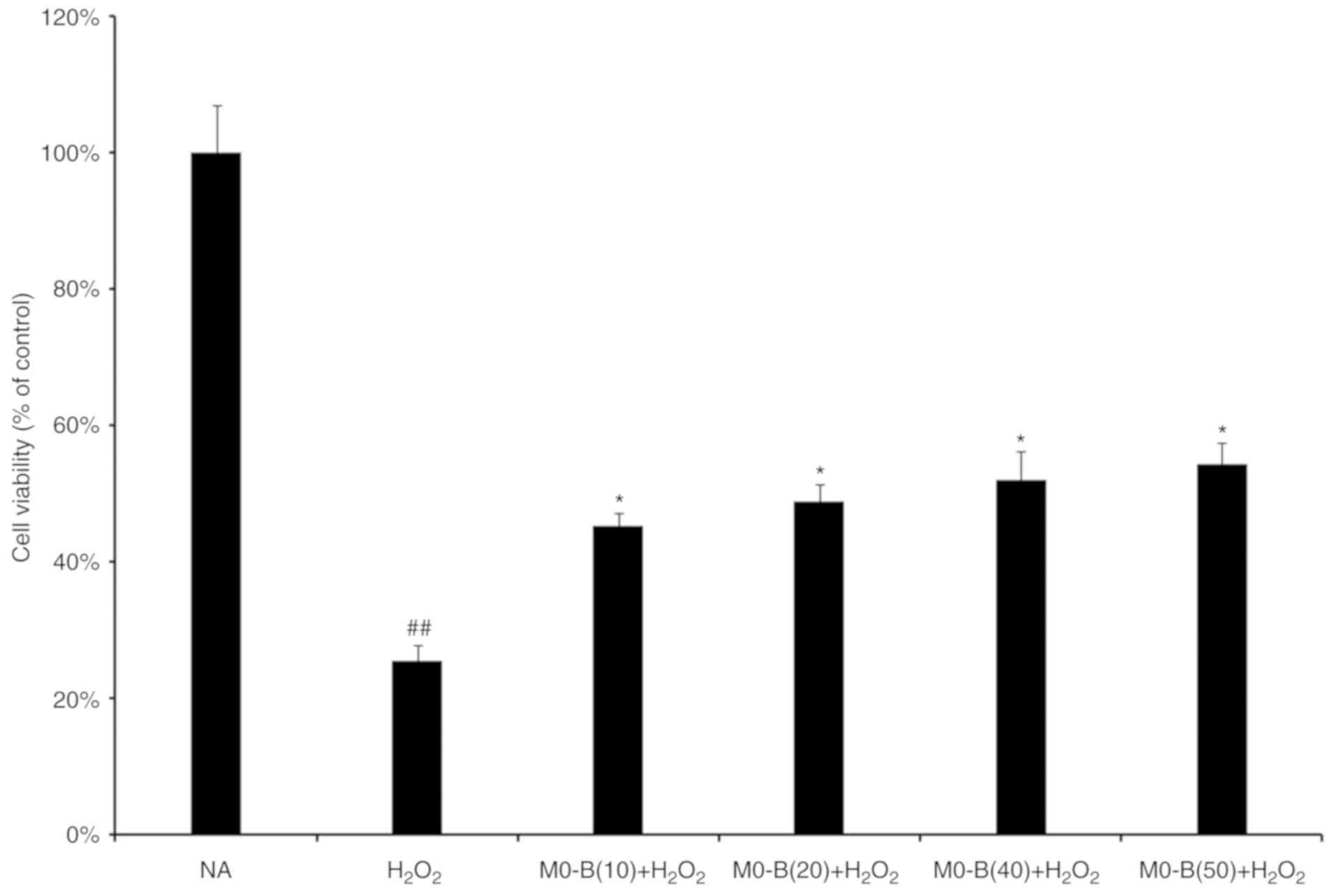
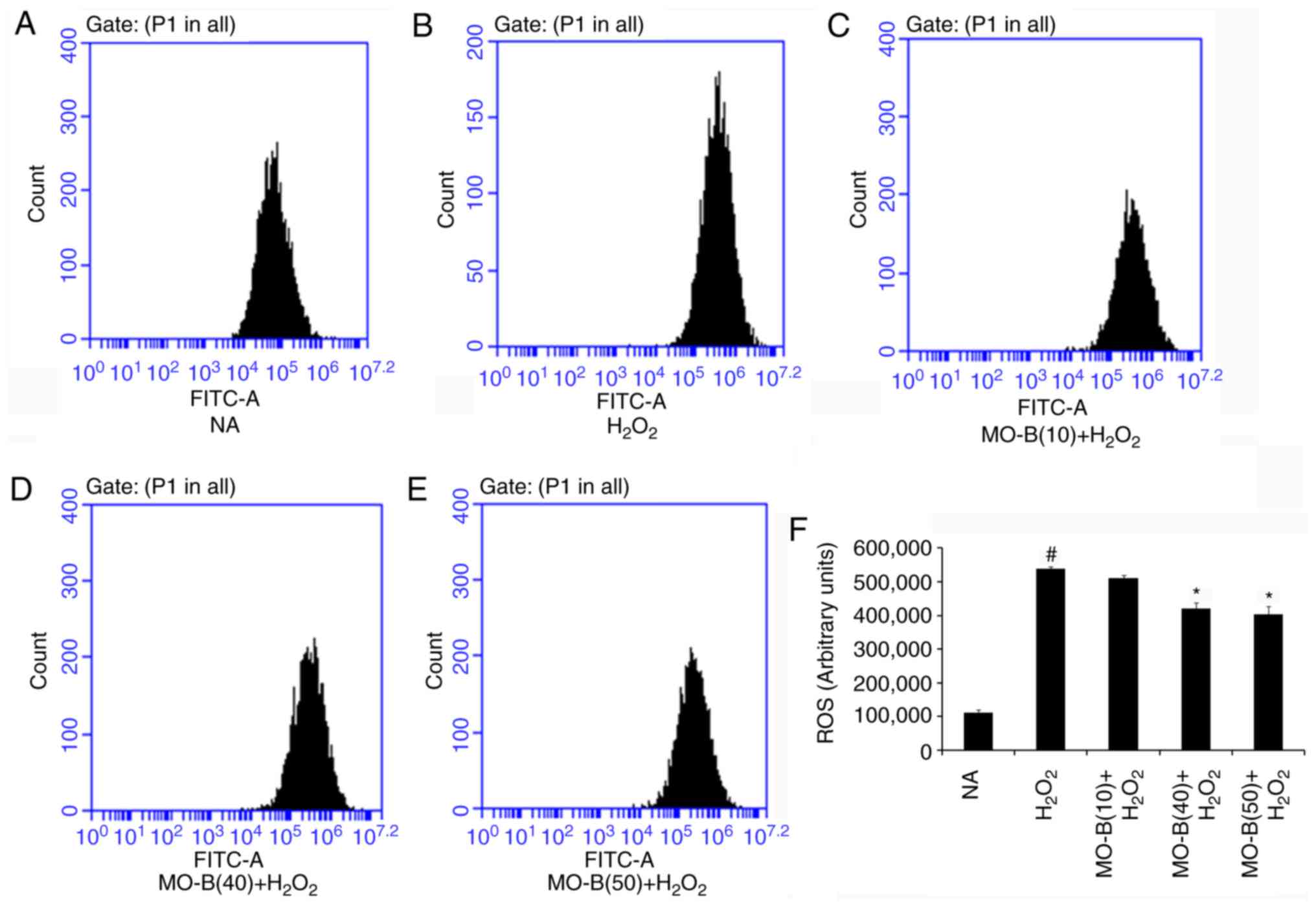
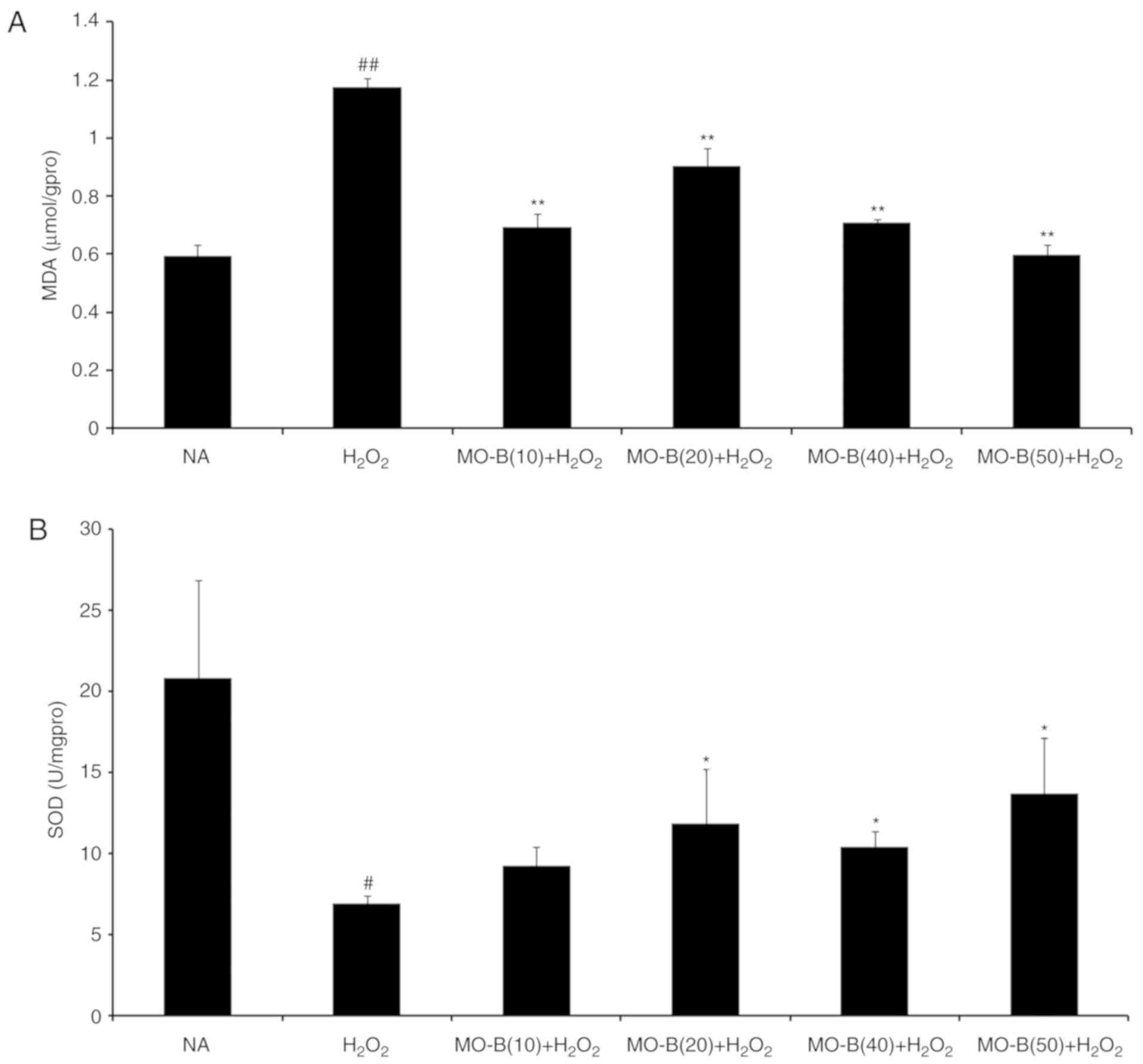
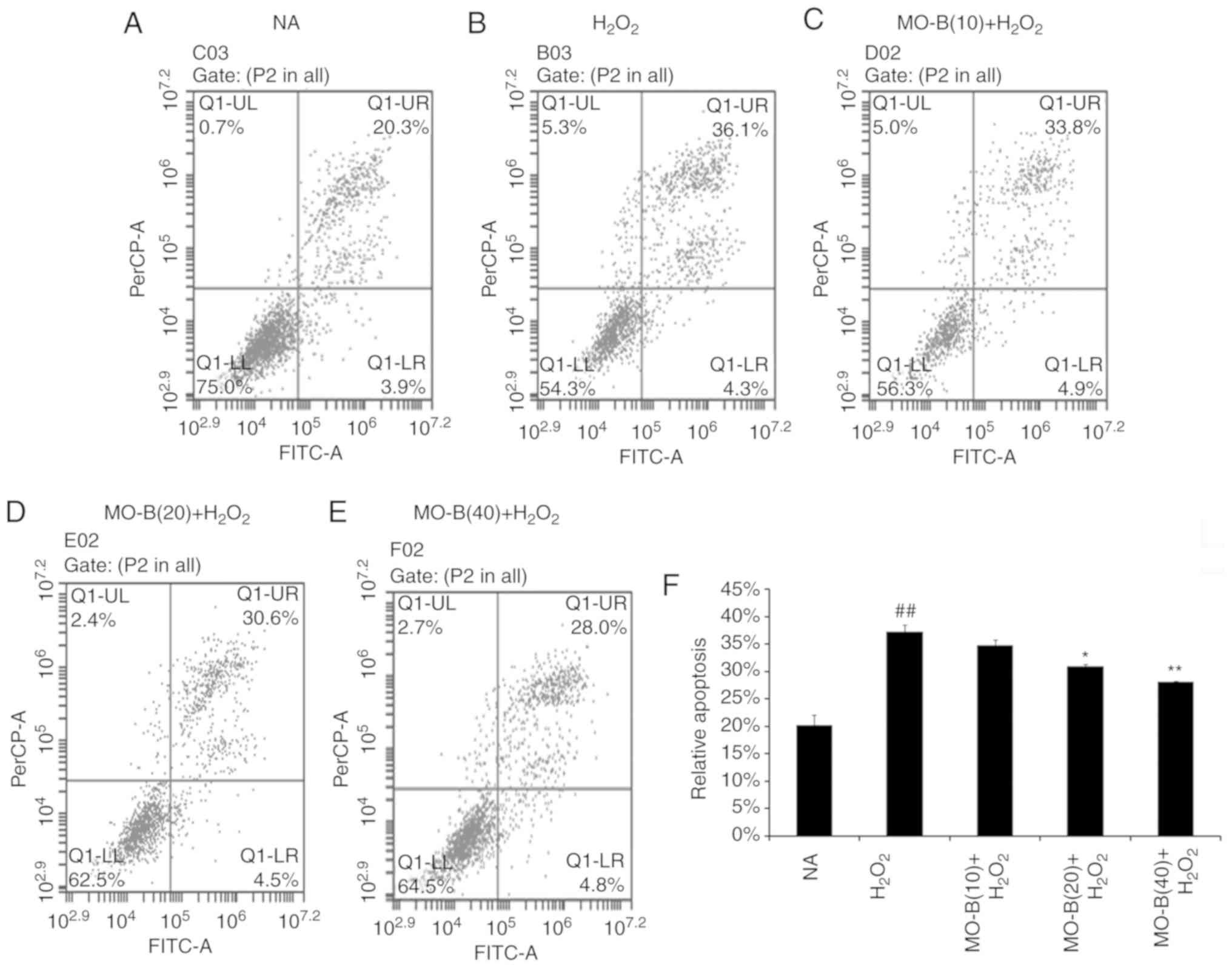
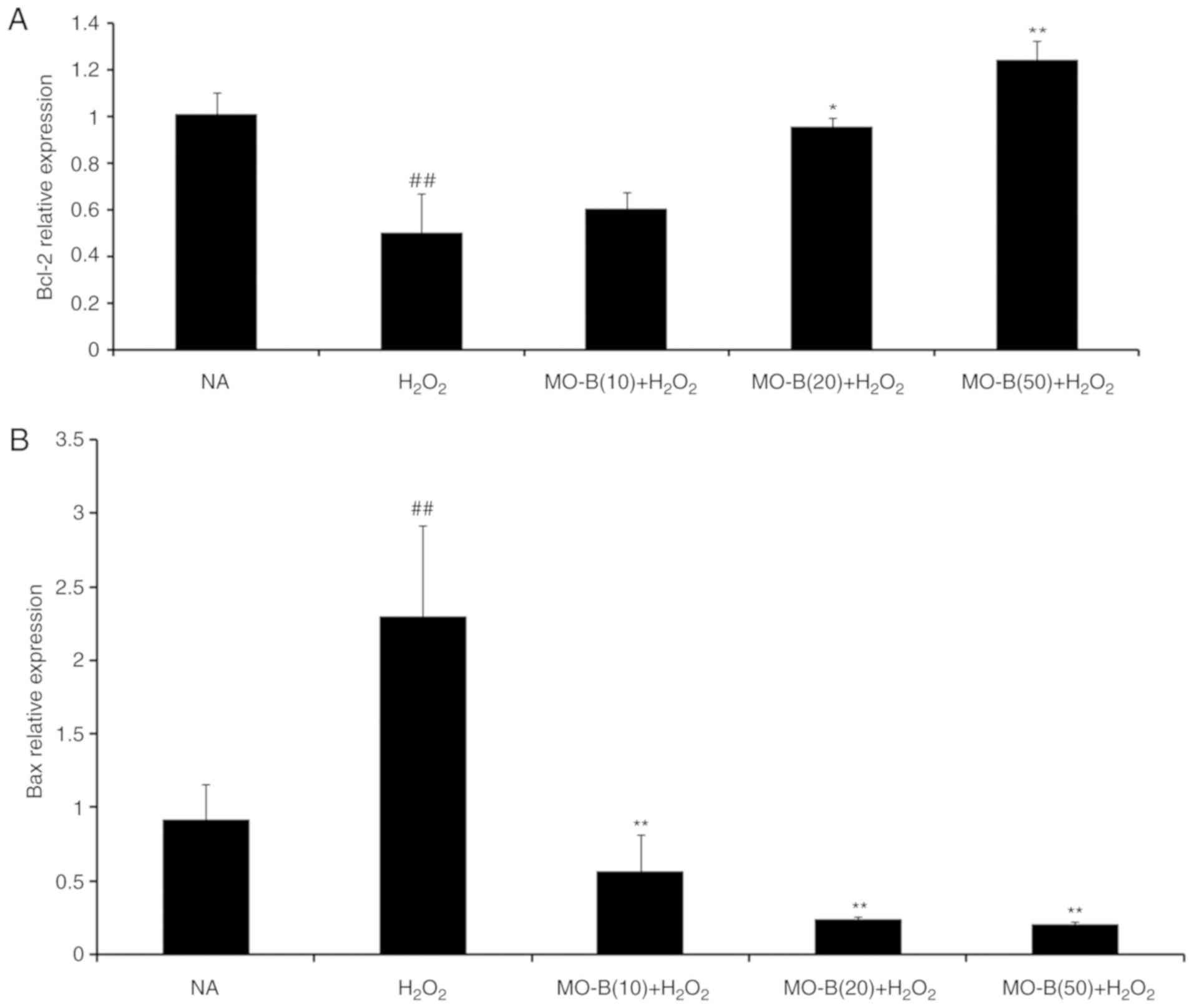
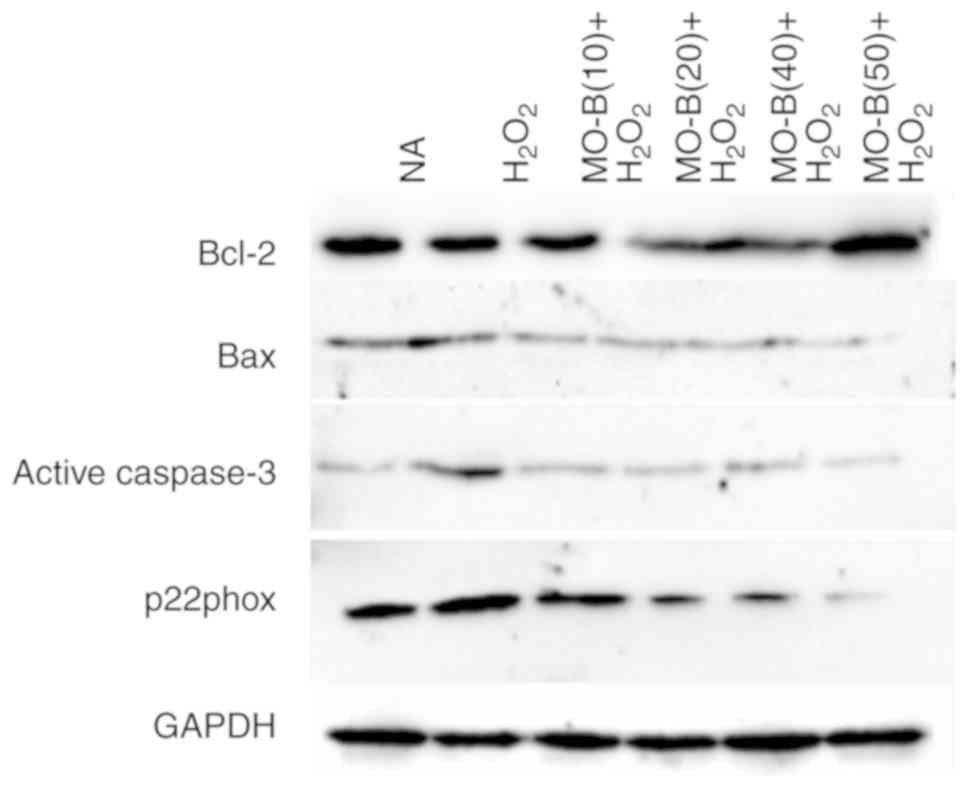
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American Heart Association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lockshin RA and Zakeri Z: Programmed cell
death and apoptosis: Origins of the theory. Nat Rev Mol Cell Biol.
2:545–550. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimmeler S, Hermann C and Zeither AM:
Apoptosis of endothelial cells: Contribution to the pathophysiology
of atherosclerosis. Eur Cytokine Netw. 9:697–698. 1998.PubMed/NCBI
|
|
4
|
Sharifpanah F and Sauer H: Reactive oxygen
species, oxidative stress, and cardiovascular diseasesOxidative
Stress and Antioxidant Protection: The Science of Free Radical
Biology and Disease. Armstrong D and Stratton RD: John Wiley &
Sons Inc.; Hoboken, NJ: pp. 281–306. 2016, View Article : Google Scholar
|
|
5
|
Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman
M and Zhu YC: Antioxidants in Chinese herbal medicines: A
biochemical perspective. Nat Prod Rep. 21:478–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu LY, Zheng GQ and Wang Y: An overview of
systematic reviews of shenmai injection for healthcare. Evid Based
Complement Alternat Med. 2014:8406502014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HD, Xie YM, Wang LX and Wu JB:
Systematic review of efficacy and safety of shenmai injection for
chronic heart failure. Zhongguo Zhong Yao Za Zhi. 39:3650–3661.
2014.(In Chinese). PubMed/NCBI
|
|
8
|
Jun F and Xu Z: Advancement in research of
pharmacological functions of Radix Ophiopogonis on cardiovascular
system. J Nanjing Univ Tradit Chin Med. 22:270–272. 2006.
|
|
9
|
Zhao M, Xu W, Shen HY, Shen PQ, Zhang J,
Wang DD, Xu H, Wang H, Yan TT, Wang L, et al: Comparison of
bioactive components and pharmacological activities of
Ophiopogon japonicas extracts from different geographical
origins. J Pharm Biomed Anal. 138:134–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu BY: Exploration on the modern research
methodology of traditional chinese medicine, basing on the systemic
research of Radix Ophiopogonis. Chin J Nat Med Jan. 5:10–14.
2007.
|
|
11
|
Chen MH, Chen XJ, Wang M, Lin LG and Wang
YT: Ophiopogon japonicas-A phytochemical, ethnomedicinal and
pharmacological review. J Ethnopharmacol. 181:193–213. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan XH, Wang Y and Cheng YY: LC/MS
fingerprinting of Shenmai injection: A novel approach to quality
control of herbal medicines. J Pharm Biomed Anal. 40:591–597. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu SL, Xu SS, Ji K, Yang QH, Lu WW, Jia YS
and Wang N: Effects of maidong on experimental myocardial
infarction and submicrostructure in myocardial hypoxia. Shanghai J
Tradit Chin Med. 3:44–45. 1983.
|
|
14
|
Wang Y, Liu F, Liang Z, Peng L, Wang B, Yu
J, Su Y and Ma C: Homoisoflavonoids and the antioxidant activity of
Ophiopogon japonicus root. Iran J Pharm Res. 16:357–365.
2017.PubMed/NCBI
|
|
15
|
Ito Y, Kanamaru AA and Akihiro T: A novel
agent, methylophiopogonanone B, promotes Rho activation and tubulin
depolymerization. Mol Cell Biochem. 297:121–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujii M, Egawa K, Hirai Y, Kondo M, Fujii
K, Uekusa H, Akita H, Nose K, Toriizuka K and Ida Y:
Dihydrochalcone designed from methylophiopogonanone B strongly
inhibits hypoxia-inducible factor (HIF)-1α activity. Heterocycles.
78:2061–2065. 2009. View Article : Google Scholar
|
|
17
|
Ito Y, Kanamaru A and Tada A: Effects of
methylophiopogonanone B on melanosome transfer and dendrite
retraction. J Dermatol Sci. 42:68–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang KW, Zhang H, Shen LQ and Wang W:
Novel steroidal saponins from liriope graminifolia (Linn.)
baker with anti-tumor activities. Carbohydr Res. 346:253–258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Wang L, Liu T, Mao Z, Ge Q and Mao
J: Isolation of homoisoflavonoids from the fibrous roots of
Ophiopogon japonicus by recycling high-speed
counter-currentchromatography and online antioxidant activity
assay. Acta Chromatogr. Oct 14–2018.(Epub ahead of print).
doi.org/10.1556/1326.2018.00509. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaweł S, Wardas M, Niedworok E and Wardas
P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek.
57:453–455. 2004.(In Polish). PubMed/NCBI
|
|
22
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cleary ML, Smith SD and Sklar AJ: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gross A, Mcdonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K
and Sun H: Luteolin protects HUVECs from TNF-α-induced oxidative
stress and inflammation via its effects on the Nox4/ROS-NF-κB and
MAPK pathways. J Atheroscler Thromb. 21:768–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang HB, Huang J, Guo MJ, Zou P and Tian
XQ: Recent advances in the study of natural homoisoflavonoids. Yao
Xue Xue Bao. 42:118–126. 2007.(In Chinese). PubMed/NCBI
|
|
30
|
Hung TM, Thu CV, Dat NT, Ryoo SW, Lee JH,
Kim JC, Na M, Jung HJ, Bae K and Min BS: Homoisoflavonoid
derivatives from the roots of Ophiopogon japonicus and their
in vitro anti-inflammation activity. Bioorg Med Chem Lett.
20:2412–2416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Damodar K, Lee J, Kim JK and Jun JG:
Synthesis and in vitro evaluation of homoisoflavonoids as potent
inhibitors of nitric oxide production in RAW-264.7 cells. Bioorg
Med Chem Lett. 28:2098–2102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siddaiah V, Maheswara M, Venkata Rao C,
Venkateswarlu S and Subbaraju GV: Synthesis, structural revision,
and antioxidant activities of antimutagenic homoisoflavonoids from
Hoffmanosseggia intricata. Bioorg Med Chem Lett. 17:1288–1290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou YF, Qi J, Zhu DN and Yu BY:
Homoisoflavonoids from Ophiopogon japonicus and its oxygen
free radicals (OFRs) scavenging effects. Chin J Nat Med. 6:201–204.
2008. View Article : Google Scholar
|
|
34
|
El-Elimat T, Rivera-Chávez J, Burdette JE,
Czarnecki A, Alhawarri MB, Al-Gharaibeh M, Alali F and Oberlies NH:
Cytotoxic homoisoflavonoids from the bulbs of Bellevalia flexuosa.
Fitoterapia. 127:201–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan CL, Kang ZY, Lin CR, Jiang Y, Liu JX
and Tu PF: Two new homoisoflavonoids from the fibrous roots of
Ophiopogon japonicus (Thunb.) Ker-Gawl. J Asian Nat Prod
Res. 11:876–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alali F, El-Elimat T, Albataineh H,
Al-Balas Q, Al-Gharaibeh M, Falkinham JO III, Chen WL, Swanson SM
and Oberlies NH: Cytotoxic homoisoflavones from the bulbs of
Bellevalia eigii. J Nat Prod. 78:1708–1715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H, Fowler MI, Messenge DJ, Terry LA,
Gu X, Zhou L, Liu R, Su J, Shi S, Ordaz-Ortiz JJ, et al:
Homoisoflavonoids are potent glucose transporter 2(GLUT2)
inhibitors: A potential mechanism for the glucose-lowering
properties of Polygonatum odoratum. J Agric Food Chem.
66:3137–3145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee B, Sun W, Lee H, Basavarajappa H,
Sulaiman RS, Sishtla K, Fei X, Corson TW and Seo SY: Design,
synthesis and biological evaluation of photoaffinity probes of
antiangiogenic homoisoflavonoids. Bioorg Med Chem Lett.
26:4277–4281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amin SA, Adhikari N, Gayen S and Jha T:
Homoisoflavonoids as potential antiangiogenic agents for retinal
neovascularization. Biomed Pharmacother. 95:818–827. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He F, Xu BL, Chen C, Jia HJ, Wu JX, Wang
XC, Sheng JL, Huang L and Cheng J: Methylophiopogonanone A
suppresses ischemia/reperfusion-induced myocardial apoptosis in
mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol
Sin. 37:763–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin M, Sun W, Gong W, Zhou Z, Ding Y and
Hou Q: Methylophiopogonanone a protects against cerebral
ischemia/reperfusion injury and attenuates blood-brain barrier
disruption in vitro. PLoS One. 10:e01245582015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Richard V, Kindt N and Saussez S:
Macrophage migration inhibitory factor involvement in breast cancer
(Review). Int J Oncol. 47:1627–1633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Günther S, Fagone P, Jalce G, Atanasov AG,
Guignabert C and Nicoletti F: Role of MIF and D-DT in
immune-inflammatory, autoimmune, and chronic respiratory diseases:
From pathogenic factors to therapeutic targets. Drug Discov Today.
24:428–439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fagone P, Mazzon E, Cavalli E, Bramanti A,
Petralia MC, Mangano K, Al-Abed Y, Bramati P and Nicoletti F:
Contribution of the macrophage migration inhibitory factor
superfamily of cytokines in the pathogenesis of preclinical and
human multiple sclerosis: In silico and in vivo evidences. J
Neuroimmunol. 322:46–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Presti M, Mazzon E, Basile MS, Petralia
MC, Bramant A, Colletti G, Bramanti P, Nicoletti F and Fagone P:
Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.PubMed/NCBI
|
|
46
|
Mangano K, Mazzon E, Basile MS, Marco R,
Bramanti P, Mammana S, Petralia MC, Fagone P and Nicoletti F:
Pathogenic role for macrophage migration inhibitory factor in
glioblastoma and its targeting with specific inhibitors as novel
tailored therapeutic approach. Oncotarget. 9:17951–17970. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kindt N, Journe F, Laurent G and Saussez
S: Involvement of macrophage migration inhibitory factor in cancer
and novel therapeutic targets. Oncol Lett. 12:2247–2253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin Y, Zhu D, Qi J, Qin M and Yu B:
Characterization of homoisoflavonoids in different cultivation
regions of Ophiopogon japonicus and related antioxidant
activity. J Pharm Biomed Anal. 52:757–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yapislar H and Taskin E: L-carnosine
alters some hemorheologic and lipid peroxidation parameters in
nephrectomized rats. Med Sci Monit. 20:399–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin Y, Liu K, Peng J, Wang C, Kang L,
Chang N and Sun H: Rhizoma Dioscoreae Nipponicae polysaccharides
protect HUVECs from H2O2-induced injury by
regulating PPARγ factor and the NADPH oxidase/ROS-NF-κB signal
pathway. Toxicol Lett. 232:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Atig F, Raffa M, Ali HB, Abdelhamid K,
Saad A and Ajina M: Altered antioxidant status and increased lipid
per-oxidation in seminal plasma of tunisian infertile men. Int J
Biol Sci. 8:139–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zafari AM, Ushio-Fukai M, Akers M, Yin Q,
Shah A, Harrison DG, Taylor WR and Griendling KK: Role of
NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular
hypertrophy. Hypertension. 32:488–495. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Drummond GR, Selemidis S, Griendling KK
and Sobey CG: Combating oxidative stress in vascular disease: NADPH
oxidases as therapeutic targets. Nat Rev Drug Discov. 10:453–471.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schramm A, Matusik P, Osmenda G and Guzik
TJ: Targeting NADPH oxidases in vascular pharmacology. Vascul
Pharmacol. 56:216–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
San José G, Fortuño A, Beloqui O, Díez J
and Zalba G: NADPH oxidase CYBA polymorphisms, oxidative stress and
cardiovascular diseases. Clin Sci (Lond). 114:173–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Djordjevic T, Pogrebniak A, BelAiba RS,
Bonello S, Wotzlaw C, Acker H, Hess J and Görlach A: The expression
of the NADPH oxidase subunit p22phox is regulated by a
redox-sensitive pathway in endothelial cells. Free Radic Biol Med.
38:616–630. 2005. View Article : Google Scholar : PubMed/NCBI
|