Introduction
Parkinson's disease (PD) is a common chronic
degenerative disease of the nervous system, which is primarily
characterized by a substantial loss of substantia nigra
dopaminergic neurons, leading to a reduction of dopamine (DA)
levels in the striata, accompanied by cognitive impairment and
functional defects (1). DA
replacement therapy is the predominant treatment for PD, although
this does not prevent or reduce dopaminergic neuron degeneration.
Therefore, the development of novel drugs that protect dopaminergic
neurons without causing dyskinesia is urgently required.
Oxidative stress is thought to be a main cause of
dopaminergic neuron degeneration in PD (2,3).
In vivo and in vitro,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is often used
to establish a model of PD. MPTP traverses the blood-brain barrier
and is decomposed into 1-methyl-4-phenylpyridinium ion
(MPP+) by monoamine oxidase B (4). MPP+ subsequently damages
the neurons in the substantia nigra, resulting in decreased
formation of DA and the production of superoxide anions. It has
been reported that MPTP induces a decline in tyrosine hydroxylase
(TH) production in PC12 cells and other cellular models, which is
the rate-limiting enzyme for the biosynthesis of DA (5–7). In
addition, MPTP induces apoptosis and the production of
intracellular reactive oxygen species (ROS) in a mouse model
(8).
Proanthocyanidins (PCs) are natural phenolic
compounds that are present in various plants. PCs have gained
increasing attention in the fields of nutrition and medicine, due
to their antioxidative, anti-inflammatory (9) and anticancer (10) effects. Epidemiological research has
suggested that PCs may reduce the risk of PD (11). Levels of antioxidative indicators,
including superoxide dismutase (SOD), catalase, glutathione and
glutathione peroxidase, as well as total antioxidant capacity, are
increased by PC intervention, whereas malondialdehyde (MDA)
concentration is decreased, in mouse models of oxidative damage
(12). In addition, it has been
reported that PCs protect rats from cisplatin-induced renal injury
and reduce toxic damage through its antioxidative effects (13). Basli et al (14) provided evidence suggesting that the
neuroprotective effects of PCs are associated with their
antioxidant activity (14).
Strathearn et al (12)
reported that neurodegeneration in a cellular model of PD is
reduced by anthocyanin- and PC-rich botanical extracts, via the
improvement of mitochondrial function (12). Therefore, it may be hypothesized
that PCs exert neuroprotective functions against the
neurodegenerative process in PD. The present study explored the
effects of PC pretreatment on MPTP-induced PD in vitro and
in vivo.
Materials and methods
Cell and drug treatments
PC12 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained at 37°C in an atmosphere
containing 5% CO2 in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 5% horse serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin. Nerve growth
factor (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), at a final
concentration of 100 ng/ml, was added to the medium 3 days prior to
drug treatment to induce neuronal differentiation. Cells were
treated with MPTP (Sigma-Aldrich; Merck KGaA) and/or PCs (cat. no.
T2849; Target Molecule Corp., Boston, MA, USA).
Cell survival
The viability of cells was measured using the MTT
assay. PC12 cells were cultured in 96-well plates at a density of
1×104 cells/well. Cells were exposed to 150 µmol/l MPTP
following treatment with 0.5, 1 or 5 µg/ml PCs for 24, 48, 72 or 96
h at 37°C. The cells were then incubated with MTT (0.25 mg/ml) at
37°C for 4 h, after which, MTT formazan products were dissolved in
dimethyl sulfoxide and the absorbance was measured at 570 nm using
a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Assessment of apoptosis by flow
cytometry
Cell apoptosis was detected using an Annexin
V/Propidium Iodide (PI) Apoptosis Detection kit (Sigma-Aldrich;
Merck KGaA), according to the manufacturer's protocol. Cells were
exposed to 150 µmol/l MPTP following treatment with 0.5, 1 or 5
µg/ml PCs for 48 h at 37°C. Following this, these cells were
harvested and 1×106 cells were fixed using 4%
polyformaldehyde for 30 min at 4°C. Following this, the cells were
resuspended in 300 ml PBS and were stained with Annexin
V-fluorescein isothiocyanate and PI (5 µg/ml each) in the dark for
15 min at 37°C. Apoptotic cells were analyzed by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). FlowJo software (version 10;
FlowJo LLC, Ashland, OR, USA) was used to calculate the apoptosis
rate.
Mitochondrial membrane potential (MMP)
detection
MMP alterations were measured using a JC-1 MMP Assay
kit (Beyotime Institute of Biotechnology, Shanghai, China),
according to the manufacturer's protocol. Briefly, cells were
exposed to 150 µmol/l MPTP following treatment with 0.5, 1 or 5
µg/ml PCs for 48 h at 37°C. The medium was then replaced with PBS,
and 1×106 cells were incubated for 24 h with the JC-1
probe (10 µg/ml) at room temperature. JC-1 fluorescence was
subsequently detected using a microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA) with an excitation and emission wavelength
of 536–620 nm.
Animals and drug treatments
All animal handling procedures were conducted in
accordance with the Guidelines for Laboratory Animal Research of
Nanjing Medical University (Nanjing, China). The present study was
approved by the Institutional Animal Care and Use Committee of
Nanjing Medical University. A total of 20 male C57BL/6 mice (age, 9
weeks; weight, 20–22 g) were purchased from the Laboratory Animal
Center of Nanjing Medical University. The mice were housed at
23±2°C and a relative humidity of 60±10% under a 12-h light/dark
cycle, with free access to water and food. Mice were assigned to
five groups: (i) Control group (n=4); (ii) MPTP (30 mg/kg) group
(n=4); (iii) MPTP (30 mg/kg) + PC (300 mg/kg/day) group (n=4); (iv)
MPTP (30 mg/kg) + PC (400 mg/kg/day) group (n=4); and (v) MPTP (30
mg/kg) + PC (500 mg/kg/day) group (n=4). PCs were intragastrically
administered at 300, 400 or 500 mg/kg/day for 14 days
consecutively, whereas the control group received an equivalent
volume of saline. Treatment began 7 days prior to the initial MPTP
treatment, from which point MPTP (20 mg/kg) dissolved in saline was
intraperitoneally injected four times daily at 2 h intervals for a
total of 7 days. All mice were sacrificed for further investigation
24 h after the last MPTP injection had been administered.
Behavioral tests
The pole test was used to measure motor behavior in
the mouse model of PD. The pole test was performed as previously
described (15), and began
following 7 days of MPTP administration. Briefly, the mice were
held on top of the pole (diameter, 8 mm; height, 55 cm; rough
surface), and the time taken for the mice to climb down and place
four feet on the floor was recorded as the time for locomotion
activity (T-LA). Each trial had a cut-off limit of 30 sec. All
measurements were performed three times to ensure accuracy.
Brain tissue preparation
Brain tissue preparation was performed as previously
described (15,16). Briefly, 24 h after the last
injection of MPTP, brains were obtained from the four mice in each
group. One side of the brain was fixed in 4% paraformaldehyde for
72 h at 4°C, followed by incubation in 0.1 M phosphate buffer (pH
7.4) containing 25% sucrose at 4°C for 2–3 days. Following this,
the brain tissues were frozen, and then substantia nigra tissues
were then cut into 25 µm sections and stored in cryoprotectant at
4°C until further use in the immunohistochemistry (IHC) and
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) experiments. For ROS and MMP assays, as well as
western blotting, the other side of the substantia nigra was
isolated and stored at −80°C until use.
TH IHC
Following three 10 min washes in PBS with 0.05%
Tween-20 (PBST), sections were incubated for 1 h at 37°C with PBST
containing 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA).
Sections were subsequently incubated overnight at 4°C with anti-TH
antibody (1:1,000; cat. no. 25859-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA), followed by incubation with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG)
secondary antibody (1:5,000; cat. no. 10285-1-AP; ProteinTech
Group, Inc.) for 1 h at 37°C and amplification with a DAB
Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA,
USA), which was performed according to the manufacturer's
instructions. Finally, sections were analyzed using a light Leica
DM2700 P microscope (magnification, ×40; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA). Quantification of TH activity was
performed by counting the number of TH-immunoreactive (TH-IR) cells
in 10 independent visual fields in the SNpc, and by measuring the
optical density of TH-IR fibers in the ST using ImageJ software
(version 1.48; National Institutes of Health, Bethesda, MD,
USA).
TUNEL staining
Tissue sections were washed in PBS and subsequently
fixed for 30 min with 4% paraformaldehyde at room temperature.
Following one wash with PBS, PBS containing 0.1% Triton X-100 was
added to the sections for 2 min in order to lyse the cells at room
temperature. Sections were subsequently washed once with PBS and
mounted onto slides, and 3% H2O2 was added to
the slides for 5 min at room temperature. Slides were then rinsed
and then incubated with 50 µl TUNEL detection solution (Roche
Diagnostics, Basel, Switzerland) for 60 min at room temperature.
The TUNEL reaction was visualized by chromogenic staining with DAB
(0.75 mg/ml; Sigma-Aldrich; Merck KGaA) at room temperature for 20
min. Sections were imaged and ten visual fields were analyzed using
a light Leica DM2700 P microscope (Leica Microsystems, Inc.). The
percentage of cell death was determined by calculating the number
of TUNEL-positive cells within a total of 100 cells in one visual
field using ImageJ software (version 1.48; National Institutes of
Health, Bethesda, MD, USA).
Measurement of ROS formation
ROS was measured with the fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
Sigma-Aldrich; Merck KGaA). Cells were exposed to 150 µmol/l MPTP
following treatment with 0.5, 1 or 5 µg/ml PCs for 48 h at 37°C.
The medium was then replaced with PBS, and 1×106 cells
were incubated with 10 µmol/l H2DCFDA at 37°C for 30
min. Substantia nigra tissues were treated with collagenase (5
mg/ml) and then the cells were dislodged in the solution using a
pipette. PC12 cells or single cell suspension of substantia nigra
homogenate was incubated with 10 µmol/l H2DCFDA at 37°C
for 30 min. The cells were subsequently washed twice with PBS and
dissolved in 1% Triton X-100. Fluorescence was measured at an
excitation wavelength of 485 nm and an emission wavelength of 530
nm, using a fluorescence microplate reader.
Western blotting
PC12 cells were exposed to 150 µmol/l MPTP following
treatment with 0.5, 1 or 5 µg/ml PCs for 48 h at 37°C. Proteins
from PC12 cells or substantia nigra were prepared as described
previously (17). Protein
concentration was measured using bicinchoninic acid assays
(Beyotime Institute of Biotechnology, Shanghai, China) and adjusted
to the same final concentration. Protein samples (20 µg/lane) were
separated by 12% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. Membranes were blocked with 5% skim milk in 50
mM Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at
room temperature, and membranes were incubated overnight at 4°C
with the following primary antibodies in the same blocking
solution: TH (cat. no. 2792), c-Jun N-terminal kinase (JNK; cat.
no. 9252), phosphorylated (p)-JNK (cat. no. 9255), c-Jun (cat. no.
9165), p-c-Jun (cat. no. 3270), B-cell lymphoma 2-like protein 11
(Bim; cat. no. 2933), cleaved caspase-3 (cat. no. 9654), cleaved
poly (ADP-ribose) polymerase (PARP; cat. no. 94885) and GAPDH
(1:2,000; cat. no. 2118) (all 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA). Subsequently, membranes were washed with
TBST and incubated with horseradish peroxidase-conjugated
goat-anti-rabbit IgG (1:10,000; cat. no. 7074; Cell Signaling
Technology, Inc.) or goat-anti-mouse IgG (1:10,000; cat. no. 7076;
Cell Signaling Technology, Inc.) for 1 h at room temperature in
TBST containing 5% skim milk. Cross-reactivity was visualized using
enhanced chemiluminescence western blotting detection reagents
(Sangon Biotech Co., Ltd., Shanghai, China) and was analyzed by
densitometry using Tanon 5200 software (Tanon Science and
Technology Co., Ltd., Shanghai, China).
Statistical analysis
All data were analyzed using Prism software 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as
the mean ± standard error of the mean. All experiments were
performed in triplicate. Statistical evaluation of the results was
performed by one-way analysis of variance followed by Bonferroni's
correction. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Effects of PCs on the proliferation
and apoptosis of MPTP-treated PC12 cells
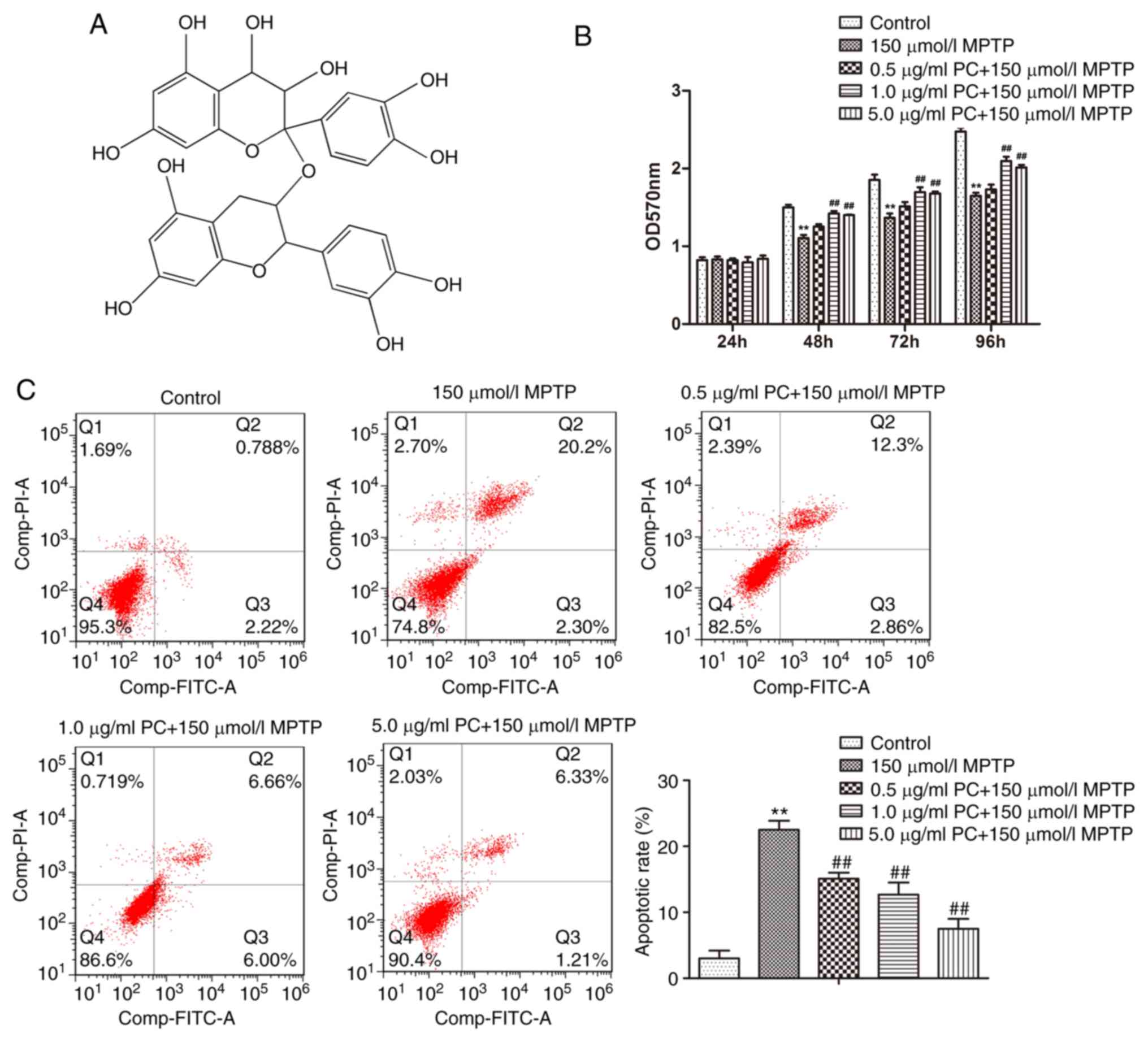
The simplest structure of PCs is a dimer formed by
catechin, L-Epicatechin or catechin and L-Epicatechin, which is
highly soluble in water and may be easily absorbed. Furthermore,
PCs also have an important role in scavenging free radicals
(18). The chemical structure of a
PCs dimer formed from catechin is presented in Fig. 1A. To exclude the possibility that
PCs induced PC12 cell toxicity, cell viability was determined in
response to various concentrations of PCs at 24, 48, 72 and 96 h
using the MTT assay, and the results revealed that PCs did not
induce toxicity in PC12 cells (data not shown). The data
demonstrated that MPTP markedly inhibited PC12 cell proliferation
compared with the control, and this effect was gradually
counteracted by increasing concentrations of PCs (Fig. 1B). Furthermore, the apoptotic rate
for each group was assessed by flow cytometry. The typical quadrant
analysis results obtained from PC12 cells, treated with or without
PCs prior to MPTP treatment, are presented in Fig. 1C. Compared with the control group
(3.0%), the percentage of apoptotic cells was significantly
increased in the MPTP treatment group (22.5%). Conversely, PC
pretreatment reduced the apoptotic percentage from 22.5 to 15.2%
(0.5 µg/ml), 12.7% (1 µg/ml) and 7.5% (5 µg/ml). It was therefore
concluded that PCs may reduce MPTP-induced apoptosis.
PCs inhibit the reduction of MMP and
accumulation of ROS induced by MPTP
Mitochondria are the major source of ROS in various
mammalian cells, and excessive production of ROS in the
mitochondria disrupts normal redox signaling. In addition, MMP is a
marker of mitochondrial function, which is also involved in
apoptosis (19). The production of
ROS in PC12 cells was analyzed using a H2DCFDA
fluorescence assay. As presented in Fig. 2A, exposure to MPTP increased ROS
levels in PC12 cells. Pretreatment with PCs significantly inhibited
the accumulation of ROS induced by MPTP. These results suggested
that PCs protected mitochondrial function and suppressed ROS
production in PC12 cells. Furthermore, enhanced MMP was observed in
the control group and treatment with MPTP significantly reduced MMP
in PC12 cells; however, pretreatment with PCs markedly restored
reduced MMP (Fig. 2B and C).
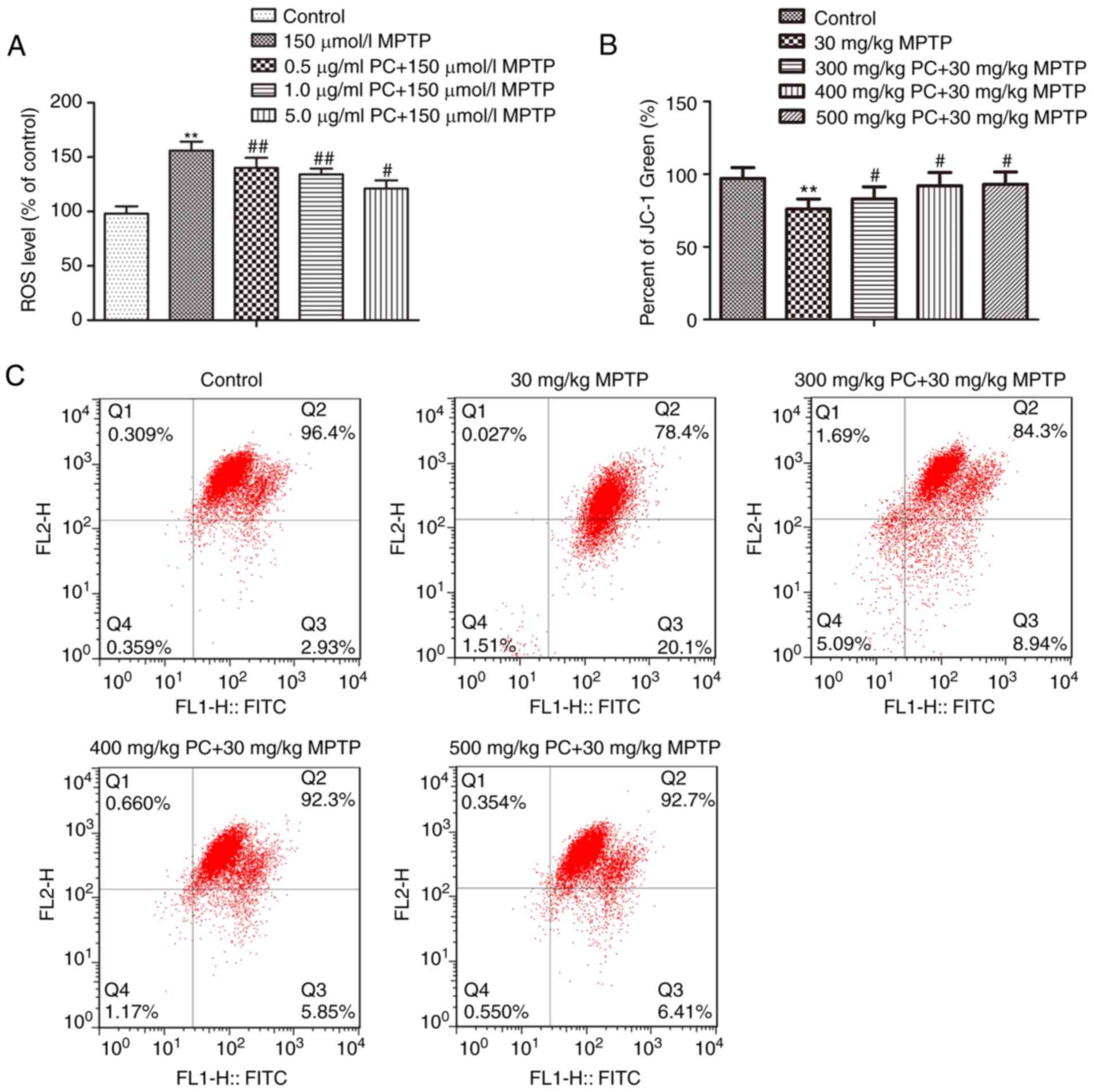 | Figure 2.Effects of PCs on MPTP-mediated ROS
generation and mitochondrial dysfunction in PC12 cells. Cells were
treated with MPTP in the absence or presence of 0.5, 1 or 5 µg/ml
PCs for 24 h. (A) ROS levels were detected with the fluorescent
probe, 2′,7′-dichlorodihydrofluorescein diacetate. (B)
Mitochondrial membrane potential was measured using the fluorescent
probe JC-1. (C) Increased MMP was observed in the control group and
treatment with MPTP significantly suppressed the MMP in PC12 cells;
however, pretreatment with PCs significantly attenuated suppressed
levels of MMP. Three independent experiments were performed.
**P<0.01 vs. untreated control cells; #P<0.05,
##P<0.01 vs. the MPTP group. FITC, fluorescein
isothiocyanate; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine;
PCs, proanthocyanidins; ROS, reactive oxygen species. |
Effects of PCs on JNK/c-Jun
signaling
JNK/c-Jun signaling is commonly activated by various
stress stimuli, and is a known mediator of cell apoptosis under
various pathophysiological conditions (20). Therefore, MPTP-induced cell
apoptosis and the potential protective effects of PCs were examined
by western blotting. Administration of MPTP significantly increased
p-JNK/JNK and p-c-Jun/c-Jun expression ratios. Furthermore,
proapoptotic proteins Bim, cleaved caspase-3 and cleaved PARP were
detected; MPTP significantly increased the expression of these
proteins, whereas PC pretreatment inhibited this increase (Fig. 3).
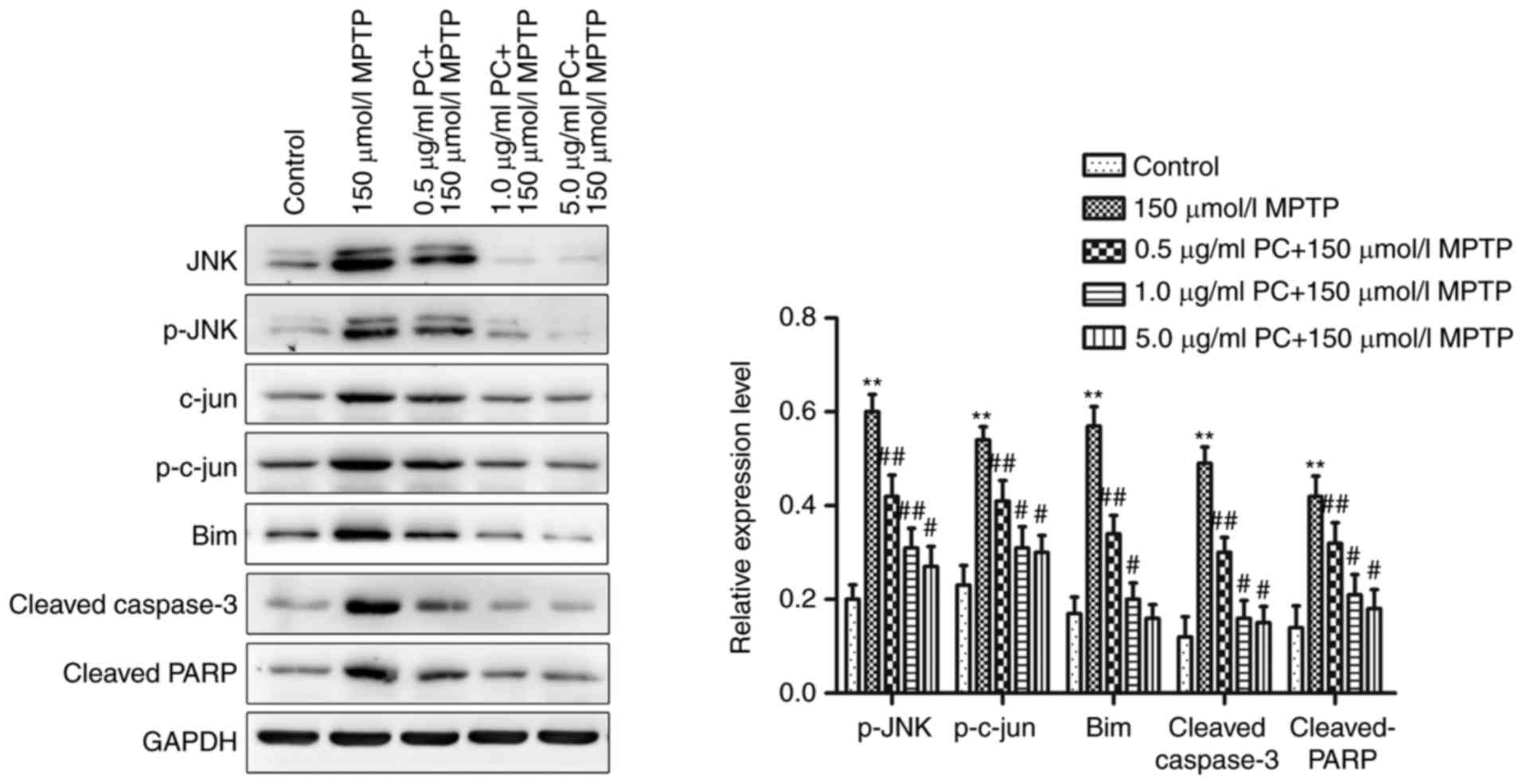 | Figure 3.Western blot analysis of the
JNK/c-Jun signaling pathway in PC12 cells. Cells were treated with
MPTP in the absence or presence of 0.5, 1 or 5 µg/ml PCs for 24 h.
Three independent experiments were performed. **P<0.01 vs.
untreated control cells; #P<0.05,
##P<0.01 vs. the MPTP group. Bim, B-cell lymphoma
2-like protein 11; JNK, c-Jun N-terminal kinase 1; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; p, phosphorylated;
PARP, poly (ADP-ribose) polymerase; PC, proanthocyanidins. |
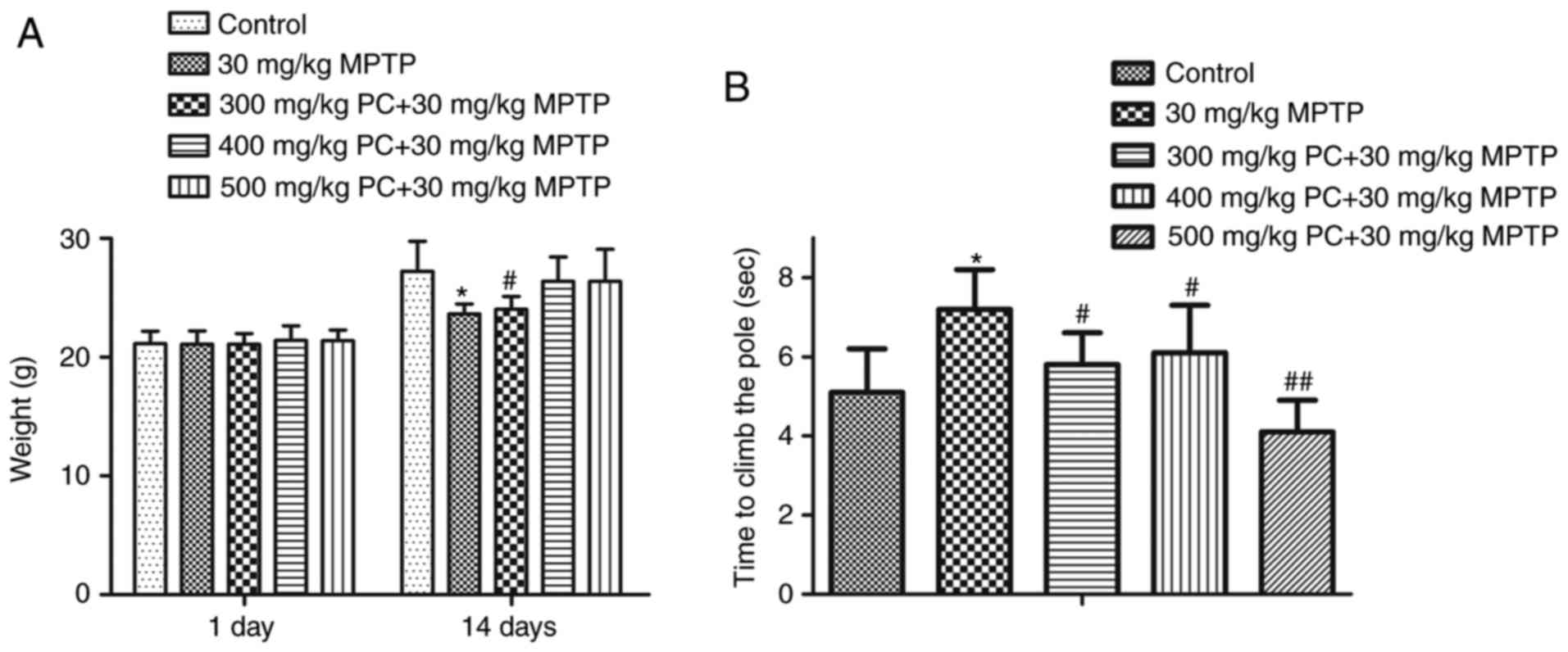
Effects of PCs against MPTP-induced
movement impairment in the pole test
As presented in Fig.
4A, the PD mouse model group were of a lower weight compared
with the control group; however, this effect was reduced following
treatment with PCs. To determine the effects of PCs on MPTP-induced
bradykinesia, a pole test was performed on day 7 after MPTP
injection. In the MPTP group, T-LA was significantly prolonged to
7.2 sec on day 7, compared with the control group. However, on day
7, T-LA was significantly shortened in the 300, 400 and 500 mg/kg
PC-treated groups to 5.9, 6.1 and 4.0 sec, respectively, compared
with MPTP alone (Fig. 4B).
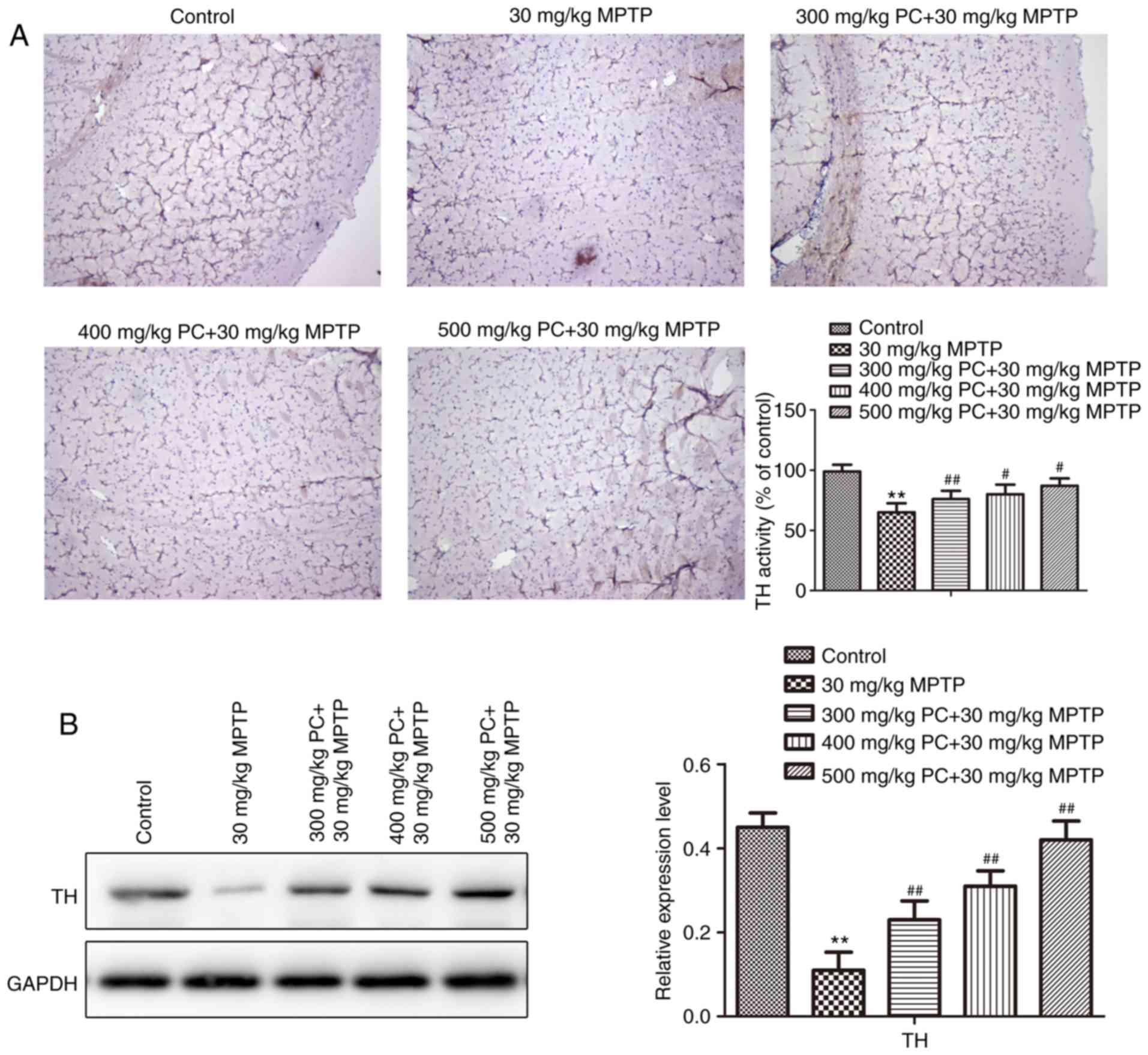
PC treatment partially protects
dopaminergic neurons
The neuroprotective action of PCs and the functional
viability of dopaminergic neurons in the substantia nigra pars
compacta were further assessed by determining the expression of the
rate-limiting enzyme for DA biosynthesis, TH. As evidenced by IHC
and western blot analysis (Fig. 5A and
B), the expression of TH was reduced in MPTP mice compared with
the control group. Conversely, TH expression in PC-pretreated mice
was more pronounced compared with in MPTP-induced mice. TH is a
specific marker protein for the identification of midbrain
dopaminergic neurons (21).
Therefore, these results demonstrated that PCs may protect against
neuronal loss in a mouse model of PD, thus suggesting that PCs
exert a neuroprotective effect in vivo.
PCs reduce MPTP-induced apoptosis via
ROS-JNK signaling
Analysis of TUNEL staining in the substantia nigra
further suggested that the control and PC-pretreated groups
presented with fewer TUNEL-positive cells compared with in the MPTP
group (Fig. 6A). ROS levels were
subsequently detected. As presented in Fig. 6B, MPTP exposure led to a
significant elevation in ROS levels in primary mice substantia
nigra cells compared with in the control group. Pretreatment with
PCs inhibited ROS generation in the MPTP group. Furthermore,
western blot analysis demonstrated that MPTP increased JNK/c-Jun
signaling pathway protein expression, whereas PCs reversed this
increase (Fig. 6C).
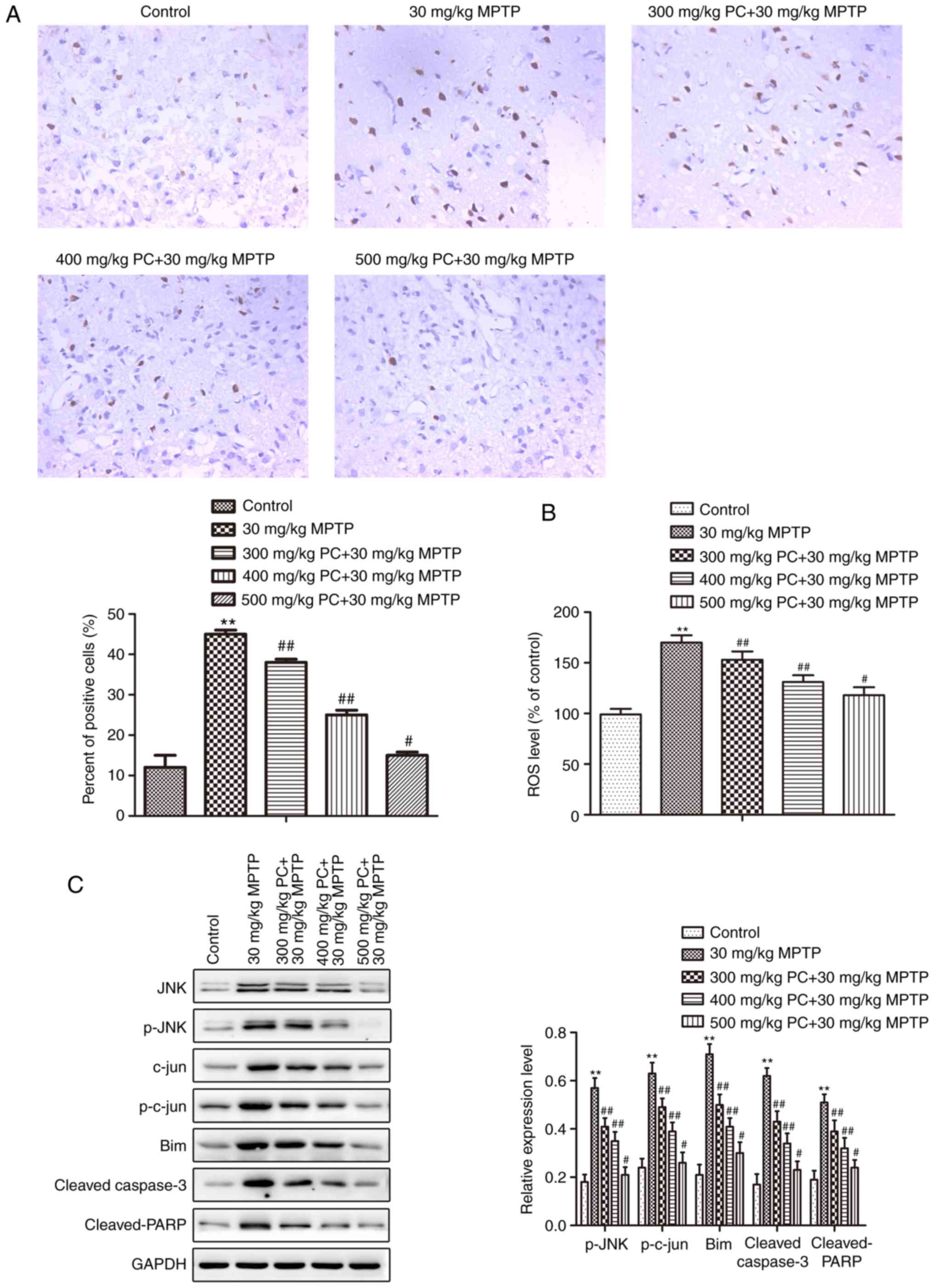 | Figure 6.Effects of PCs on MPTP-induced
apoptosis. (A) A TUNEL assay was performed to detect apoptosis, and
TUNEL-positive cells were detected (magnification, ×200). (B) ROS
levels were measured using the fluorescent probe,
2′,7′-dichlorodihydrofluorescein diacetate. (C) JNK/c-Jun signaling
pathway proteins were detected via western blot analysis. Three
independent experiments were performed. **P<0.01 vs. the
untreated control group; #P<0.05,
##P<0.01 vs. the MPTP group. Bim, B cell lymphoma
2-like protein 11; JNK, c-Jun N-terminal kinase 1; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; p, phosphorylated;
PARP, poly (ADP-ribose) polymerase; PCs, proanthocyanidins; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling. |
Discussion
In order to investigate the effects of PCs on
dopaminergic neurons, an MPTP-induced experimental model of PD was
established in vitro and in vivo. The results
demonstrated that, in vitro, PCs significantly protected
PC12 cells against MPTP-induced toxicity, apoptosis and high ROS
levels. In vivo, the data revealed that treatment with PCs
prevented neuronal loss in the substantia nigra and prevented
apoptosis in a dose-dependent manner. Furthermore, western blotting
and immunohistochemical analysis for dopaminergic TH expression
revealed that PCs prevented the decrease in TH induced by MPTP.
Western blot analysis also revealed that the ROS/JNK signaling
pathway was involved in the action of PCs. The results of the
present study consistently demonstrated that PCs protected neurons
from the impairments induced by MPTP treatment via the ROS/JNK
signaling pathway.
PD is a movement disorder characterized by
progressive loss of nigrostriatal dopaminergic neurons. Therapeutic
strategies that slow or stop the neurodegenerative processes of PD
are urgently required. The identification of polyphenolic compounds
or polyphenols with potential neuroprotective properties has
increased considerably during the last few years. Catechins, such
as epigallocatechin-3-gallate, have been reported to exert several
actions on the CNS, including anxiolytic, sedative and
neuroprotective effects on animal models of Alzheimer's disease and
PD. Notably, PCs are composed of catechin and epicatechin oligomers
(22,23). Hartley et al (24) reported that PCs prevent the early
motor and non-motor symptoms of PD, and may represent a promising
therapeutic tool in PD via their neuroprotective potential
(14). The neuroprotective effects
of PCs are exerted via decreasing MDA and SOD levels, in
vitro and in vivo (25). Recently, PCs have been reported to
possess neuroprotective effects by targeting β-amyloid
fibrillization and neurotoxicity (26). The results of the present study
also revealed that PCs exerted neuroprotective effects in
vitro and in vivo.
Overwhelming evidence has indicated that the
apoptotic death of nigrostriatal dopaminergic neurons is initiated
by oxidative stress (27).
Oxidative stress is self-propagating, in that initial oxidative
damage creates additional free radicals and damages mitochondria,
leading to further ROS production (28,29).
Mitochondrial dysfunction and the overproduction of ROS may also
enhance neuronal excitability and increase seizure susceptibility
(13). Mitochondrial dysfunction
is a common trigger for apoptosis, by inducing the sequential
activation of proapoptotic caspase-3 and PARP (30).
Evidence indicates that activation of JNK regulates
ROS-induced neuronal apoptosis (31,32).
MPP+ is selectively transported to the cell through the
high affinity DA transporter, and is absorbed by the mitochondria
within dopaminergic neurons. By inhibiting the mitochondrial
electron transfer complex I, it destroys the process of phosphoric
oxide phosphorylation and increases cellular ROS expression levels
(24). Large amounts of ROS in the
mitochondria are released into the cytoplasm, which stimulates JNK
phosphorylation and activates signal cascades. The activated JNK
subsequently enters the nucleus to activate c-Jun, which further
regulates Bim, caspase-3 and PARP to promote the apoptosis of cells
(33,34), eventually leading to the death of
dopaminergic neurons.
In conclusion, PCs may represent a safe and
affordable intervention for the clinical treatment of PD. PCs
effectively prevented mitochondrial apoptosis, ROS production and
JNK activation in neurons. The results of the present study
provided experimental evidence to support the potential use of PCs
as a therapeutic agent in PD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX, HC and JX conceived and designed the study. HC,
JX, YL, PH and CL performed the experiments. JJ, XM and SL analyzed
the data. XX and XM wrote the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keeney PM, Xie J, Capaldi RA and Bennett
JP Jr: Parkinson's disease brain mitochondrial complex I has
oxidatively damaged subunits and is functionally impaired and
misassembled. J Neurosci. 26:5256–5264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53 Suppl 3:S26–S38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reynolds A, Laurie C, Mosley RL and
Gendelman HE: Oxidative stress and the pathogenesis of
neurodegenerative disorders. Int Rev Neurobiol. 82:297–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharpe MA, Han J, Baskin AM and Baskin DS:
Design and synthesis of a MAO-B-selectively activated prodrug based
on MPTP: A mitochondria-targeting chemotherapeutic agent for
treatment of human malignant gliomas. ChemMedChem. 10:621–628.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chalimoniuk M, Snoek GT, Adamczyk A,
Małecki A and Strosznajder JB: Phosphatidylinositol transfer
protein expression altered by aging and Parkinson disease. Cell Mol
Neurobiol. 26:1151–1164. 2006. View Article : Google Scholar
|
|
6
|
Lee WS, Tsai WJ, Yeh PH, Wei BL and Chiou
WF: Divergent role of calcium on Abeta- and MPTP-induced cell death
in SK-N-SH neuroblastoma. Life Sci. 78:1268–1275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalia A, Neff NH and Hadjiconstantinou M:
Tyrosine hydroxylase and aromatic L-amino acid decarboxylase in
mesencephalic cultures after MPP+: The consequences of treatment
with GM1 ganglioside. Brain Res. 742:260–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MJ, Kim DW, Jeong HJ, Sohn EJ, Shin
MJ, Ahn EH, Kwon SW, Kim YN, Kim DS, Park J, et al: Tat-Frataxin
protects dopaminergic neuronal cells against MPTP-induced toxicity
in a mouse model of Parkinson's disease. Biochimie. 94:2448–2456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rocarodríguez MM, Lópeztinoco C, Murri M,
Fernández-Deudero A, García-Palacios MV, García-Valero MA,
Tinahones-Madueño FJ and Aguilar-Diosdado M: Postpartum development
of endothelial dysfunction and oxidative stress markers in women
with previous gestational diabetes mellitus. J Endocrinol Invest.
37:503–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Cassidy A, Schwarzschild MA, Rimm
EB and Ascherio A: Habitual intake of dietary flavonoids and risk
of Parkinson disease. Neurology. 78:1138–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strathearn KE, Yousef GG, Grace MH, Roy
SL, Tambe MA, Ferruzzi MG, Wu QL, Simon JE, Lila MA and Rochet JC:
Neuroprotective effects of anthocyanin- and proanthocyanidin-rich
extracts in cellular models of Parkinson's disease. Brain Res.
1555:60–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saad AA, Youssef MI and Elshennawy LK:
Cisplatin induced damage in kidney genomic DNA and nephrotoxicity
in male rats: The protective effect of grape seed proanthocyanidin
extract. Food Chem Toxicol. 47:14992009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basli A, Soulet S, Chaher N, Mérillon JM,
Chibane M, Monti JP and Richard T: Wine polyphenols: Potential
agents in neuroprotection. Oxid Med Cell Longev. 2012:8057622012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park G, Park YJ, Yang HO and Oh MS:
Ropinirole protects against 1-methyl-4-phenyl-1, 2, 3,
6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice via
anti-apoptotic mechanism. Pharmacol Biochem Behav. 104:163–168.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HG, Ju MS, Shim JS, Kim MC, Lee SH,
Huh Y, Kim SY and Oh MS: Mulberry fruit protects dopaminergic
neurons in toxin-induced Parkinson's disease models. Br J Nutr.
104:8–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamine A, Létourneau M, Doan ND, Maucotel
J, Couvineau A, Vaudry H, Chatenet D, Vaudry D and Fournier A:
Characterizations of a synthetic pituitary adenylate
cyclase-activating polypeptide analog displaying potent
neuroprotective activity and reduced in vivo cardiovascular side
effects in a Parkinson's disease model. Neuropharmacology.
108:440–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ariga T and Asao Y: Isolation,
identification and organoleptic astringency of dimeric
proanthocyanidins occurring in Azuki Beans. J Agric Chem Soc Japan.
45:2709–2712. 2014.
|
|
19
|
Celardo I, Martins LM and Gandhi S:
Unravelling mitochondrial pathways to Parkinson's disease. Br J
Pharmacol. 171:1943–1957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leppä S and Bohmann D: Diverse functions
of JNK signaling and c-Jun in stress response and apoptosis.
Oncogene. 18:6158–6162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Freund TF, Bolam JP, Björklund A, Stenevi
U, Dunnett SB, Powell JF and Smith AD: Efferent synaptic
connections of grafted dopaminergic neurons reinnervating the host
neostriatum: A tyrosine hydroxylase immunocytochemical study. J
Neurosci. 5:603–16. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dragicevic N, Smith A, Lin X, Yuan F,
Copes N, Delic V, Tan J, Cao C, Shytle RD and Bradshaw PC: Green
tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce
Alzheimer's amyloid-induced mitochondrial dysfunction. J Alzheimers
Dis. 26:507–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laschober GT, Ruli D, Hofer E, Muck C,
Carmona-Gutierrez D, Ring J, Hutter E, Ruckenstuhl C, Micutkova L,
Brunauer R, et al: Identification of evolutionarily conserved
genetic regulators of cellular aging. Aging Cell. 9:1084–1097.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartley A, Stone JM, Heron C, Cooper JM
and Schapira AH: Complex I inhibitors induce dose-dependent
apoptosis in PC12 cells: Relevance to Parkinson's disease. J
Neurochem. 63:1987–1990. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuchta K, Qiao HX, Huang HB, Fang L, Chen
Y and Wang RW: The neuroprotective activity of a proanthocyanidin
enriched Ginkgo biloba L. leaves extract in vitro and in vivo.
Planta Medica. 81 Suppl 1:S1–S381. 2016.
|
|
26
|
Li L, Zhang Y, Sun B, Zhang H, Tao W, Tian
J, Ye X and Chen S: The neuroprotective effects of Chinese bayberry
leaves proanthocyanidins. J Funct Foods. 40:554–563. 2018.
View Article : Google Scholar
|
|
27
|
Agrawal S, Singh A, Tripathi P, Mishra M,
Singh PK and Singh MP: Cypermethrin-induced nigrostriatal
dopaminergic neurodegeneration alters the mitochondrial function: A
proteomics study. Mol Neurobiol. 51:448–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang QR, Li Q, Chen YH, Li L, Liu LL, Lei
SH, Chen HP, Peng WJ and He M: Involvement of anion exchanger-2 in
apoptosis of endothelial cells induced by high glucose through an
mPTP-ROS-Caspase-3 dependent pathway. Apoptosis. 15:693–704. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu M, Su C, Qiao C, Bian Y, Ding J and Hu
G: Metformin prevents dopaminergic neuron death in MPTP/P-induced
mouse model of Parkinson's disease via autophagy and mitochondrial
ROS clearance. Int J Neuropsychopharmacol. 19:pyw0472016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stefanis L, Burke RE and Greene LA:
Apoptosis in neurodegenerative disorders. Curr Opin Neurol.
10:299–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoehn MM and Yahr MD: Parkinsonism: Onset,
progression, and mortality. Neurology. 17:427–442. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SY, Kim MY, Mo JS, Park JW and Park
HS: SAG protects human neuroblastoma SH-SY5Y cells against
1-methyl-4-phenylpyridinium ion (MPP+)-induced cytotoxicity via the
downregulation of ROS generation and JNK signaling. Neurosci Lett.
413:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voss T and Ravina B: Neuroprotection in
Parkinson's disease: Myth or reality? Curr Neurol Neurosci Rep.
8:304–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Xing Y, Ye CF, Ai HX, Wei HF and
Li L: Learning-memory deficit with aging in APP transgenic mice of
Alzheimer's disease and intervention by using tetrahydroxystilbene
glucoside. Behav Brain Res. 173:246–254. 2006. View Article : Google Scholar : PubMed/NCBI
|