Introduction
Cisplatin (cis-dichlorodiammine platinum) is a
platinum-based chemotherapeutic agent used for the treatment of
several types of cancer. It binds to DNA and inhibits DNA
replication, resulting in apoptosis (1,2).
Cisplatin has a strong anti-cancer effect and synergistic action
with various anti-tumor drugs and is therefore one of the most
commonly used drugs in combination chemotherapy (3,4). It
is primarily used in the treatment of ovarian, prostate,
testicular, lung and gastric cancer (3,4).
Cisplatin is associated with dose-dependent side effects (5,6),
including gastrointestinal reactions (7), bone marrow suppression (8), liver and kidney damage (9), ototoxicity (10) and neurotoxicity (11). Renal toxicity is frequently
encountered, with a clinical incidence rate of 25–35% (12–14).
The side effects associated with cisplatin have limited its use and
clinical efficacy (15,16). Therefore, strategies to reduce
these side effects are required in the field of antitumor
therapy.
Gastric cancer, which is a malignant tumor
originating from the gastric mucosal epithelium (17), is the fourth most common cancer and
the second most common cause of cancer-associated mortality
worldwide (18). According to
global cancer statistics in 2012, gastric cancer accounted for
approximately one million new cases and over 700,000 mortalities,
representing 8% of all cancer cases and 9.7% of all cancer
mortalities, and almost half of these patients came from China
(19). Although the morbidity and
mortality of gastric cancer has decreased in recent years, the
5-year survival rate remains low (<30%) (20,21).
Radiotherapy and chemotherapy combined with surgical resection is
the main treatment for gastric cancer, but the recurrence rate is
very high (20,21). Cisplatin is currently a first line
chemotherapeutic agent for gastric cancer (22,23),
particularly for patients with advanced gastric cancer.
Cisplatin has a better curative effect for gastric
cancer. The target microRNAs (miRNAs) of cisplatin remain unknown.
In the current study, the human gastric cancer cell line NCI-N87
was used to identify differentially expressed miRNAs following
exposure to cisplatin. High-throughput sequencing and
bioinformatics analysis identified 49 differentially expressed
miRNAs, including 33 upregulated miRNAs and 16 downregulated miRNAs
following treatment with cisplatin. Reverse-transcription
quantitative polymerase chain reaction (RT-qPCR) results revealed
that the expression level of hsa-miR-1246 and hsa-miR-892b were
consistent with the microarray data. The results obtained in the
current study suggested that cisplatin may serve a role in the
treatment of gastric cancer by regulating the expression of the
aforementioned miRNAs, which may allow the development of novel
therapeutic agents for gastric cancer.
Materials and methods
Cell culture and cisplatin
stimulation
The human gastric cancer cell line NCI-N87 (American
Type Culture Collection) was cultured in Dulbecco's Modified Eagles
Medium (DMEM; HyClone; GE Healthcare Life Sciences) supplemented
with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences)
and 1% penicillin and streptomycin (Suzhou Zeke Biotech, Co.,
Ltd.). Cells were maintained in an incubator at 5% CO2
and 37°C. When the cell confluence reached 70–80%, the cells were
treated 0, 7.5, 15, 30 and 60 µg/ml cisplatin for 48 h at 37°C.
Cell viability was subsequently assessed using an MTT assay and
applied for further investigation.
MTT assay
A total of 1×104 NCI-N87 cells were
seeded in 96-well plates with complete DMEM and incubated at 37°C
for 48 h. Cells were treated with MTT (Changchun Keygen Biological
Products Co., Ltd.) according to the manufacturer's instructions
and incubated in an incubator at 5% CO2 and 37°C for 4
h. The purple formazan crystals were subsequently dissolved in 150
µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min, and analyzed at a wavelength of 490 nm
using a microplate reader.
High-throughput sequencing
The total RNA of NCI-N87 cells treated with and
without cisplatin was extracted using the Takara MiniBEST Universal
RNA Extraction Kit (cat. no. 9767; Takara Bio, Inc.) according to
the manufacturer's protocol and sent to Shanghai Personal
Biotechnology Co., Ltd. for high-throughput sequencing using the
Illumina NextSeq500 platform (Illumina, Inc.).
RT-qPCR assay
The total RNA was extracted using the Takara
MiniBEST Universal RNA Extraction Kit (cat. no. 9767; Takara Bio,
Inc.). Total RNA was reverse transcribed and qPCR was performed
using a PrimeScript One Step RT-PCR Kit Ver.2 (cat. no. RR057A;
Takara Bio, Inc.) according to the manufacturers' instructions. The
reaction mixture, which consisted of 2 µl 5X PrimeScript RT Master
mix (included in the PrimeScript One Step RT-PCR Kit), 500 ng total
RNA, RNase-free dH2O up to 10 µl, was prepared and
reacted at 37°C for 15 min, followed by 85°C for 5 sec and 4°C
indefinitely. qPCR was subsequently performed using the following
mixture: 10 µl of 2× SYBR Premix Ex Taq II (included in the
PrimeScript One Step RT-PCR Kit), 0.8 µl of forward primer, 0.8 µl
of reverse primer, 0.4 µl of ROX Reference Dye II (included in the
kit), 2 µl of cDNA and 6 µl of ddH2O. U6 was used as
reference gene. The thermocycling conditions were as follows: One
cycle of 95°C for 30 sec, 40 cycles of 95°C for 5 sec, 60°C for 34
sec and 4°C indefinitely. The data was analyzed using SDS software
(version 1.4; Applied Biosystems; Thermo Fisher Scientific, Inc.)
based on the 2−ΔΔCq method (24,25),
and histogram analysis was performed using Origin software (version
10.5.30; http://www.originlab.com/index.aspx?go=PRODUCTS/Origin).
All primers are listed in Table
I.
 | Table I.Primers used for the
reverse-transcription polymerase chain reaction. |
Table I.
Primers used for the
reverse-transcription polymerase chain reaction.
| Primer | Sequence
(5′→3′) |
|---|
| hsa-miR-3609-F |
5′-ACACTCCAGCTGGGCAAAGTGATGAGTAATAC-3′ |
| hsa-miR-3609-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCAGCCAGT-3′ |
| hsa-miR-4497-F |
5′-ACACTCCAGCTGGGCTCCGGGACGG-3′ |
| hsa-miR-4497-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGCCCAGCC-3′ |
| hsa-miR-1246-F |
5′-ACACTCCAGCTGGGAATGGATTTTTGG-3′ |
| hsa-miR-1246-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCTGCTCC-3′ |
| hsa-miR-4301-F |
5′-ACACTCCAGCTGGGTCCCACTACTTCAC-3′ |
| hsa-miR-4301-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCACAAGT-3′ |
|
hsa-miR-6724-5p-F |
5′-ACACTCCAGCTGGGCTGGGCCCGCGGCGGGC-3′ |
|
hsa-miR-6724-5p-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCCACGC-3′ |
| hsa-miR-4508-F |
5′-ACACTCCAGCTGGGGCGGGGCTGGG-3′ |
| hsa-miR-4508-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCGCGCC-3′ |
|
hsa-miR-33b-3p-F |
5′-ACACTCCAGCTGGGCAGTGCCTCGGCAGTG-3′ |
|
hsa-miR-33b-3p-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGGCTGCA-3′ |
|
hsa-miR-1268b-F |
5′-ACACTCCAGCTGGGCGGGCGTGGTGGTG-3′ |
|
hsa-miR-1268b-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCACCCCCA-3′ |
|
hsa-miR-153-5p-F |
5′-ACACTCCAGCTGGGTCATTTTTGTGATGTT-3′ |
|
hsa-miR-153-5p-F |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGCTGCAA-3′ |
| hsa-miR-892b-F |
5′-ACACTCCAGCTGGGCACTGGCTCCTTTCTG-3′ |
| hsa-miR-892b-R |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCTACCCA-3′ |
| U6-F |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6-R |
5′-AACGCTTCACGAATTTGCGT-3′ |
Bioinformatics analysis of
differentially expressed miRNAs in NCI-N87 cells following
cisplatin treatment
High-throughput sequencing was performed with >3
biological replicates in each group, and t-tests were used to
select differentially expressed genes using fold change (FC). FC
differences in the cisplatin-treated (3 replicates) and control (3
replicates) groups were investigated. P<0.05 and log2 (FC)>2
(upregulated) or log2(FC)<-2 (downregulated) were used to
identify differentially expressed miRNAs. The differentially
expressed miRNAs were used to construct heatmaps and perform
volcano analysis using R (version 3.5, www.R-project.org). The distribution frequency of the
miRNAs following cisplatin treatment was determined by the adjusted
P-value (Padj) according to the analysis of differentially
expressed miRNAs (26).
Gene Ontology (GO) clustering
The GO database (geneontology.org) contains three aspects of functional
information: The biological process (BP), the cellular component
(CC), and the molecular function (MF) (27,28).
These functions were organized into the ‘Directed Acyclic Graph’
(DAG) structure according to the size of the concept. Genes are
considered significantly enriched based on the ratio of the
observed GO term for all genes/GO term for a single gene set. In
the current study, each gene annotated to a GO term was extensively
annotated to all parent nodes of that node. Each GO term-enriched
P-value was calculated using the hyper-geometric distribution
method, and the P-value was corrected using the false discovery
rate. P<0.05 was considered to indicate a statistically
significant difference. Redundant GO terms within the threshold
were removed; GO terms terminal to the DAG graph which were
significantly enriched were selected.
Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway clustering
The KEGG database (https://www.genome.jp/kegg/) is the world's largest
database for analyzing gene function and genomic information from a
biological pathway perspective system (29). It contains metabolites, enzymes,
biochemical reactions, gene regulation and protein interactions. In
the current study, the hypergeometric distribution method was used
to study biological pathways. The F-value was used to correct the
P-value and the threshold was set to 0.05. Finally, a KEGG
signaling pathway in which differentially expressed genes were
significantly enriched was determined. The network was analyzed
using the Cytoscape software (version 3.7.1; http://cytoscape.org/download.html).
Target prediction
miR-1246-regulated genes were obtained using
TargetScan (version 7.2; http://www.targetscan.org/vert_72/) (30) and the miRDB database (http://www.mirdb.org/) (31).
Statistical analysis
All data were expressed as mean ± standard
deviation. One-way ANOVA followed by the Least Significant
Difference test was performed. The Student's t-test was used to
analyze two groups. Statistical analysis was performed using SPSS
software (version 22.0; IBM Corp.). P<0.05 and P<0.01 were
considered to indicate significant and highly significant
statistical differences, respectively.
Results
Differential expression profile
analysis of cisplatin-regulated miRNAs
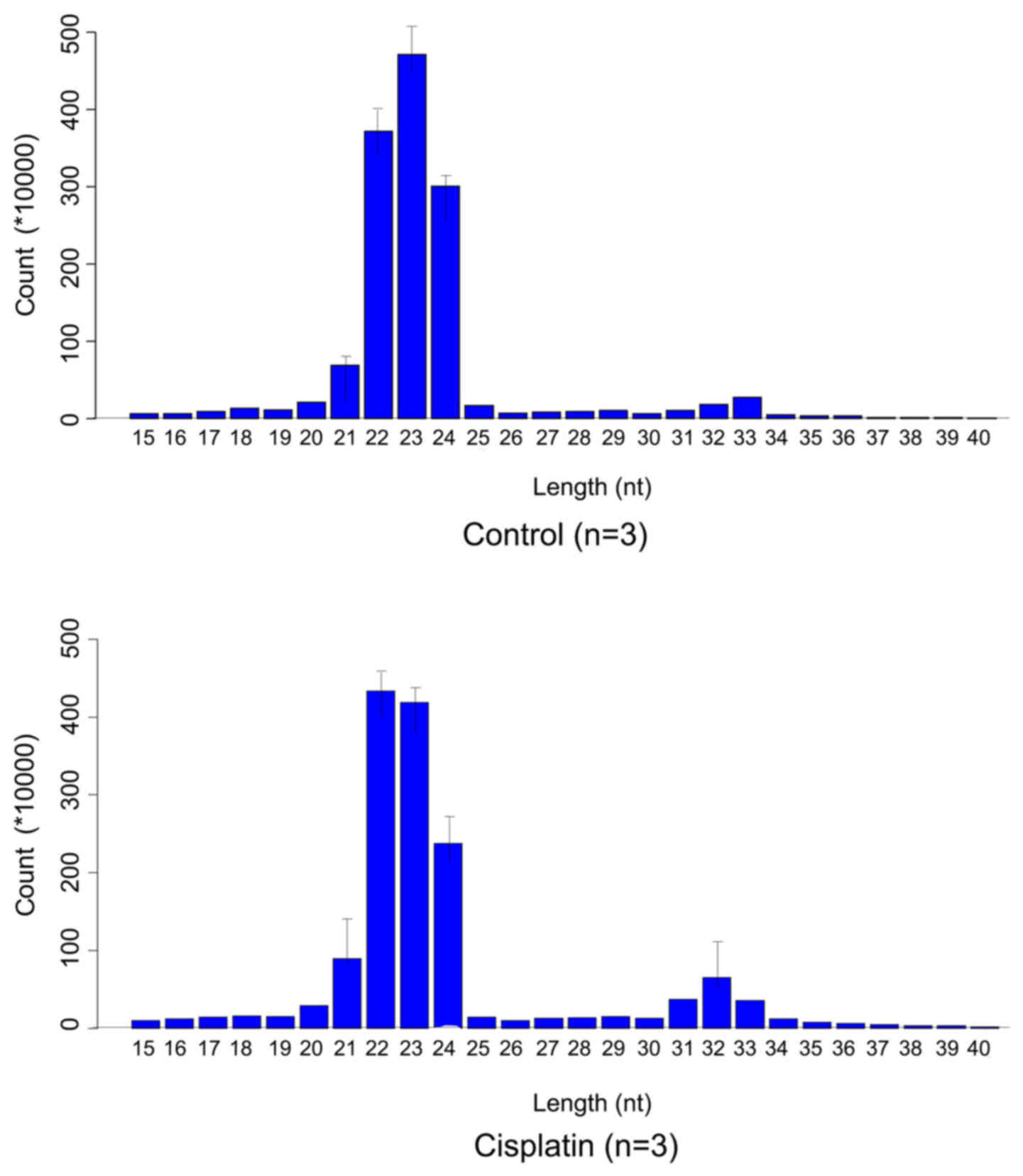
According to the quantile normalization exhibited in
Fig. 1, the length of the miRNAs
identified was 19–25 nucleotides. Bioinformatics analysis revealed
a total of 33 upregulated miRNAs and 16 downregulated miRNAs in
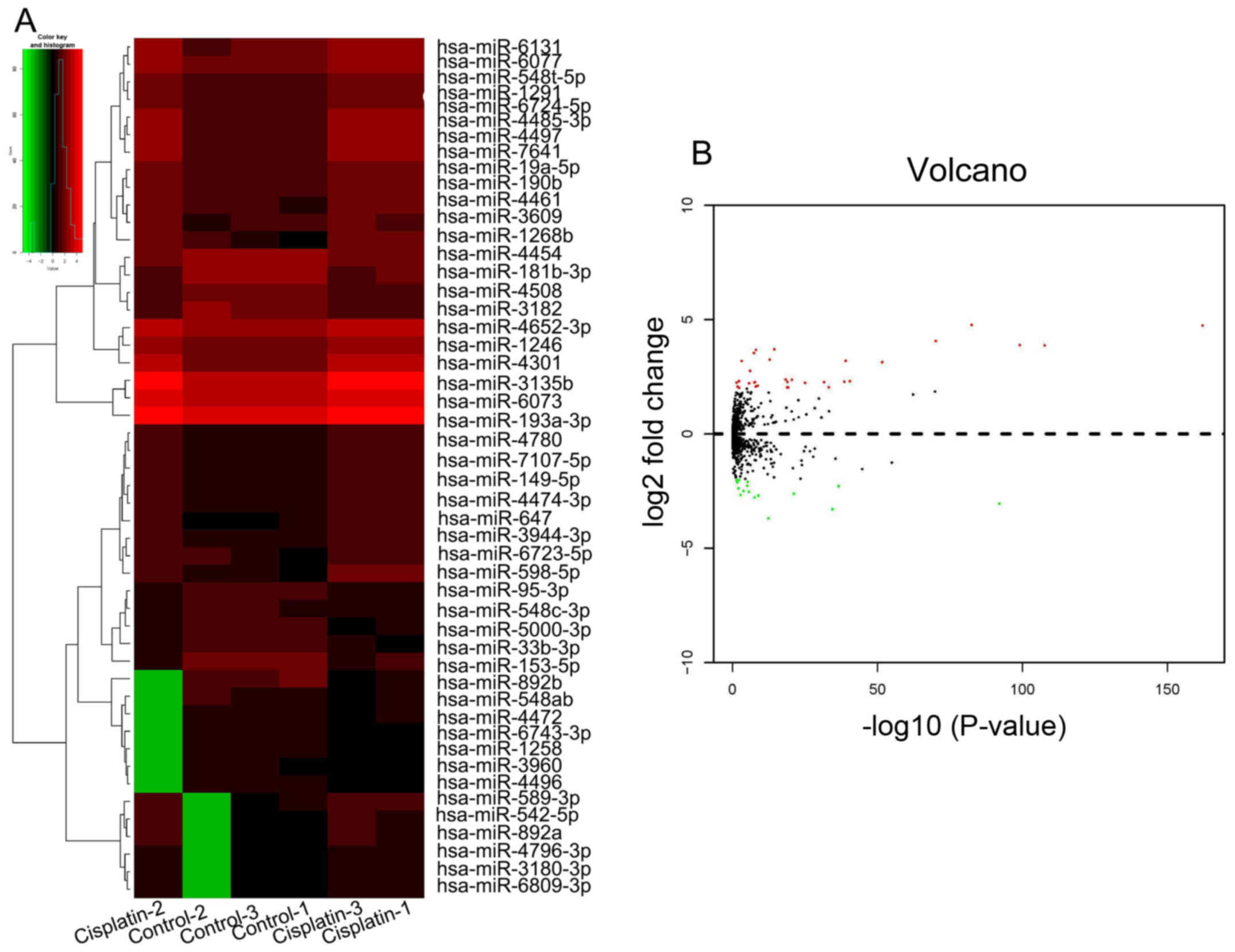
NCI-N87 cells following cisplatin treatment (Table II). A heatmap was generated based
on these differentially expressed miRNAs, where red represented the
upregulated miRNAs and green represented the downregulated miRNAs
(Fig. 2A). The distribution
frequency of the miRNAs following cisplatin treatment was
determined by the Padj according to the analysis of differentially
expressed miRNAs. The volcano plot presented in Fig. 2B reflected the similarities and the
differences in gene expression among the miRNAs regulated by
cisplatin treatment.
 | Table II.Microarray expression profile
analysis of microRNAs in NCI-N87 gastric cancer cells following
cisplatin treatment. |
Table II.
Microarray expression profile
analysis of microRNAs in NCI-N87 gastric cancer cells following
cisplatin treatment.
| miR | Normalized mean
value | Normalized mean
value in the control group | Normalized mean
value in the cisplatin treatment group | Fold change | P-value | Adjusted
P-value |
|---|
| hsa-miR-3609 | 93 | 7 | 180 | 27.1907 |
3.32×10−83 |
5.94×10−81 |
| hsa-miR-4497 | 287 | 21 | 553 | 26.7058 |
7.36×10−163 |
6.59×10−160 |
| hsa-miR-1246 | 1,530 | 173 | 2,887 | 16.6663 |
7.95×10−71 |
1.19×10−68 |
| hsa-miR-4301 | 15,355 | 1,951 | 28,759 | 14.7441 |
7.76×10−100 |
2.32×10−97 |
|
hsa-miR-6724-5p | 192 | 25 | 359 | 14.6075 |
2.13×10−108 |
9.51×10−106 |
| hsa-miR-598-5p | 31 | 4 | 58 | 12.9564 |
3.79×10−15 |
6.64×10−14 |
|
hsa-miR-4485-3p | 185 | 27 | 343 | 12.7338 |
8.22×10−09 |
7.08×10−08 |
| hsa-miR-542-5p | 6 | 1 | 12 | 11.6069 |
3.93×10−08 |
3.00×10−07 |
| hsa-miR-647 | 12 | 2 | 22 | 9.4955 |
1.45×10−13 |
1.96×10−12 |
| hsa-miR-6073 | 45,471 | 8,943 | 81,999 | 9.1686 |
9.15×10−40 |
6.30×10−38 |
| hsa-miR-892a | 5 | 1 | 9 | 9.0982 | 0.00074172 | 0.00301745 |
| hsa-miR-3135b | 8,839 | 1,807 | 15,871 | 8.7814 |
2.23×10−52 |
2.00×10−50 |
| hsa-miR-589-3p | 7 | 2 | 12 | 6.7428 |
8.38×10−07 |
5.28×10−06 |
|
hsa-miR-4652-3p | 427 | 138 | 715 | 5.1846 |
4.70×10−19 |
9.34×10−18 |
| hsa-miR-190b | 33 | 11 | 55 | 5.1624 |
3.19×10−21 |
7.32×10−20 |
|
hsa-miR-4796-3p | 3 | 1 | 5 | 4.9350 | 0.00614119 | 0.01850627 |
|
hsa-miR-548t-5p | 107 | 36 | 178 | 4.9321 |
3.05×10−41 |
2.27×10−39 |
| hsa-miR-1291 | 102 | 35 | 169 | 4.8621 |
2.77×10−39 |
1.77×10−37 |
| hsa-miR-4461 | 32 | 11 | 52 | 4.8275 |
1.14×10−19 |
2.44×10−18 |
| hsa-miR-6077 | 158 | 54 | 261 | 4.7968 |
2.65×10−32 |
1.19×10−30 |
| hsa-miR-4780 | 11 | 4 | 19 | 4.7869 |
1.76×10−08 |
1.46×10−07 |
|
hsa-miR-193a-3p | 11 | 4 | 19 | 4.7675 |
2.97×10−08 |
2.31×10−07 |
|
hsa-miR-3180-3p | 3 | 1 | 5 | 4.7199 | 0.04119246 | 0.09570207 |
| hsa-miR-6131 | 159 | 56 | 263 | 4.6951 |
9.21×10−26 |
2.84×10−24 |
|
hsa-miR-4474-3p | 13 | 4 | 21 | 4.6427 |
1.96×10−06 |
1.19×10−05 |
|
hsa-miR-3944-3p | 17 | 6 | 28 | 4.2961 |
2.18×10−09 |
2.04×10−08 |
|
hsa-miR-7107-5p | 16 | 6 | 27 | 4.2217 |
9.13×10−09 |
7.79×10−08 |
| hsa-miR-149-5p | 14 | 5 | 23 | 4.2044 |
9.25×10−09 |
7.81×10−08 |
|
hsa-miR-6809-3p | 3 | 1 | 4 | 4.1852 | 0.01789444 | 0.04669248 |
| hsa-miR-3182 | 720 | 282 | 1,159 | 4.1092 |
6.49×10−34 |
3.06×10−32 |
| hsa-miR-7641 | 52 | 20 | 83 | 4.0835 |
1.45×10−19 |
3.02×10−18 |
| hsa-miR-19a-5p | 39 | 16 | 63 | 4.0720 |
6.52×10−20 |
1.42×10−18 |
|
hsa-miR-6723-5p | 17 | 7 | 27 | 4.0387 | 0.00518868 | 0.01601334 |
| hsa-miR-548ab | 6 | 9 | 2 | 0.2471 | 0.00544169 | 0.01673648 |
| hsa-miR-4496 | 2 | 4 | 1 | 0.2460 | 0.04127486 | 0.09570207 |
| hsa-miR-95-3p | 9 | 14 | 3 | 0.2335 |
5.91×10−06 |
3.35×10−05 |
| hsa-miR-3960 | 3 | 4 | 1 | 0.2299 | 0.02518227 | 0.06285282 |
|
hsa-miR-548c-3p | 9 | 14 | 3 | 0.2067 |
8.12×10−06 |
4.41×10−05 |
|
hsa-miR-181b-3p | 91 | 152 | 31 | 0.2048 |
2.38×10−37 |
1.42×10−35 |
|
hsa-miR-6743-3p | 3 | 5 | 1 | 0.1925 | 0.00920966 | 0.02633433 |
| hsa-miR-4472 | 5 | 9 | 2 | 0.1774 | 0.0001624 | 0.00074159 |
|
hsa-miR-5000-3p | 8 | 14 | 2 | 0.1724 |
2.20×10−06 |
1.32×10−05 |
| hsa-miR-4454 | 169 | 290 | 47 | 0.1624 |
7.04×10−22 |
1.75×10−20 |
| hsa-miR-1258 | 4 | 6 | 1 | 0.1573 | 0.00176885 | 0.00646173 |
| hsa-miR-4508 | 103 | 179 | 28 | 0.1541 |
1.19×10−09 |
1.14×10−08 |
| hsa-miR-33b-3p | 18 | 31 | 5 | 0.1453 |
2.76×10−08 |
2.23×10−07 |
| hsa-miR-1268b | 267 | 477 | 57 | 0.1204 |
9.38×10−93 |
2.10×10−90 |
| hsa-miR-153-5p | 50 | 91 | 9 | 0.1019 |
3.28×10−35 |
1.73×10−33 |
| hsa-miR-892b | 20 | 37 | 3 | 0.0773 |
3.90×10−13 |
5.14×10−12 |
Expression of hsa-miR-1246 is
significantly increased and that expression of hsa-miR-892b is
significantly decreased following cisplatin treatment
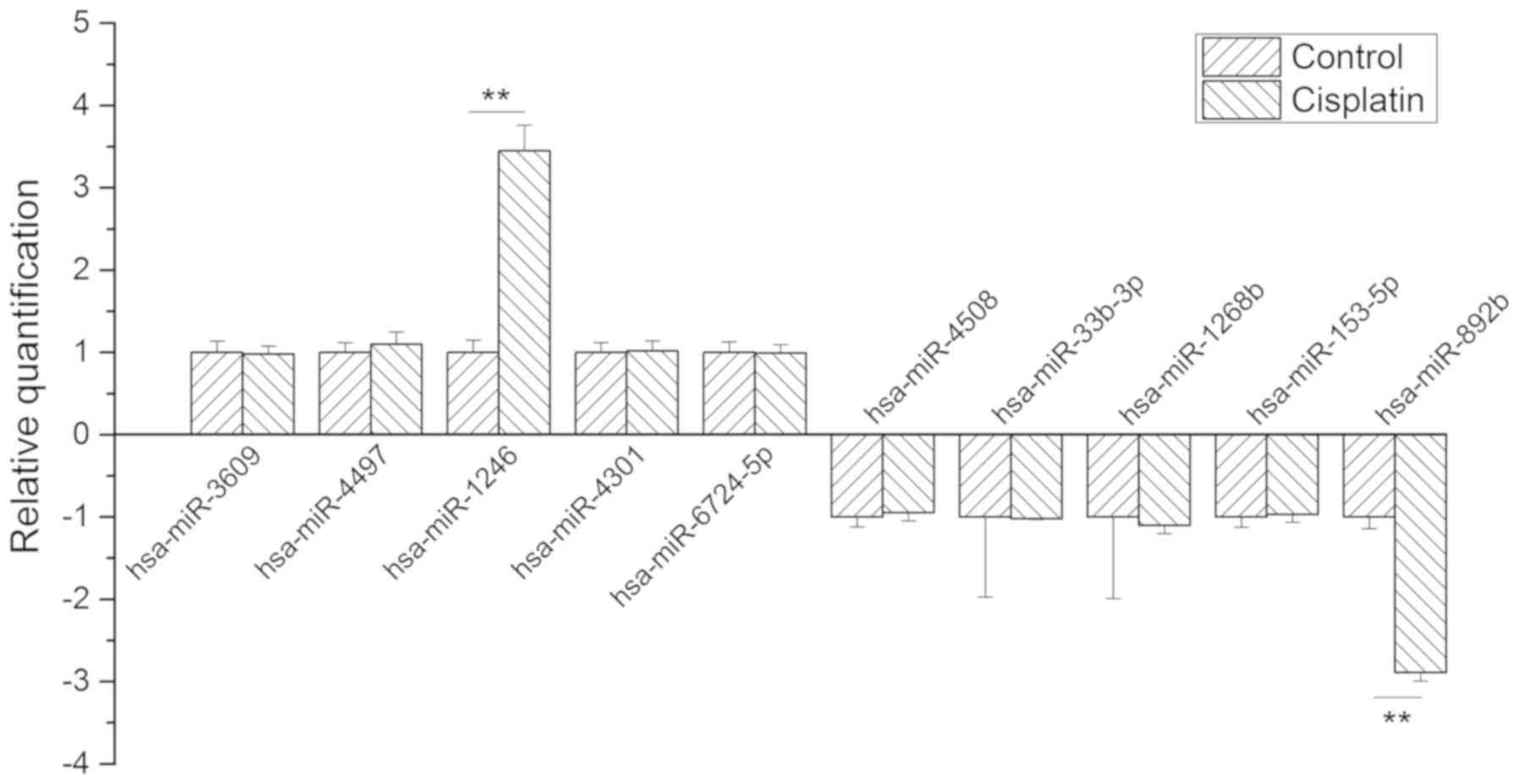
To further validate the differently expressed miRNAs
identified in the microarray, the top five significantly
upregulated miRNAs, including hsa-miR-3609, hsa-miR-4497,
hsa-miR-1246, hsa-miR-4301 and hsa-miR-6724-5p, and the top five
significantly downregulated miRNAs, including hsa-miR-4508,
hsa-miR-33b-3p, hsa-miR-1268b, hsa-miR-153-5p and hsa-miR-892b,
were selected for RT-qPCR analysis. The expression level of
hsa-miR-1246 was significantly increased, and that of hsa-miR-892b
was significantly decreased, following treatment of cisplatin
compared with the control (Fig.
3), consistent with the results obtained from the microarray.
Therefore, hsa-miR-1246 and hsa-miR-892b were selected for
subsequent analysis.
GO clustering of hsa-miR-1246 and
hsa-miR-892b-regulated genes
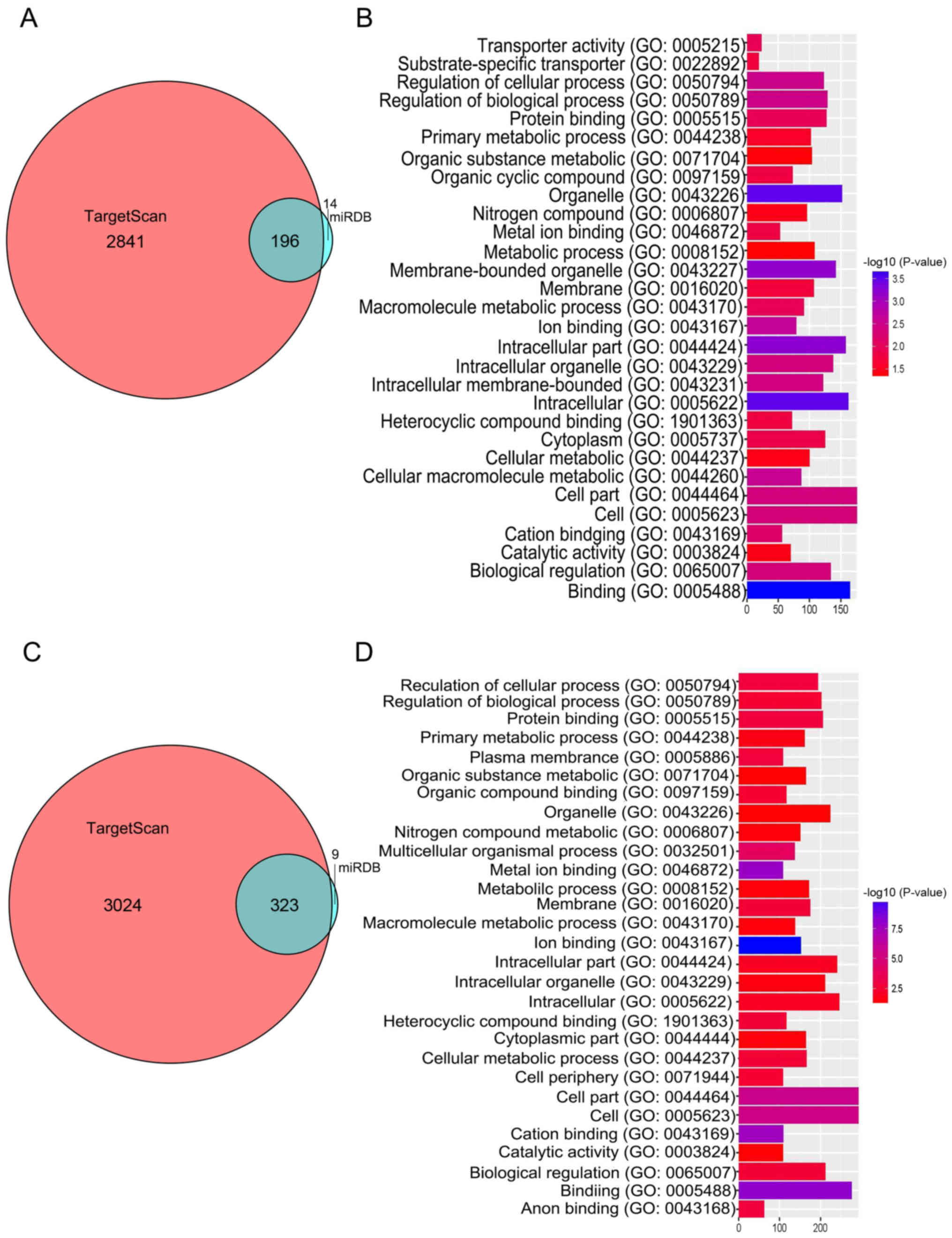
For GO analysis, hsa-miR-1246-regulated genes were
obtained using TargetScan and the miRDB database. A total of 14
intersecting genes were obtained as presented in the Venn diagram
in Fig. 4A. GO clustering was
performed to investigate MF, BF and CC associated with the miRs. As
presented in Fig. 4B, 10 BPs were
identified, including ‘transporter activity’, ‘substrate-specific
transporter activity’, ‘regulation of cellular process’,
‘regulation of BP’, ‘protein binding’, ‘primary metabolic process’,
‘organic substance metabolic process’, ‘organic cyclic compound
binding’, ‘organelle’ and ‘nitrogen compound metabolic process’.
Similarly, for hsa-miR-892b regulated genes, a total of 9
intersecting genes was obtained as presented in Venn diagram in
Fig. 4C. The GO clustering
revealed six processes, including ‘regulation of cellular process’,
‘regulation of biological process’, ‘protein binding’, ‘primary
metabolic process’, ‘plasma membrane’ and ‘organic substance
metabolic process’ (Fig. 4D). The
results obtained indicated that cisplatin-regulated miRNAs
participate in a variety of BPs and may have important regulatory
roles in the tumorigenesis of gastric cancer.
KEGG pathway clustering of
hsa-miR-1246 and hsa-miR-892b regulated genes
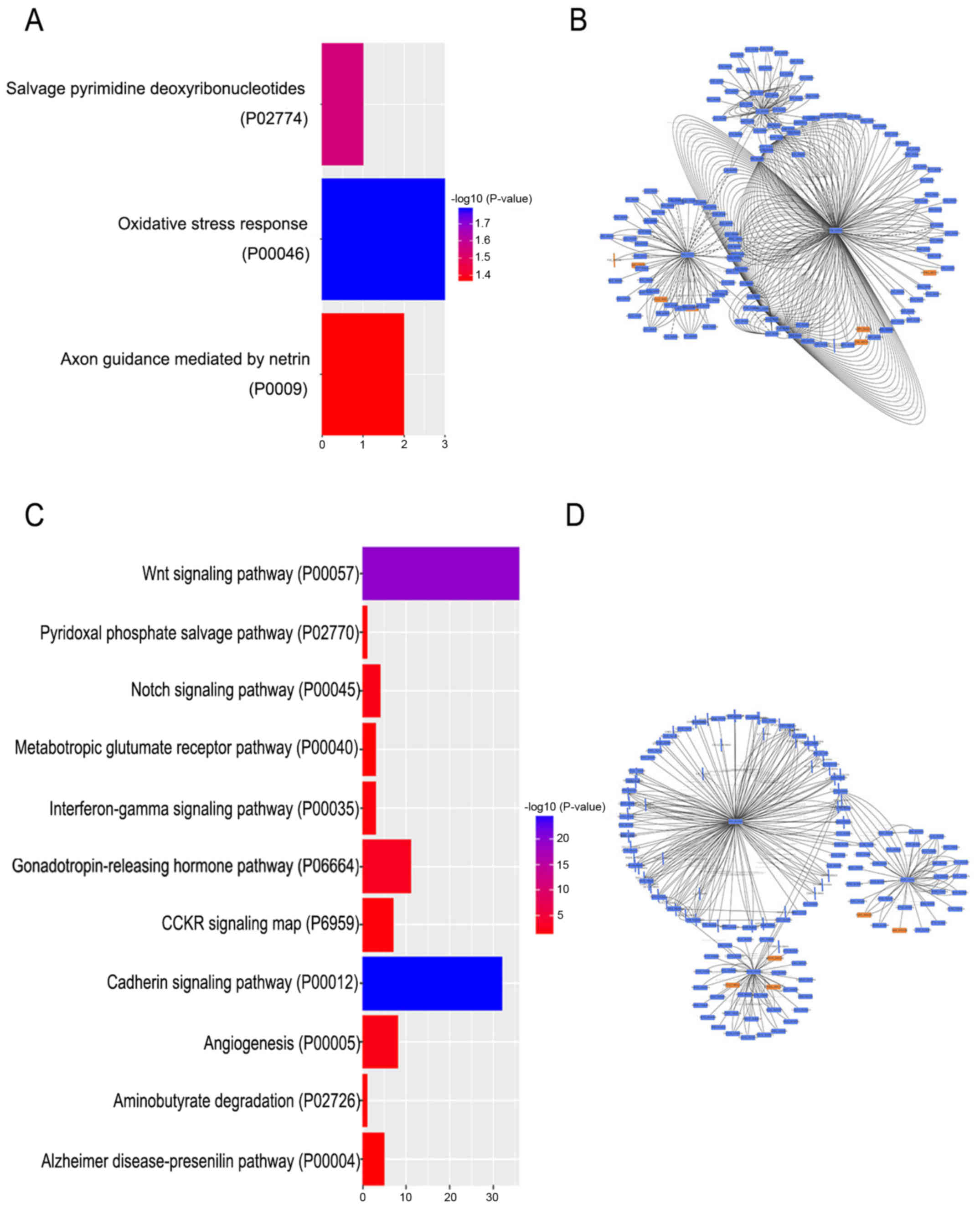
As presented in Fig.
5A, the KEGG pathway enrichment based on hsa-miR-1246 regulated
genes revealed three pathways, including ‘oxidative stress
response’, ‘axon guidance mediated by netrin’ and ‘salvage
pyrimidine deoxyribonucleotides’. Cytoscape software was used to
further analyze the hsa-miR-1246 regulated genes (Fig. 5B).
In addition, as presented in Fig. 5C, the KEGG pathway clustering based
on the hsa-miR-892b regulated genes were involved in 11 pathways,
including ‘Wnt signaling pathway’, ‘cadherin signaling pathway’ and
‘notch signaling pathway’. Cytoscape software was used to further
analyze the hsa-miR-892b regulated genes (Fig. 5D).
Discussion
In the current study, the human gastric cancer cell
line NCI-N87 was cultured with the 15 µg/ml of cisplatin, and
high-throughput sequencing combined with RT-qPCR was used to detect
cisplatin-regulated genes. Finally, miRNAs that were positively and
negatively associated with cisplatin treatment were analyzed
statistically, and confirmed 33 positively- and 16
negatively-associated miRNAs. The top five significantly
upregulated and top five significant down-regulation miRNAs were
selected to further verify the results obtained from the microarray
analysis. It was revealed that the expression levels of
hsa-miR-1246 and hsa-miR-892b were consistent with microarray
analysis. GO and KEGG pathway clustering of cisplatin-regulated
miRNAs was performed, and it was demonstrated that the two miRNAs
were involved in several BPs and signaling pathways. Therefore,
cisplatin may exert an important anticancer effect in gastric
cancer by affecting biological processes and signal pathways;
however further investigation is required.
Gastric cancer is the second leading cause of
cancer-associated mortalities in the world, and has a poor
prognosis as the majority of patients are diagnosed at an advanced
stage (18). Although there have
been improvements in the early diagnosis and treatment of gastric
cancer, the prognosis of patients with gastric cancer is generally
not favorable (32,33). One of the main reasons for this
observation is primary or secondary drug resistance during
chemotherapy (34). A number of
mechanisms have been proposed to explain the phenomenon of drug
resistance in cancer cells (34).
Previous studies revealed that tumor drug-resistance mechanisms
included oncogene activation, anti-oncogene inactivation, reduced
intracellular drug concentration, drug target molecular changes,
metabolism detoxification, enhanced DNA damage repair function and
inhibition of tumor cell apoptosis (28–30).
Therefore, elucidating the mechanisms underlying the occurrence and
development of gastric cancer drug resistance in order to reverse
this process may improve treatment outcomes in gastric cancer.
Cisplatin is one of the most commonly used drugs in the treatment
of advanced cancer, including gastric cancer (35). Despite the high sensitivity of
patients with gastric cancer to cisplatin at initial
administration, a substantial number of patients develop drug
resistance, which is one of the major causes of treatment failure
(36). Cisplatin-based combination
chemotherapy is the most effective treatment for metastatic gastric
cancer (37). However,
chemoresistance remains an obstacle for the effective treatment of
the disease (38).
miRNAs are small non-coding RNAs that function as
endogenous silencers of various target genes (39). Mature miRNAs bind to the
3′untranslated regions of target mRNA, leading to the silencing of
mRNA (39). miRNAs regulate
biological functions, including cell proliferation, differentiation
and apoptosis. A number of studies have demonstrated that miRNAs
are involved in the occurrence, development, diagnosis and
treatment of cancer (40,41). Certain miRNAs, such as miR-34a, are
downregulated in various types of cancer and act as tumor
suppressors (42,43). Conversely, miRNAs such as miR-155
are reportedly overexpressed in various types of cancer, and act as
oncogenes (44). Changes in miRNA
expression contribute to the initiation and progression of cancer
(45,46). The association between miRNAs and
tumors suggests that miRNAs may be altered in patients with gastric
cancer (47,48). A previous study demonstrated that
miR-218 increased chemosensitivity to cisplatin in vitro and
in vivo by inducing apoptosis (18). The current study revealed that
cisplatin upregulated hsa-miR-1246 and downregulated hsa-miR-892b.
Few studies have investigated the role of hsa-miR-1246 in cancer;
however, it has been reported that miR-1246 is associated with the
apoptosis and migration of cancer cells (49). Furthermore, miR-1246 has a
p53-responsive element in its promoter region; p53 was determined
to induced hsa-miR-1246 expression (49). In non-small cell lung cancer,
hsa-miR-1246 plays an important role in the invasion of cancer
cells by promoting proliferation, and sphere and colony formation
(50); however, its role in
gastric cancer has not been reported. The role of hsa-miR-892b in
tumor progression, particularly in gastric cancer, remains
unclear.
The results obtained in the current study suggested
that hsa-miR-1246 and hsa-miR-892b may play important roles in the
progression of gastric cancer via certain BPs and signaling
pathways; however, future studies are required to confirm this. The
high incidence of false positives obtained in the current study may
be associated with the small sample size and the use of only one
cell line. A larger sample size and other cell lines are required
for future work.
The current study investigated cisplatin-regulated
genes in NCI-N87 cells. The results obtained may provide a
theoretical basis for a novel approach to further study the
association between miRNA and cisplatin resistance in gastric
cancers, as well as its possible mechanism. Importantly,
differential miRNA expression patterns may provide a solid basis
for further functional studies to identify potential oncogenic or
tumor suppressor miRNAs in gastric cancer during cisplatin
resistance. Of note, as our study was conducted in a preliminary
matter, further investigation is required to determine the direct
target genes of cisplatin and the mechanism underlying the effects
of cisplatin on gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY and XM conceived and designed the experiments.
CY, XZ, HX and HL performed the experiments. CY, XZ, MG and KY
contributed to the acquisition and analysis of data. CY and XM
wrote, reviewed and edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Podratz JL, Lee H, Knorr P, Koehler S,
Forsythe S, Lambrecht K, Arias S, Schmidt K, Steinhoff G, Yudintsev
G, et al: Cisplatin induces mitochondrial deficits in Drosophila
larval segmental nerve. Neurobiol Dis. 97:60–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bobylev I, Joshi AR, Barham M, Neiss WF
and Lehmann HC: Depletion of mitofusin-2 causes mitochondrial
damage in cisplatin-induced neuropathy. Mol Neurobiol.
55:1227–1235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shkalim-Zemer V, Ash S, Toledano H,
Kollender Y, Issakov J, Yaniv I and Cohen IJ: Highly effective
reduced toxicity dose-intensive pilot protocol for non-metastatic
limb osteogenic sarcoma (SCOS 89. Cancer Chemother Pharmacol.
76:909–916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma G, Cai H, Gao L, Wang M and Wang H:
sCLU regulates cisplatin chemosensitivity of lung cancer cells in
vivo. World J Surg Oncol. 13:802015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li P, Yang X, Cheng Y, Zhang X, Yang C,
Deng X, Li P, Tao J, Yang H, Wei J, et al: MicroRNA-218 increases
the sensitivity of bladder cancer to cisplatin by targeting Glut1.
Cell Physiol Biochem. 41:921–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen JC, Kirschner A, Scarpato KR and
Morgans AK: Current management of refractory germ cell tumors and
future directions. Curr Oncol Rep. 19:82017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pellat A, Wislez M, Svrcek M, Hammel P,
Afchain P and Andre T: Therapeutic management of poorly
differentiated neuroendocrine lung tumors and neuroendocrine
carcinomas of the digestive system. Bull Cancer. 103:880–895. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durusu IZ, Husnugil HH, Atas H, Biber A,
Gerekci S, Gulec EA and Özen C: Anti-cancer effect of clofazimine
as a single agent and in combination with cisplatin on U266
multiple myeloma cell line. Leuk Res. 55:33–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Starkov AK, Zamay TN, Savchenko AA,
Ingevatkin EV, Titova NM, Kolovskaya OS, Luzan NA, Silkin PP and
Kuznetsova SA: Antitumor effect of arabinogalactan and platinum
complex. Dokl Biochem Biophys. 467:92–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jamesdaniel S, Rathinam R and Neumann WL:
Targeting nitrative stress for attenuating cisplatin-induced
downregulation of cochlear LIM domain only 4 and ototoxicity. Redox
Biol. 10:257–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leo M, Schmitt LI, Erkel M, Melnikova M,
Thomale J and Hagenacker T: Cisplatin-induced neuropathic pain is
mediated by upregulation of N-type voltage-gated calcium channels
in dorsal root ganglion neurons. Exp Neurol. 288:62–74. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee J, Kim HH, Ro SM and Yang JH:
Capecitabine and cisplatin (XP) combination systemic chemotherapy
in heavily pre-treated HER2 negative metastatic breast cancer. PLoS
One. 12:e01716052017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vergaro V, Papadia P, Petrini P, Fanizzi
FP, De Pascali SA, Baldassarre F, Pastorino L and Ciccarella G:
Nanostructured polysaccharidic microcapsules for intracellular
release of cisplatin. Int J Biol Macromol. 99:187–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cherniawsky H, Merchant N, Sawyer M and Ho
M: A case report of posterior reversible encephalopathy syndrome in
a patient receiving gemcitabine and cisplatin. Medicine
(Baltimore). 96:e58502017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hassan SM, Khalaf MM, Sadek SA and
Abo-Youssef AM: Protective effects of apigenin and myricetin
against cisplatin-induced nephrotoxicity in mice. Pharm Biol.
55:766–774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karafakioglu YS, Bozkurt MF, Hazman O and
Fidan AF: Efficacy of safranal to cisplatin-induced nephrotoxicity.
Biochem J. 474:1195–1203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sokolova O and Naumann M: NF-κB signaling
in gastric cancer. Toxins. 9(pii): E1192017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen XZ, Chen H, Castro FA, Hu JK and
Brenner H: Epstein-Barr virus infection and gastric cancer: A
systematic review. Medicine (Baltimore). 94:e7922015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Zhang J, Cai M, Zhu Z, Gu W, Yu Y
and Zhang X: DBGC: A database of human gastric cancer. PLoS One.
10:e01425912015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long ZW, Yu HM, Wang YN, Liu D, Chen YZ,
Zhao YX and Bai L: Association of IL-17 polymorphisms with gastric
cancer risk in Asian populations. World J Gastroenterol.
21:5707–5718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang D, Duan H, Huang H, Tong X, Han Y,
Ru G, Qu L, Shou C and Zhao Z: Cisplatin resistance in gastric
cancer cells is associated with HER2 upregulation-induced
epithelial-mesenchymal transition. Sci Rep. 6:205022016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu W, Wang S, Chen Q, Zhang Y, Ni P, Wu X,
Zhang J, Qiang F, Li A, Roe OD, et al: TXNL1-XRCC1 pathway
regulates cisplatin-induced cell death and contributes to
resistance in human gastric cancer. Cell Death Dis. 5:e10552014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korobkina EA, Knyazeva MS, Kil YV, Titov
SE and Malek AV: Comparative analysis of RT-qPCR based
methodologies for microRNA detection. Klin Lab Diagn. 63:722–728.
2018.PubMed/NCBI
|
|
25
|
Kumar D, Das PK and Sarmah BK: Reference
gene validation for normalization of RT-qPCR assay associated with
germination and survival of rice under hypoxic condition. J Appl
Genet. 59:419–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou J, Dong X, Li Y, Tong S, Wang J, Liao
M and Huang G: Deep sequencing identification of differentially
expressed miRNAs in the spinal cord of resiniferatoxin-treated rats
in response to electroacupuncture. Neurotox Res. May 23–2019.(Epub
ahead of print). View Article : Google Scholar
|
|
27
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
The Gene Ontology Consortium: The Gene
Ontology Resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife:. 4:e050052015. View Article : Google Scholar :
|
|
31
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res 43 (Database Issue). D146–D152. 2015. View Article : Google Scholar
|
|
32
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kamata S, Kishimoto T, Kobayashi S,
Miyazaki M and Ishikura H: Possible involvement of persistent
activity of the mammalian target of rapamycin pathway in the
cisplatin resistance of AFP-producing gastric cancer cells. Cancer
Biol Ther. 6:1036–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He J, Qi H, Chen F and Cao C: MicroRNA-25
contributes to cisplatin resistance in gastric cancer cells by
inhibiting forkhead box O3a. Oncol Lett. 14:6097–6102.
2017.PubMed/NCBI
|
|
35
|
Li X, Liang J, Liu YX, Wang Y, Yang XH,
Luan BH, Zhang GL, Du J and Wu XH: miR-149 reverses cisplatin
resistance of gastric cancer SGC7901/DDP cells by targeting FoxM1.
Die Pharmazie. 71:640–643. 2016.PubMed/NCBI
|
|
36
|
Silberman H: Perioperative adjunctive
treatment in the management of operable gastric cancer. J Surg
Oncol. 90:174–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ajani JA, Buyse M, Lichinitser M,
Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha
R, Carrato A, et al: Combination of cisplatin/S-1 in the treatment
of patients with advanced gastric or gastroesophageal
adenocarcinoma: Results of noninferiority and safety analyses
compared with cisplatin/5-fluorouracil in the First-Line Advanced
Gastric Cancer Study. Eur J Cancer. 49:3616–3624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SC and Chun HJ: Chemotherapy for
advanced gastric cancer: Review and update of current practices.
Gut Liver. 7:385–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia S, Guo J, Li J, Zhou L and Zhao Y:
Application of miRNAs in the occurrence and early diagnosis of
pancreatic cancer. Zhonghua Wai Ke Za Zhi. 52:198–201. 2014.(In
Chinese). PubMed/NCBI
|
|
41
|
Wang CM, Yang XL, Liu MH, Cheng BH, Chen J
and Bai B: High-throughput sequencing analysis of differentially
expressed miRNAs and target genes in ischemia/reperfusion injury
and apelin-13 neuroprotection. Neural Regen Res. 13:265–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun X, Huang T, Liu Z, Sun M and Luo S:
LncRNA SNHG7 contributes to tumorigenesis and progression in breast
cancer by interacting with miR-34a through EMT initiation and the
Notch-1 pathway. Eur J Pharmacol. 856:1724072019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu M, Zheng Z, Huang J, Ma X, Huang C, Wu
R, Li X, Liang Z, Deng F, Wu J, et al: Modulation of miR-34a in
curcumin-induced antiproliferation of prostate cancer cells. J Cell
Biochem. May 1–2019.(Epub ahead of print).
|
|
44
|
Fukuda K, Arigami T, Yanagita S,
Matsushita D, Okubo K, Kijima T, Uenosono Y, Ishigami S and
Natsugoe S: A case of advanced gastric cancer showing pathological
complete response after neoadjuvant chemotherapy with S-1 and
oxaliplatin. Gan To Kagaku Ryoho. 46:471–473. 2019.PubMed/NCBI
|
|
45
|
Kalapanida D, Zagouri F, Gazouli M,
Zografos E, Dimitrakakis C, Marinopoulos S, Giannos A, Sergentanis
TN, Kastritis E, Terpos E, et al: Evaluation of pre-mir-34a
rs72631823 single nucleotide polymorphism in triple negative breast
cancer: A case-control study. Oncotarget. 9:36906–36913. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Orosz E, Kiss I, Gyongyi Z and Varjas T:
Expression of circulating miR-155, miR-21, miR-221, miR-30a,
miR-34a and miR-29a: Comparison of colonic and rectal cancer. In
Vivo. 32:1333–1337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anauate AC, Leal MF, Wisnieski F, Santos
LC, Gigek CO, Chen ES, Calcagno DQ, Assumpcao PP, Demachki S,
Arasaki CH, et al: Analysis of 8q24.21 miRNA cluster expression and
copy number variation in gastric cancer. Future Med Chem. May
29–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Z, Dong Y, Hua J, Xue H, Hu J, Jiang
T, Shi L and Du J: A five-miRNA signature predicts survival in
gastric cancer using bioinformatics analysis. Gene. 699:125–134.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hibino S, Saito Y, Muramatsu T, Otani A,
Kasai Y, Kimura M and Saito H: Inhibitors of enhancer of zeste
homolog 2 (EZH2) activate tumor-suppressor microRNAs in human
cancer cells. Oncogenesis. 3:e1042014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim G, An HJ, Lee MJ, Song JY, Jeong JY,
Lee JH and Jeong HC: Hsa-miR-1246 and hsa-miR-1290 are associated
with stemness and invasiveness of non-small cell lung cancer. Lung
Cancer. 91:15–22. 2016. View Article : Google Scholar : PubMed/NCBI
|