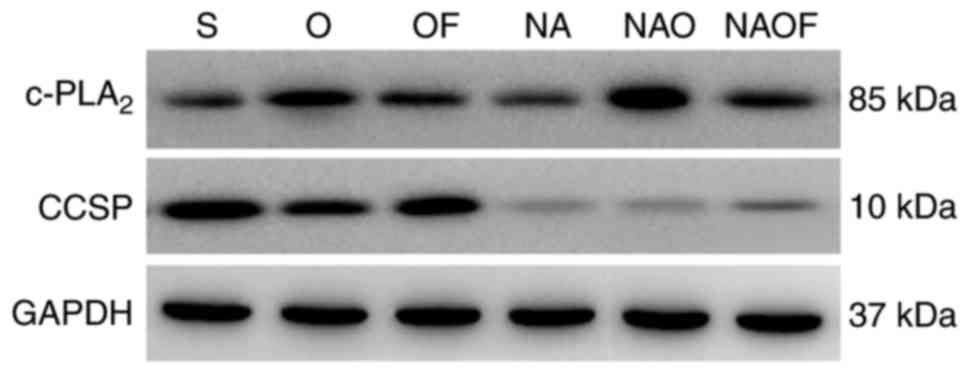
In the group O, c-PLA2 expression was
significantly increased compared with the sham group (Fig. 1A), whereas CCSP expression was
decreased following OLV (P<0.05 vs. group S; Fig. 1B). Following OLV, SVF inhalation
(group OF) significantly reduced c-PLA2 expression
(Fig. 1A), and CCSP expression
increased (vs. group O; Fig.
1B).
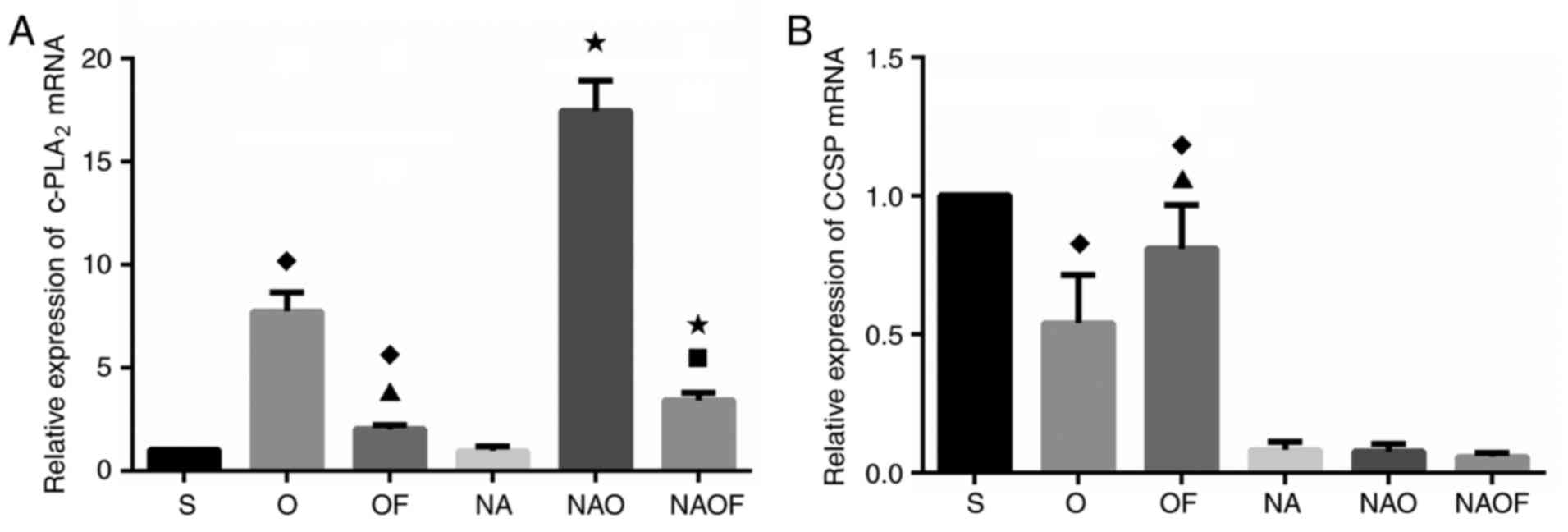 | Figure 1.Effects of OLV and sevoflurane
inhalation on the expression levels of c-PLA2 and CCSP
mRNA in rabbit lung. Relative expression levels of (A)
c-PLA2, and (B) CCSP quantified by reverse
transcription-quantitative polymerase chain reaction.
♦P<0.05 vs. S; ▲P<0.05 vs. O;
*P<0.05 vs. NA; ■P<0.05 vs. NAO. OLV, one-lung
ventilation; c-PLA2, cytosolic phospholipase
A2; S, sham-operated group; O, OLV group; OF, OLV +
sevoflurane group; NA, club cells exfoliated + sham-operated group;
NAO, club cells exfoliated + OLV group; NAOF, club cells exfoliated
+ OLV + sevoflurane group; CCSP, club cell secretory protein. |
The effects of SVF on the expressional changes of
CCSP, an endogenous inhibitor of PLA2, was further
analyzed using the club cell-exfoliated model exposed to
naphthalene vapor at a concentration of 100 mg/l for 12 h (groups
NA, NAO and NAOF). Low expression levels of CCSP were detected at
the mRNA and protein level in groups NA, NAO and NAOF, indicating
that club cells were successfully exfoliated (Figs. 1B and 2). As the endogenous inhibitor of
c-PLA2 was not present, the expression of
c-PLA2 was the highest in group NAO. However, following
SVF inhalation in the group NAOF, the expression of
c-PLA2 was significantly reduced compared with group NAO
(P<0.05; Fig. 1A). A similar
pattern of c-PLA2 and CCSP protein expression was
observed in the lungs of the three groups of rabbits (Fig. 2).
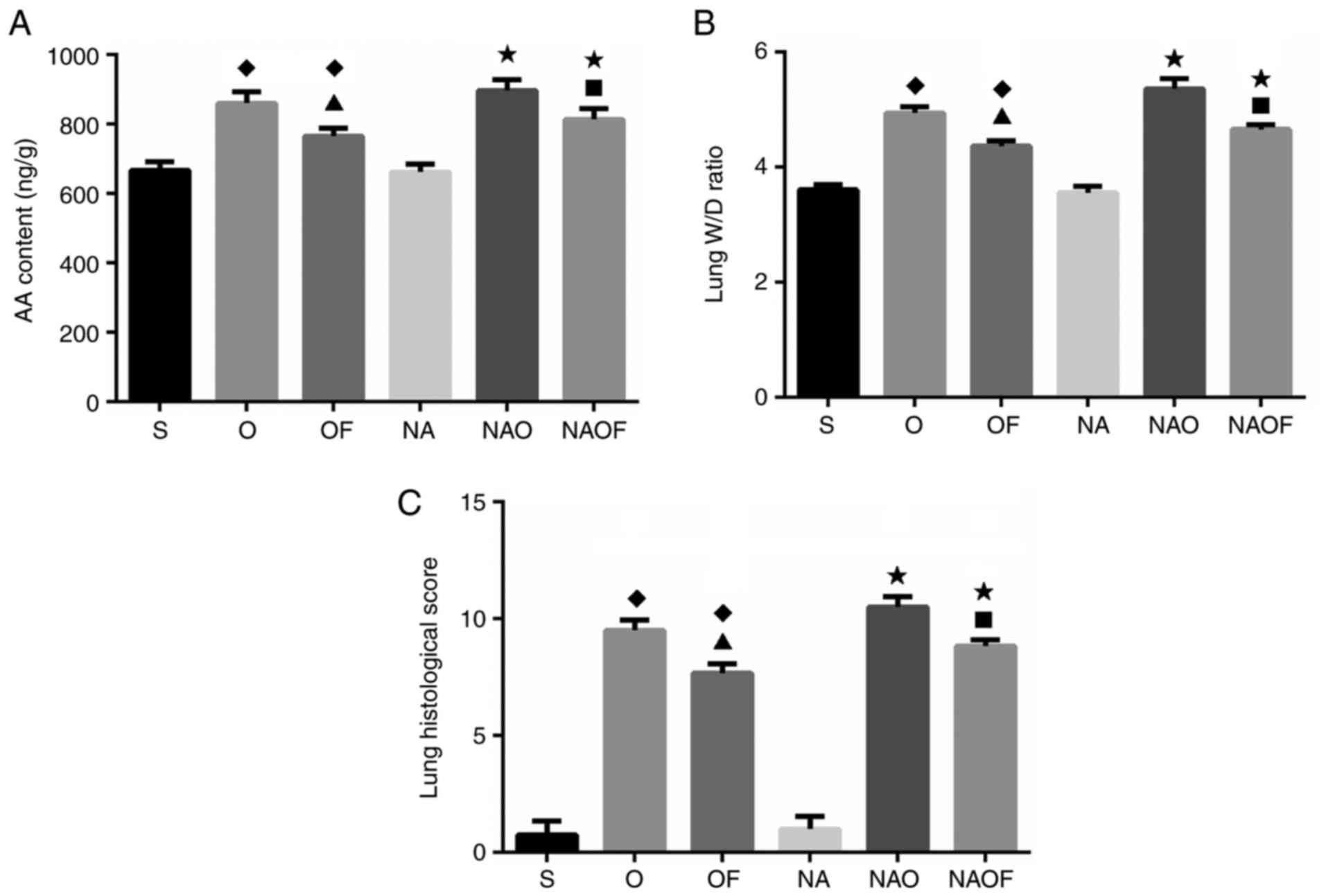
Evaluation of pulmonary AA content,
W/D ratio and histological scores
Pulmonary AA was determined using an ELISA kit
(Fig. 3A). No significant
difference in AA content was observed between group S and group NA.
The concentrations of AA were significantly increased in all groups
following OLV, compared with group S and NA. As expected, the AA
contents was reduced following SVF inhalation in the groups OF, NA
and NAOF, compared with the groups O and NAO (Fig. 3A). The W/D ratio was used as an
indicator of edema formation following OLV. The data revealed that
the W/D ratio did not differ significantly between groups S and NA.
The W/D ratio increased by 13.3% in group O compared with that in
group S. SVF inhalation reduced the W/D ratio in group OF compared
with in group O (Fig. 3B). The
highest W/D ratio was observed in the group NAO, while SVF
inhalation reduced the W/D ratio in the group NAOF compared with
group NAO. The histological lung injury scores of different groups
are presented in Fig. 3C. OLV
increased lung injury scores by 8 times in the O group compared
with S group, and 10 times in the NAO group compared with NA group.
Following SVF inhalation, the histological lung injury scores were
significantly reduced in groups OF and NAOF, compared with in
groups O and NAO, respectively.
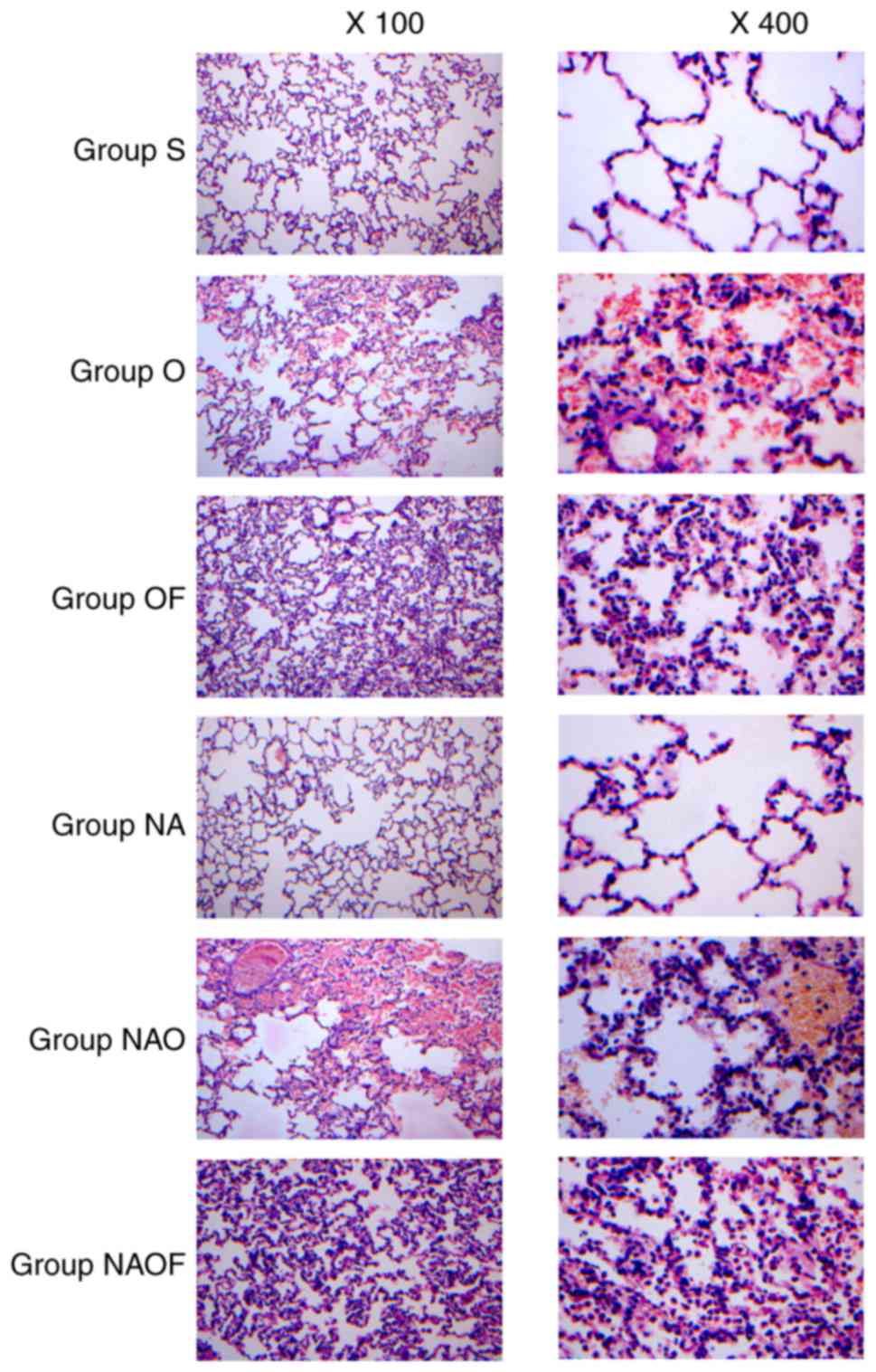
Assessment of lung histopathological
changes
On examining hematoxylin and eosin-stained lung
tissue sections, no significant pathological changes were observed
in the sham-operated group (group S) and club cells exfoliated +
sham-operated group (group NA; Fig.
4). OLV caused severe lesions in the group O, manifesting as
lung hyperemia and hemorrhage, alveolar wall thickening and
increased exudation, with significant infiltration of red blood and
inflammatory cells in the alveolar spaces. The pathological changes
in the group NAO were more extensive compared with those in the
group O. In groups OF and NAOF, those in the pathological lesions
of lung tissues were significantly alleviated compared with group O
and NAO, respectively. However, the pathological lesions in group
NAOF were more pronounced compared with those in group OF.
Discussion
In the present study, SVF inhalation was
demonstrated to attenuate OLV-induced ALI, which was associated
with c-PLA2 downregulation by regulating the endogenous
inhibitor of PLA2. With the advances in thoracic surgery
techniques, OLV has been routinely used to facilitate surgical
exposure; however, OLV further increases lung injury and leukocyte
recruitment, independent of the administration of propofol or
desflurane anesthesia (21,22).
PLA2 is a key enzyme that hydrolyzes
membrane phospholipids. It serves a crucial role in pulmonary
inflammation through modulating membrane signal transduction,
membrane stability and formation of leukocyte-endothelial cell
adhesion (23). c-PLA2
is an 85-kDa protein, widely distributed in cells, and it is
activated by phosphorylation through the mitogen-activated protein
kinase pathway during ventilation to release AA (12,24,25).
AA is further metabolized to generate inflammatory mediators,
including prostaglandins and thromboxanes. Therefore, inhibition of
c-PLA2 may alleviate ventilation-induced lung injury by
reducing these AA downstream products, which cause edema during
acute lung inflammation (26).
CCSP is an endogenous modulator of lung inflammation. In
vitro, CCSP inhibits the activation of c-PLA2 by
binding Ca2+, a cofactor of c-PLA2 activation
(6). A previous study demonstrated
that after 2 and 4 h high-PIP ventilation in CCSP−/−
mice, the W/D ratios were significantly higher compared with those
in wild-type mice, indicating a greater vascular permeability
increase in CCSP−/− mice (27). This evidence was consistent with
the results of the present study, which demonstrated that OLV
indeed caused lung inflammation with evidence of upregulated
c-PLA2, higher AA concentration and higher W/D ratios.
It is hypothesized that the upregulated c-PLA2 in group
O may be associated with the activation of nuclear factor-κB and
the production of pro-inflammatory cytokines induced by OLV. In the
NA, NAO and NAOF groups, CCSP was reduced compared with groups S, O
and OF, as the club cells were damaged by exposure to naphthalene
vapor (club cells exfoliated). Thus, the expression of
c-PLA2 in the NAO group was increased compared with that
in the O group.
Clinical and experimental research has demonstrated
that inhalation of SVF may reduce inflammation in patients with ALI
(28–30). SVF is a widely used anesthetic
agent, and the protective effect of SVF under conditions of hypoxia
or endotoxemia has been extensively studied (31). Rodríguez-González et al
(32) suggested that SVF helped to
preserve endothelial integrity in severe endotoxemia. Shen et
al (30) also demonstrated
that repeated SVF inhalation significantly reduced airway
inflammation in the lungs of allergic mice. Therefore, the ability
of SVF to act protectively against inflammation expands its
therapeutic value beyond its anesthetic properties.
In the present study, it was observed that lung
injury was alleviated following SVF inhalation in animals with
OLV-induced-acute lung injury. SVF inhalation also reduced the
expression of c-PLA2, the lung W/D ratio and
histological scores in a concentration-dependent manner. However,
the expression of CCSP exhibited no significant changes. These
results are notable as CCSP is a well-established endogenous
modulator of c-PLA2. In the current study, a club cell
exfoliated rabbit model was also used to investigate the potential
association between SVF, c-PLA2 and CCSP. Little CCSP
expression was detected in the club cell exfoliated model rabbits,
which indicated that the club cells were successfully exfoliated.
Furthermore, no significant changes were observed between the
groups S and NA. The data indicated that exfoliation of club cells
may not cause lung inflammation. The data revealed that, in club
cell exfoliated animals, the expression of c-PLA2, the
concentration of AA, the W/D ratio and the lung injury scores were
significantly decreased in group NAOF compared with group NAO. The
results suggested that the protective role of SVF under OLV may not
be associated with CCSP. Other inflammation modulators may
participate in the SVF signaling pathway; however, further studies
are required.
In conclusion, the results of the present study
indicated that OLV caused lung inflammation through CCSP and
c-PLA2 regulation. The inhalation of SVF reduced lung
inflammation in a CCSP-independent manner.
Acknowledgements
The authors thank Yunnan Labreal Biotechnology Co.,
Ltd. (Kunming, China) for their technical support.
Funding
The present study was supported by Science
Department of Yunnan Province combined with Kunming Medical
University (grant no. 2014FB098 and 2017FE467).
Availability of data and materials
The datasets generated and analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, WFW and RL wrote and critically revised the
manuscript. YHL and LSL performed the animal experiments. XG, YHL
and RL performed the PCR, ELISA and western blot analyses. RL was
in charge of the design and conception of the manuscript. YY and
WFW performed HE stain and lung histopathological assessment. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study complied with the Guidelines for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication No. 85-23, revised 1996). All
experimental procedures were approved by the Animal Ethics
Committee and Research Ethics Committee of Yunnan Province (license
no. KM3476-821) and Kunming Medical University (license no.
KY976222-m23).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AA
|
arachidonic acid
|
|
ALI
|
acute lung injury
|
|
CCSP
|
club cell secretory protein
|
|
c-PLA2
|
cytosolic phospholipase
A2
|
|
OLV
|
one lung ventilation
|
|
PIPs
|
peak inspiratory pressure
|
|
PLA2
|
phospholipase A2
|
|
SVF
|
sevoflurane
|
|
W/D
|
wet-to-dry lung weight
|
References
|
1
|
Zeldin RA, Normandin D, Landtwing D and
Peters RM: Postpneumonectomy pulmonary edema. J Thorac Cardiovasc
Surg. 87:359–365. 1984.PubMed/NCBI
|
|
2
|
Della Rocca G and Coccia C: Acute lung
injury in thoracic surgery. Curr Opin Anaesthesiol. 26:40–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hegeman MA, Hennus MP, Heijnen CJ, Specht
PA, Lachmann B, Jansen NJ, van Vught AJ and Cobelens PM:
Ventilator-induced endothelial activation and inflammation in the
lung and distal organs. Crit Care. 13:R1822009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kilpatrick B and Slinger P: Lung
protective strategies in anaesthesia. Br J Anaesth. 105 Suppl
1:i108–i116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ,
Juffermans NP and Schultz MJ: Mechanical ventilation using
non-injurious ventilation settings causes lung injury in the
absence of pre-existing lung injury in healthy mice. Crit Care.
13:R12009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andersson O, Nordlund-Möller L, Barnes HJ
and Lund J: Heterologous expression of human
uteroglobin/polychlorinated biphenyl-binding protein. Determination
of ligand binding parameters and mechanism of phospholipase A2
inhibition in vitro. J Biol Chem. 269:19081–19087. 1994.PubMed/NCBI
|
|
7
|
Magrioti V and Kokotos G: Phospholipase A2
inhibitors as potential therapeutic agents for the treatment of
inflammatory diseases. Expert Opin Ther Pat. 20:1–18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitsiouli E, Nakos G and Lekka ME:
Phospholipase A2 subclasses in acute respiratory distress syndrome.
Biochim Biophys Acta. 1792:941–953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leslie CC: Regulation of the specific
release of arachidonic acid by cytosolic phospholipase A2.
Prostaglandins Leukot Essent Fatty Acids. 70:373–376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kapoor M, Clarkson AN, Sutherland BA and
Appleton I: The role of antioxidants in models of inflammation:
Emphasis on L-arginine and arachidonic acid metabolism.
Inflammopharmacology. 12:505–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samuelsson B: Arachidonic acid metabolism:
Role in inflammation. Z Rheumatol. 50 Suppl 1:S3–S6. 1991.
|
|
12
|
Murakami M and Kudo I: Phospholipase A2. J
Biochem. 131:285–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reynolds SD and Malkinson AM: Clara cell:
Progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol.
42:1–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harrod KS, Mounday AD, Stripp BR and
Whitsett JA: Clara cell secretory protein decreases lung
inflammation after acute virus infection. Am J Physiol.
275:L924–L930. 1998.PubMed/NCBI
|
|
15
|
Jorens PG, Sibille Y, Goulding NJ, van
Overveld FJ, Herman AG, Bossaert L, De Backer WA, Lauwerys R,
Flower RJ and Bernard A: Potential role of Clara cell protein, an
endogenous phospholipase A2 inhibitor, in acute lung injury. Eur
Respir J. 8:1647–1653. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steurer M, Schläpfer M, Steurer M,
Z'Graggen BR, Booy C, Reyes L, Spahn DR and Beck-Schimmer B: The
volatile anaesthetic sevoflurane attenuates
lipopolysaccharide-induced injury in alveolar macrophages. Clin Exp
Immunol. 155:224–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong HY, Zhu SM, Wang LQ, He Y, Xie HY and
Zheng SS: Sevoflurane protects against acute kidney injury in a
small-size liver transplantation model. Am J Nephrol. 32:347–55.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu R, Yang Y, Li Y, Li J, Ma Q, Zhao Y
and Wang D: Effects of sevoflurane on pulmonary cytosolic
phospholipase A2 and clara cell secretory protein
expressions in rabbits with one-lung ventilation-induced lung
injury. Nan Fang Yi Ke Da Xue Xue Bao. 33:469–473. 2013.PubMed/NCBI
|
|
19
|
The Guidelines for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health. NIH Publication 85–23. revised. 1996.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bauer P: Postpneumonectomy pulmonary
oedema revisited. Eur Respir J. 15:629–630. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kozian A, Schilling T, Röcken C, Breitling
C, Hachenberg T and Hedenstierna G: Increased alveolar damage after
mechanical ventilation in a porcine model of thoracic surgery. J
Cardiothorac Vasc Anesth. 24:617–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burke JE and Dennis EA: Phospholipase A2
structure/function, mechanism, and signaling. J Lipid Res. 50
Suppl:S237–S242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burke JE and Dennis EA: Phospholipase A2
biochemistry. Cardiovasc Drugs Ther. 23:49–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang CM, Lee IT, Chi PL, Cheng SE, Hsiao
LD and Hsu CK: TNF-α induces cytosolic phospholipase A2 expression
via Jak2/PDGFR-dependent Elk-1/p300 activation in human lung
epithelial cells. Am J Physiol Lung Cell Mol Physiol.
306:L543–L551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park GY and Christman JW: Involvement of
cyclooxygenase-2 and prostaglandins in the molecular pathogenesis
of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol.
290:L797–L805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshikawa S, Miyahara T, Reynolds SD,
Stripp BR, Anghelescu M, Eyal FG and Parker JC: Clara cell
secretory protein and phospholipase A2 activity modulate acute
ventilator-induced lung injury in mice. J Appl Physiol (1985).
98:1264–1271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe K, Iwahara C, Nakayama H,
Iwabuchi K, Matsukawa T, Yokoyama K, Yamaguchi K, Kamiyama Y and
Inada E: Sevoflurane suppresses tumour necrosis factor-α-induced
inflammatory responses in small airway epithelial cells after
anoxia/reoxygenation. Br J Anaesth. 110:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song SY, Zhou B, Yang SM, Liu GZ, Tian JM
and Yue XQ: Preventive effects of sevoflurane treatment on lung
inflammation in rats. Asian Pac J Trop Med. 6:53–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen QY, Fang L, Wu HM, He F, Ding PS and
Liu RY: Repeated inhalation of sevoflurane inhibits airway
inflammation in an OVA-induced mouse model of allergic airway
inflammation. Respirology. 20:258–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matchett GA, Allard MW, Martin RD and
Zhang JH: Neuroprotective effect of volatile anesthetic agents:
Molecular mechanisms. Neurol Res. 31:128–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodríguez-González R, Baluja A, Del Río
Veiras S, Rodríguez A, Rodríguez J, Taboada M, Brea D and Álvarez
J: Effects of sevoflurane postconditioning on cell death,
inflammation and TLR expression in human endothelial cells exposed
to LPS. J Transl Med. 11:872013. View Article : Google Scholar : PubMed/NCBI
|