Introduction
Lung cancer is one of the leading causes of
cancer-associated mortalities worldwide, with a 5-year survival
rate of <20% (1). Approximately
1.8 million new cases are diagnosed annually, of which 80% present
with an advanced stage disease. Furthermore, ~50% of the patients
are aged >65 years, while 30–40% are aged >70 years and are
ineligible for surgery (2). In
clinical practice, chemotherapy is the primary treatment modality
for lung cancer. However, the majority of patients acquire
chemoresistance and metastatic progression, which leads toward the
failure of cancer-targeted therapies.
Several advances in tumor immunology in the past
decade have aided the body's natural immune system in combating
cancer. The tumor microenvironment (TME), characterized by the lack
of nutrients, acidic and hypoxic environment, consists of cancerous
and non-cancerous cells supporting tumor growth, invasion and
metastasis (3). Furthermore,
immune cells lose their anti-tumorigenic ability and antagonize
antitumour activity. The mutual conversion of tumor-associated
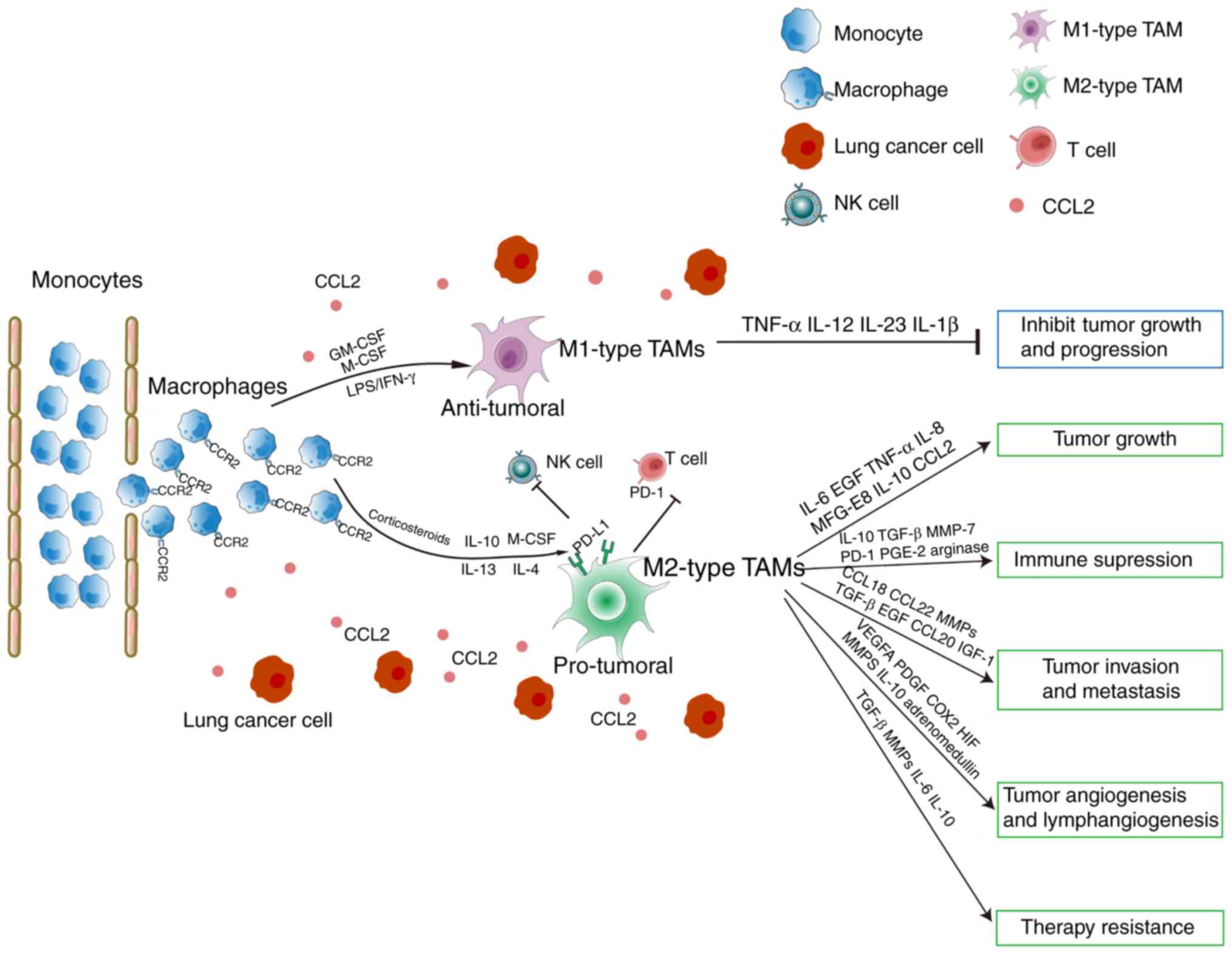
macrophages (TAMs), an abundant population of leukocytes in lung
cancer, are determined by the TME (4). The TAM phenotypes dynamically alter
during tumor progression. The M1-like macrophages are initially
activated, and they produce chemokines and cytokines to recruit the
cytotoxic CD8+ T and NK cells, which express high levels
of IFN-γ and other cytokines to destroy the tumor cells (4). However, during tumor progression, the
M2-like TAMs protect the cancer cells from anti-tumor immune
responses, and promote their proliferation, angiogenesis, and
metastasis. These M2-like TAMs secrete TGF-β to impede the
cytotoxicity of NK cells, and express high levels of programmed
cell death ligand 1 (PD-L1) to restrict the anti-tumor activity of
T cells (5,6).
Clinical studies have suggested that increased TAM
density correlates with a poor prognosis in solid tumors (5,7,8)
Several animal model experiments have validated this observation by
demonstrating that increased TAM density is associated with tumor
progression and metastasis, and overexpression of macrophage growth
factors or chemokines (9,10). The deletion or re-differentiation
of TAMs enhances immune cell-mediated anti-tumor responses and
benefits from chemotherapy (11–13).
Therefore, targeting TAMs may be at the forefront of lung cancer
research and a novel strategy for lung cancer therapy. The present
review provides an overview of TAM biology and proposes a
therapeutic strategy for targeting TAMs in lung cancer.
Macrophage plasticity in lung cancer
development
Origin of TAMs in lung cancer
Accumulating evidence has suggested that TAMs
originate from blood monocytes, and are recruited at tumor sites by
tumor-derived chemotactic signals, including monocyte
chemo-attractant protein-1 (MCP-1), which is also known as CCL2
(11–13). Furthermore, a small wave develops
from in situ monocyte-macrophage proliferation and splenic
monocytes. However, lung cancer exhibits a high proportion of
tissue-resident macrophages, named alveolar macrophages (AMs),
which are different to other solid tumors. The AMs are also derived
from peripheral blood monocytes, but di-erentiated in response to
interferon-γ (IFN-γ) and lipopolysaccharide (LPS) (14). The peripheral monocytes and
resident mature monocytes significantly contribute toward the
origin of TAMs in lung cancer. Furthermore, the functional
diversity of TAMs is affected by local TME, and macrophage
polarization occurs at any point in the tumorigenic process.
Opposite properties of M1 and M2
macrophages
Similarly to two polarized sets of T helper 1/2
(Th1/Th2) cells, the TAMs are divided by dichotomy as classically
activated M1 macrophages and alternatively activated M2
macrophages. The classical or M1 macrophages are activated by
microbial products or interferon-γ (IFN-γ), conferring
pro-inflammatory and microbicidal functions, and the capacity to
facilitate tumor cell destruction (15). The microbial products or IFN-γ
activate signal transducer and activator of transcription 1
(STAT1), interferon regulatory factor (IRF) 3, IRF5, and NF-κB,
enable M1 macrophages to generate additional pro-inflammatory
mediators (16). These are
characterized by high production of nitric oxide (NO) and reactive
oxygen intermediates (ROI), secretion of pro-inflammatory
cytokines, including TNF-α, IL-1, IL-12 and IL-23, and high levels
of MHC molecules (15,17) (Fig.
1).
Additionally, Th2 cytokines, including IL-4 and
IL-13, stimulate monocytes or macrophages to transform into the M2
phenotype (15). This macrophage
subset triggers allergic reactions, promotes inflammation
resolution and wound healing, and favors angiogenesis and tissue
remodeling in cancer (Fig. 1).
Apart from IL-4 and IL-13, other stimuli and signaling pathways,
including IL-10, glucocorticoid hormones and IL-1R may also induce
M2 macrophage polarization. There are central transcription
regulators that activate the M2 phenotype, including STAT1, STAT3,
STAT6, peroxisome proliferator-activated receptor (PPAR-γ), cAMP
response element binding protein (CREB)-CCAAT/enhancer binding
protein (C/EBP), hypoxia-inducible factor (HIF), IRF4 and PI3K/Akt
(18–21).
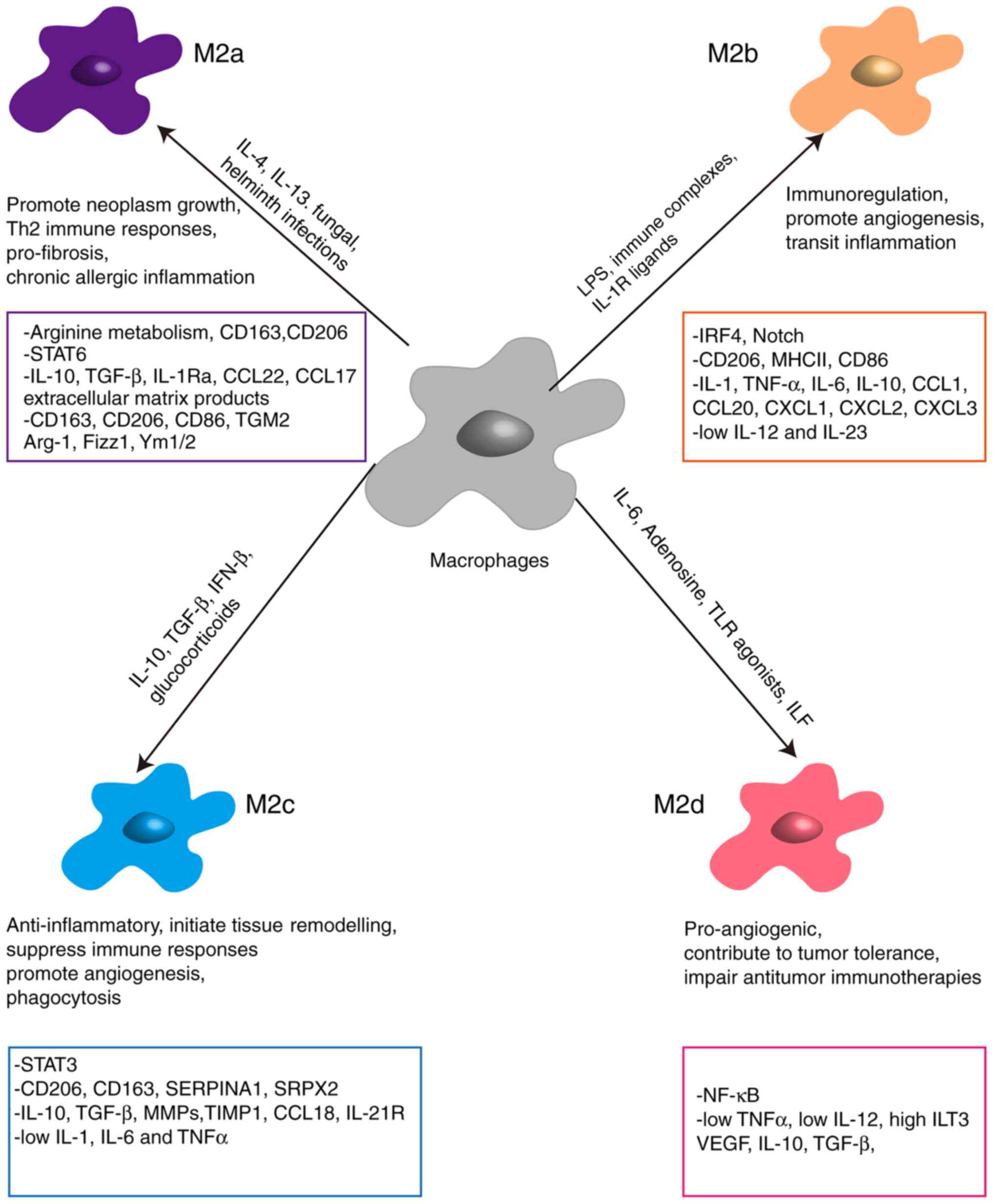
Based on their functions, M2 macrophages are further
classified into M2a, M2b, M2c and M2d (Fig. 2). M2a, induced by IL-4 or IL-13, as
well as fungal and helminth infections, express high levels of
mannose receptor (CD206), CD209, IL-4R and FcεR, and secrete large
amounts of TGF-β and insulin-like growth factor, which contribute
toward wound healing and tissue repair (22). M2b, stimulated in response to
immune complexes, IL-1β and bacterial LPS, are high producers of
IL-10, IL-1β, IL-6 and TNF-α, which exert anti-inflammatory effects
(23). M2c, induced by IL-10,
TGF-β and glucocorticoids, are considered to be involved in
immunosuppression, tissue repair and matrix remodeling (24). These macrophages exhibit increased
expression of RAGE, CD163 and CD206, and secrete large amounts of
IL-10 and TGF-β (25). Finally,
M2d, activated by leukocyte inhibitory factor, TLR ligands and
adenosine, express low levels of CD206, but produce significant
amounts of IL-10, TGF-β and VEGF to promote tumor progression by
facilitating immunosuppression and angiogenesis (26).
TAMs display pro-tumor M2 type
macrophages
Compared to M1 macrophages, TAMs produce fewer ROIs
and inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-12, CCL3 and
CCL4) (27). While the NF-κB
pathway is a key regulator of inflammation, TAMs display defective
NF-κB activation, indicating low expression of NF-κB-dependent
cytotoxic mediators and inflammatory cytokines (16). By contrast, typical M2 markers,
including the scavenger receptor-A (SR-A), mannose receptor (MR),
arginase-I (Arg-I), YM1 and FIZZ1, and MGL2 showed higher
expression in TAMs (16). Previous
studies have suggested that TAMs present M2-associated function by
secreting pro-angiogenic and tumor-inducing chemokines, including
epidermal growth factor (EGF), VEGF and TGF-β (28,29).
Therefore, the notion that TAMs resemble M2 macrophages has been
supported in vitro and in vivo (30).
The M2-type macrophages may be reversed to M1-type
under certain conditions. Macrophages are highly plastic cells that
may be differentiated into several phenotypes. Polarization is
dynamic and affected by the TME. The dichotomy of M1 and M2
subtypes is over-generalized and only partially represents the
continuity of polarization. For example, 5% of the AMs from lung
cancer express M1 and M2 markers (31), and mixed polarization phenotypes
(displaying M1 and M2 characteristics, HLA-DR, IL-1β, TNF-α, CD163
and IL10) have been described (32). Therefore, M1 and M2 markers may be
used to distinguish macrophage populations to a certain extent.
Functional aspects of macrophages in lung
cancer
TAMs in lung cancer initiation and
progression
TAMs provide a suitable microenvironment to support
growth, immunosuppression, invasion and therapeutic resistance in
lung cancer, primarily by secreting TGF-β, IL-10, CCL18, matrix
metalloproteases (MMPs), VEGF, COX2 and PDGF-B (Fig. 1).
IL-10
In vitro, the TAMs derived from THP-1 cells
co-cultured with A549 and H1299 cells promoted
epithelial-to-mesenchymal transition (EMT) and invasion in lung
cancer cells (33,34). Furthermore, TAMs may activate and
protect cancer stem cells (CSCs) to promote tumor progression by
secreting IL-10 (35). When tumor
cell proliferation is uncontrolled, oxygen and nutrition are
limited, leading to hypoxia. Hypoxia skews macrophages to the
M2-like phenotype with increased expression of IL-10, HIF1α and
VEGF (36). Hypoxia then drives
macrophage diversity to facilitate lung cancer cell metastasis,
angiogenesis, and immune evasion in vitro and in vivo
(36,37). Clinical data have demonstrated that
increased gene expression of macrophage-derived IL-10 in tumor
tissues was significantly correlated with stage, tumor size, lymph
node metastasis, lymphovascular invasion, or histologically poor
differentiation (38).
IL-6
The macrophages derived from THP-1 exhibit high
expression of IL-6 when co-cultured with human non-small cell lung
cancer (NSCLC) A549 or H1299 cells, and enhances the invasive
ability of lung cancer cells by regulating EMT (34). Additionally, IL-6 may stimulate
macrophages to express higher levels of IL-10, and together, IL-6
and IL-10 induce M2 macrophage differentiation in an IL-4-dependent
manner via STAT3 activation (39);
while, IL-6-induced macrophage infiltration proceeds via the
CCL2/CCL5 pathway in NSCLC. Abrogation or suppression of IL-6
expression may inhibit TAM-induced invasion and angiogenesis in
lung cancer cells (34,40).
TGF-β
TGF-β, together with its co-receptor endoglin,
serves a vital role in tissue repair, and angiogenesis and
lymphangiogenesis. A previous study reported an increase in the
levels of endoglin during the process of monocyte transition to
macrophages (41). Furthermore,
macrophages and pro-inflammatory cytokines are significantly
downregulated in Eng+/− mice (42). The TGF-β, released by tumor cells
and M2 type macrophages, may suppress M1 polarized macrophages, and
stimulate mature macrophages to polarize to the pro-tumor M2 type.
Maeda et al (43) reported
that IL-10 expression in macrophages is positively associated with
TGF-β expression, and that TGF-β enhances Mφ to secrete IL-10,
promoting tumor progression in tumor-bearing mice (43). A previous study has shown that
TGF-β secreted by TAMs promotes EMT, and upregulates the expression
of SOX9, which enhances tumor cell proliferation, migration and
invasion (44). Furthermore,
suppressing the expression of TGF-β may inhibit TGF-β1-induced EMT
in A549 lung cancer cells (45).
MMPs
Furthermore, TAMs induce lung cancer cell invasion
by producing MMPs, including MMP-9 and MMP-2, and degrading the
extracellular matrix. MMP-9 expression is associated with lymph
node metastases, tumor progression and prognosis (46). IL-10-induced macrophages enhance
MMP-9 and MMP-2 expression and promote cancer cell invasion and
migration (47). Therefore,
inhibition of MMP production may reverse macrophage-mediated cancer
cell invasion and migration activity (46–48).
Chemokines
Chemokines are a family of soluble and chemotactic
cytokines that are secreted by and mediate the chemotaxis and
migration of immune or tumor cells. Recent advances have indicated
that chemokines originating from TAMs, including CCL18, MIP-3α,
CCL5, CXCL8, and CCL22, serve critical roles in cancer progression
by binding to their cognate receptors in carcinoma cells (49–51).
Early evidence has suggested that CCL22 is highly expressed in lung
cancer, and is a predictive marker for disease-free survival
duration and tumor recurrence (49–52).
CCL22 may promote the bone metastasis of lung cancer cells that
express CCR4 (53). CXCL8, an
M2-related chemokine secreted by TAMs, also serves a role in lung
cancer. Previous studies have suggested that CXCL8 may induce EMT,
and accelerate invasion and migration via the MAPK/NF-κB and
JAK2/STAT3 signaling pathways (54,55).
Therefore, therapies or drugs targeting CXCL8 may attenuate cell
proliferation, invasion, and migration in lung cancer (55,56).
Angiogenesis
TAMs serve a key role in facilitating angiogenesis
by producing pro-angiogenic factors, including IL-8, VEGF,
urokinase plasminogen activator (uPA), and MMPs, (Fig. 1). TAM density is associated with
intra-tumoral microvessel counts in NSCLC (57). Chen et al (58) reported that the THP-1-derived
M2-type macrophages may promote angiogenesis in NSCLC, by producing
proangiogenic factors, including IL-8, and supporting the
generation of blood vessels (58).
Hypoxia is a local attractant for TAMs in the TME, which induces
the expression of HIF-1 and HIF-2; and HIF-2 may upregulate VEGF
expression (59,60). Additionally, VEGF is also a
chemoattractant for TAMs, which forms a positive feedback loop to
promote tumor angiogenesis (61).
Immunosuppression
In TME, macrophages not only lose their anti-cancer
properties, but also impede the immunoregulatory functions of other
immune cells. The TAMs upregulate the expression of PD-L1 to
suppress T-cell toxicity and inhibit phagocytosis (5,62).
The CD8+ T cells are excluded by TAMs, and thus cannot
act near the cancer cells (63).
Furthermore, TAMs produce cytokines and other proteins to maintain
immunosuppression, including CCL-22, CCL-17, TGF-β, arginase 1 and
galectin-3 (28,29). The AMs stimulated by the Th2 cells
produce immunosuppressive cytokines, including IL-10 and TGF-β in
the lung TME to reduce the number of tumor-infiltrating lung
dendritic cells (DCs) and block their maturation (64,65).
Furthermore, IL-10 triggers the immunosuppression of T cells by
upregulating PD-L1 expression in tumor macrophages (38,66).
The blockade or deficiency of IL-10 may induce CD8+ T
cell cytotoxicity and promote tumor-resident CD8+ T cell
expansion (66). Additionally, the
macrophage-derived CCL22 promotes an immunosuppressive tumor
microenvironment by recruiting Tregs (67). Furthermore, Young et al
(68) indicated that NK cell
cytotoxicity is also suppressed and facilitates pulmonary
metastases (68). Depletion of the
AM or reversal of M2 polarization may relieve immunosuppression
imposed by the macrophages, and strengthen local Th1 anticancer
activity (64).
Chemotherapy resistance
Resistance to chemotherapy increases the difficulty
of therapeutic efficacy, and drives tumor progression, recurrence,
and distant, bone and lymph node metastasis. A strong correlation
has been demonstrated between TAMs and chemotherapy resistance
(13,69). A previous study reported that
abundant CD68+ and CD163+ macrophages
accumulate inside or adjacent to tumors following chemotherapy
(69). In a mouse Lewis lung
carcinoma model (LLC1s), treatment with chemotherapeutic agents
induces neoplastic cells that release CXCL12, which enhances the
infiltration of CD206+ TAMs, inhibits tumor cell death,
and assists in tumor relapse (13). Additionally, treatment with
cisplatin or carboplatin induces tumor cells to secrete IL-6 and/or
prostaglandin E2 (PGE2), which mediates M2 macrophage polarization
via activation of the STAT3, STAT1 and STAT6 signaling pathways,
and resists cytotoxic chemotherapy (70,71).
Furthermore, DeNardo et al (72) illustrated that paclitaxel treatment
boosts the infiltration of macrophages, which limits the
recruitment and efficacy of CD8+ cytotoxic T cells, and
inhibits the antitumor activity of paclitaxel (72). Recent large cohort clinical studies
have reported a close correlation between the infiltration of
M2-macrophages, poor response to chemotherapy, and poor clinical
outcomes (73,74). The elimination of TAMs by
anti-CSF-1 or anti-CCL2 antibodies, preventing M2-differentiation
by COX inhibitors, and/or anti-IL-6R antibodies may enhance the
cytotoxic effects of chemotherapeutic agents, including taxol,
cisplatin, and doxorubicin (75,76).
Therefore, concomitant therapy with an intervention strategy that
reduces macrophage population or inhibits M2 polarization may
amplify the antitumor activity of chemotherapeutic agents.
Clinical implications of TAMs in lung
cancer
Clinical studies have suggested that the density of
macrophages, particularly M2 type, is associated with a poor
prognosis in almost all human cancer types (7,8).
However, there are conflicting data with regards to lung cancer.
CD68, a common monocyte/macrophage marker, when used to label TAMs,
indicated it to act as an independent prognostic factor, and a
higher percentage of tumor islets were found to be correlated with
improved outcomes (77,78). However, other studies observed no
association between CD68+ macrophage densities and tumor
islets or stroma with patients' survival duration (79,80).
This is possibly due to involvement of the margin or central
macrophages.
Usually, the CD68+CD163+ or
CD68+CD206+ markers are used to identify M2
macrophages. Zhang et al (81) indicated that levels of M2-type
(CD68+CD206+) were positively associated with
peritumoral lymphatic microvessel density, but negatively
associated with patients' prognoses (81). In line with this, emerging research
has suggested that the accumulation of CD163+
macrophages is closely correlated with a poor prognosis in lung
cancer. Furthermore, an increased density of
CD68+CD163+ macrophages in tumor nests and
stroma was associated with lymph node metastases (81), but no such association was observed
with recurrence-free survival (RFS), overall survival (OS), and TNM
stages (80,82). However, Cao et al (7) found that levels of
CD68+CD163+M2 were correlated with OS and DFS
in NSCLC (7). Furthermore,
increased infiltration of macrophages was observed in patients with
lung squamous cell carcinoma (LUSC), wild-type EGFR, and
smoking habits (7).
Additionally, M2-TAMs labeled with CD204+
serve a role in prognosis. High infiltration of
CD204+TAMs in the stroma may be correlated with TNM
stages, presence of vascular and pleural invasion, and OS and RFS
in patients with stage II LUSC. However, no association was
observed between the levels of CD204+ macrophages and
poor patient outcomes (83).
Taken together, the different data or contradictory
results of previous studies may be explained by the tumor
histological type and origin in patients, methodologies applied in
counting TAMs, and definition of islet and stroma. Furthermore, a
recent meta-analysis reported that M2-type TAMs or M1/M2
polarization in the lung cancer islets or stroma are associated
with tumor progression. Therefore, targeting TAMs may be considered
as a newer anti-tumor strategy in lung cancer.
TAM-targeted therapeutics
TAMs, the major component of leukocyte infiltration
in tumors, serve an important role in tumor behavior, and thus
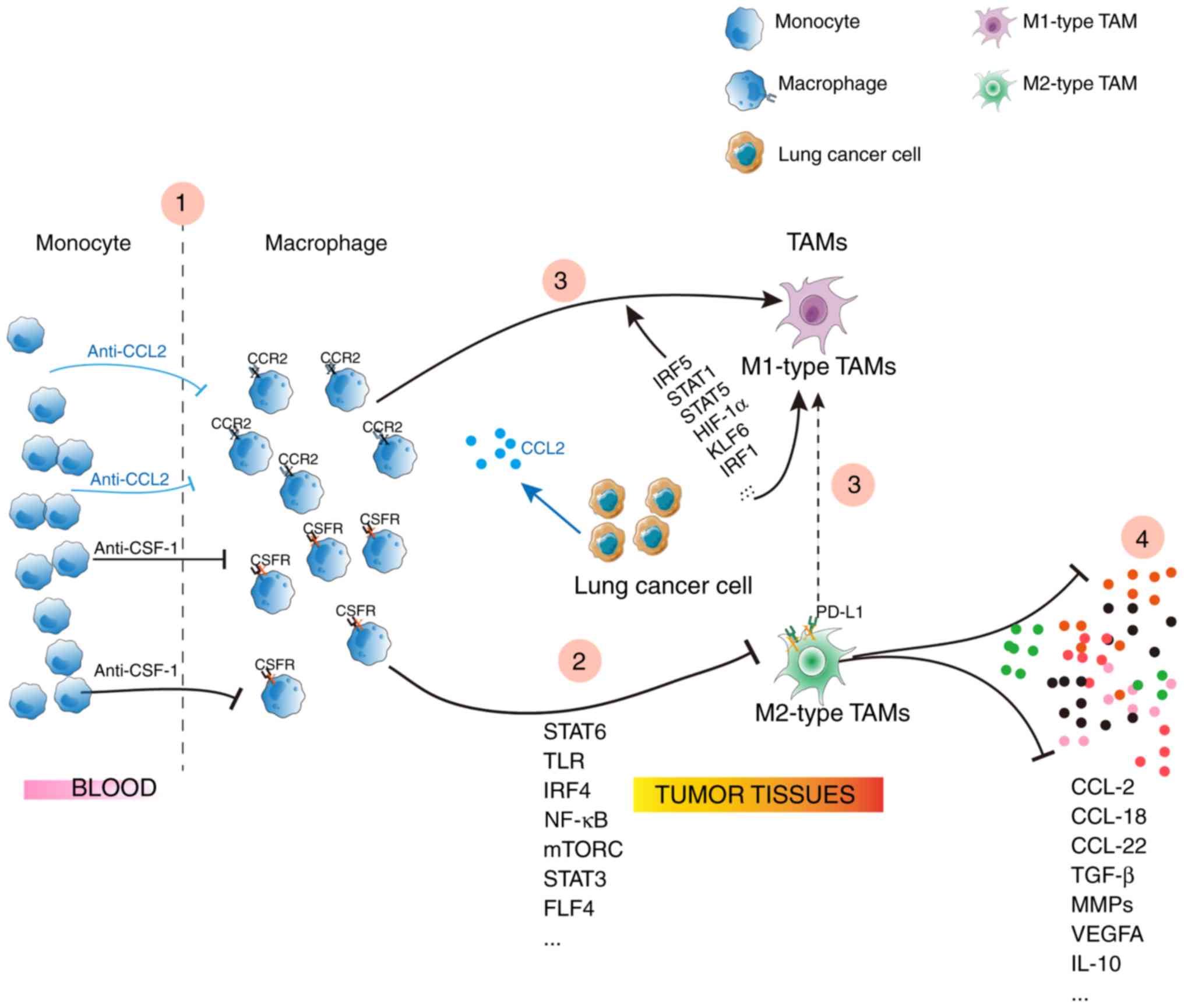
therapies targeting TAMs are employed. To begin with, inhibition of
macrophages infiltrating the tumor; CSF1-CSF1R and CCL2-CCR2 may
induce macrophage recruitment, and blockade of CCL2-CCR2 or
CSF1-CSF1R may decrease TAM infiltration, reversing the
immunosuppressive status (84)
(Fig. 3), but anti-CCL2 therapy
may aggravate metastasis (85). A
second strategy is that blockade of TAMs repolarize into the
M2-type: Few signaling components regulate M2 macrophage
polarization, including the Toll-like receptors (TLR), STAT6 and
NK-κB. When these signals are intervened, TAMs lose their
‘alternative’ activated phenotype. A third strategy would involve
reeducating TAMs to M1-type or switching M2 to M1: Several drugs,
including BTH1677 (a yeast β-glucan immunomodulator),
hydroxychloroquine, and celecoxib, switch M2-like TAMs to an
antitumor phenotype, or M1-like TAMs (86–88).
A final strategy is based on the fact that decreasing the levels of
critical TAM-secreted cytokines involved in tumor biology: For
example, CCL18, CCL22, and MIP-3α, mainly produced by the M2-type
macrophages, confer malignant behaviors (9,10,49).
Blockade of CCL18, CCL22, or MIP-3α weakens the TAM-mediated
pro-tumor ability (9,10,89).
The aforementioned strategies provide enhanced and
promising therapeutic effects, although there are a few major
issues or side effects that require attention, including the
efficiency of specific drug delivery and nontargeting TAMs.
Evidence has indicated that nanoparticles or nanoparticle-based
drug delivery are more reliable and effective in regulating the
macrophage phenotype by ensuring that the drug reaches the cancer
site without off-target activity. Several studies have demonstrated
that nanodrugs offer superiority in mediating the polarization of
macrophages with increased drug uptake. For instance, curcumin
(Cur), baicalin (Bai), and ginseng-derived nanoparticles have been
reported to alter TAM polarization without discernible toxicity
(90–92). Compared to the drugs themselves,
their nanoparticle derivatives showed improved pharmacokinetics and
bioavailability in systemic circulation, and thus contributed
toward excellent antitumor responses (90–92).
Furthermore, few materials used in nanoparticle production,
including TiO2 and Ag, may preferentially polarize TAMs
towards an M1 phenotype (93,94).
We hypothesize that every immune cell serves an
equal role in the body, and macrophages have dual property;
therefore, eliminating or decreasing macrophages is not a rational
approach and has other disadvantages. By contrast, ‘reeducating’
the macrophages or targeting the tumorigenic cytokines or
chemokines secreted by the macrophages should be studied as a
preferred strategy for combating cancer.
Conclusions
Several experimental and clinical studies have
demonstrated that TAMs serve a seminal role in the growth,
angiogenesis, metastasis, and invasion in lung cancer. Furthermore,
TAMs confer chemotherapy resistance and immunosuppression.
Therefore, TAMs are now considered a promising target in the
treatment of lung cancer. However, no appropriate drugs have been
administered in the patients, and newer treatment approaches may
ascertain improved clinical outcomes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Development
Project of Shanghai Peak Disciplines-Integrative Medicine and
Western Medicine (grant no. 20150407), National Natural Science
Program of China (grant nos. 81673916 and 81403148) and the
Development Plan of Shandong Medical and Health Technology (grant
no. 2019WS581).
Availability of data and materials
Not applicable.
Authors' contributions
FX and YW wrote the manuscript; ZT made
contributions to the figures; BL and JD contributed toward the
literature review and revised the manuscript. All authors read and
appvoed the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
References
|
1
|
Brenman JE and Temple BR: Opinion:
Alternative views of AMP-activated protein kinase. Cell Biochem
Biophys. 47:321–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gridelli C, Perrone F and Monfardini S:
Lung cancer in the elderly. Eur J Cancer. 33:2313–2314. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goswami KK, Ghosh T, Ghosh S, Sarkar M,
Bose A and Baral R: Tumor promoting role of anti-tumor macrophages
in tumor microenvironment. Cell Immunol. 316:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sumitomo R, Hirai T, Fujita M, Murakami H,
Otake Y and Huang CL: PD-L1 expression on tumor-infiltrating immune
cells is highly associated with M2 TAM and aggressive malignant
potential in patients with resected non-small cell lung cancer.
Lung Cancer. 136:136–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao L, Che X, Qiu X, Li Z, Yang B, Wang S,
Hou K, Fan Y, Qu X and Liu Y: M2 macrophage infiltration into tumor
islets leads to poor prognosis in non-small-cell lung cancer.
Cancer Manag Res. 11:6125–6138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sumitomo R, Hirai T, Fujita M, Murakami H,
Otake Y and Huang CL: M2 tumor-associated macrophages promote tumor
progression in non-small-cell lung cancer. Exp Ther Med.
18:4490–4498. 2019.PubMed/NCBI
|
|
9
|
Zhou Z, Peng Y, Wu X, Meng S, Yu W, Zhao
J, Zhang H, Wang J and Li W: CCL18 secreted from M2 macrophages
promotes migration and invasion via the PI3K/Akt pathway in
gallbladder cancer. Cell Oncol (Dordr). 42:81–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarode P, Zheng X, Giotopoulou GA, Weigert
A, Kuenne C, Günther S, Friedrich A, Gattenlöhner S, Stiewe T,
Brüne B, et al: Reprogramming of tumor-associated macrophages by
targeting β-catenin/FOSL2/ARID5A signaling: A potential treatment
of lung cancer. SCI ADV. 6:eaaz61052020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura Y, Sumiyoshi M and Baba K:
Antitumor and antimetastatic activity of synthetic hydroxystilbenes
through inhibition of lymphangiogenesis and M2 macrophage
differentiation of tumor-associated macrophages. Anticancer Res.
36:137–148. 2016.PubMed/NCBI
|
|
13
|
Hughes R, Qian BZ, Rowan C, Muthana M,
Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons
M, et al: Perivascular M2 macrophages stimulate tumor relapse after
chemotherapy. Cancer Res. 75:3479–3491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flaherty DM, Monick MM and Hinde SL: Human
alveolar macrophages are deficient in PTEN. The role of endogenous
oxidants. J Biol Chem. 281:5058–5064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biswas SK, Gangi L, Paul S, Schioppa T,
Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F,
et al: A distinct and unique transcriptional program expressed by
tumor-associated macrophages (defective NF-kappaB and enhanced
IRF-3/STAT1 activation). Blood. 107:2112–2122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klimp AH, Hollema H, Kempinga C, van der
Zee AG, de Vries EG and Daemen T: Expression of cyclooxygenase-2
and inducible nitric oxide synthase in human ovarian tumors and
tumor-associated macrophages. Cancer Res. 61:7305–7309.
2001.PubMed/NCBI
|
|
18
|
Nam S and Lim J: Essential role of
interferon regulatory factor 4 (IRF4) in immune cell development.
Arch Pharm Res. 39:1548–1555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong M, Zhuo X and Ma A: STAT6
Upregulation promotes M2 macrophage polarization to suppress
atherosclerosis. Med Sci Monit Basic Res. 23:240–249. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vergadi E, Ieronymaki E, Lyroni K,
Vaporidi K and Tsatsanis C: Akt signaling pathway in macrophage
activation and M1/M2 polarization. J Immunol. 198:1006–1014. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue J, Schmidt SV, Sander J, Draffehn A,
Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L,
et al: Transcriptome-based network analysis reveals a spectrum
model of human macrophage activation. Immunity. 40:274–288. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson MP, Christmann BS, Dunaway CW,
Morris A and Steele C: Experimental Pneumocystis lung infection
promotes M2a alveolar macrophage-derived MMP12 production. Am J
Physiol Lung Cell Mol Physiol. 303:L469–L475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Xu W and Xiong S: Blockade of
Notch1 signaling alleviates murine lupus via blunting macrophage
activation and M2b polarization. J Immunol. 184:6465–6478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koscsó B, Csóka B, Kókai E, Németh ZH,
Pacher P, Virág L, Leibovich SJ and Haskó G: Adenosine augments
IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol.
94:1309–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olmes G, Büttner-Herold M, Ferrazzi F,
Distel L, Amann K and Daniel C: CD163+ M2c-like macrophages
predominate in renal biopsies from patients with lupus nephritis.
Arthritis Res Ther. 18:902016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Ni H, Lan L, Wei X, Xiang R and
Wang Y: Fra-1 protooncogene regulates IL-6 expression in
macrophages and promotes the generation of M2d macrophages. Cell
Res. 20:701–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watkins SK, Egilmez NK, Suttles J and
Stout RD: IL-12 rapidly alters the functional profile of
tumor-associated and tumor-infiltrating macrophages in vitro and in
vivo. J Immunol. 178:1357–1362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schoppmann SF, Birner P, Stöckl J, Kalt R,
Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K and Kerjaschki
D: Tumor-associated macrophages express lymphatic endothelial
growth factors and are related to peritumoral lymphangiogenesis. Am
J Pathol. 161:947–956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hotchkiss KA, Ashton AW, Klein RS, Lenzi
ML, Zhu GH and Schwartz EL: Mechanisms by which tumor cells and
monocytes expressing the angiogenic factor thymidine phosphorylase
mediate human endothelial cell migration. Cancer Res. 63:527–533.
2003.PubMed/NCBI
|
|
30
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohri CM, Shikotra A, Green RH, Waller DA
and Bradding P: Macrophages within NSCLC tumour islets are
predominantly of a cytotoxic M1 phenotype associated with extended
survival. Eur Respir J. 33:118–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Helm O, Held-Feindt J, Grage-Griebenow E,
Reiling N, Ungefroren H, Vogel I, Krüger U, Becker T, Ebsen M,
Röcken C, et al: Tumor-associated macrophages exhibit pro- and
anti-inflammatory properties by which they impact on pancreatic
tumorigenesis. Int J Cancer. 135:843–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Che D, Zhang S, Jing Z, Shang L, Jin S,
Liu F, Shen J, Li Y, Hu J, Meng Q and Yu Y: Macrophages induce EMT
to promote invasion of lung cancer cells through the IL-6-mediated
COX-2/PGE(2)/β-catenin signalling pathway. Mol Immunol. 90:197–210.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dehai C, Bo P, Qiang T, Lihua S, Fang L,
Shi J, Jingyan C, Yan Y, Guangbin W and Zhenjun Y: Enhanced
invasion of lung adenocarcinoma cells after co-culture with
THP-1-derived macrophages via the induction of EMT by IL-6. Immunol
Lett. 160:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L, Dong Y, Li Y, Wang D, Liu S, Wang
D, Gao Q, Ji S, Chen X, Lei Q, et al: IL-10 derived from M2
macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1
pathway in non-small cell lung cancer. Int J Cancer. 145:1099–1110.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Cao J, Ma S, Dong R, Meng W, Ying
M, Weng Q, Chen Z, Ma J, Fang Q, et al: Tumor hypoxia enhances
Non-Small Cell Lung Cancer metastasis by selectively promoting
macrophage M2 polarization through the activation of ERK signaling.
Oncotarget. 5:9664–9677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laoui D, Van Overmeire E, Di Conza G,
Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe
E, Elkrim Y, et al: Tumor hypoxia does not drive differentiation of
tumor-associated macrophages but rather fine-tunes the M2-like
macrophage population. Cancer Res. 74:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang R, Lu M, Zhang J, Chen S, Luo X, Qin
Y and Chen H: Increased IL-10 mRNA expression in tumor-associated
macrophage correlated with late stage of lung cancer. J Exp Clin
Cancer Res. 30:622011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng
YS, Yu PW, Zhuang Y and Zhao YL: Interleukin 6 induces M2
macrophage differentiation by STAT3 activation that correlates with
gastric cancer progression. Cancer Immunol Immunother.
66:1597–1608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Yang X, Tsai Y, Yang L, Chuang KH,
Keng PC, Lee SO and Chen Y: IL-6 mediates macrophage infiltration
after irradiation via up-regulation of CCL2/CCL5 in non-small cell
lung cancer. Radiat Res. 187:50–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Cortie K, Russell NS, Coppes RP,
Stewart FA and Scharpfenecker M: Bone marrow-derived macrophages
incorporate into the endothelium and influence vascular and renal
function after irradiation. Int J Radiat Biol. 90:769–777. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Russell NS, Floot B, van Werkhoven E,
Schriemer M, de Jong-Korlaar R, Woerdeman LA, Stewart FA and
Scharpfenecker M: Blood and lymphatic microvessel damage in
irradiated human skin: The role of TGF-β, endoglin and macrophages.
Radiother Oncol. 116:455–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maeda H, Kuwahara H, Ichimura Y, Ohtsuki
M, Kurakata S and Shiraishi A: TGF-beta enhances macrophage ability
to produce IL-10 in normal and tumor-bearing mice. J immunol.
155:4926–4932. 1995.PubMed/NCBI
|
|
44
|
Zhang S, Che D, Yang F, Chi C, Meng H,
Shen J, Qi L, Liu F, Lv L, Li Y, et al: Tumor-associated
macrophages promote tumor metastasis via the TGF-β/SOX9 axis in
non-small cell lung cancer. Oncotarget. 8:99801–99815. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HJ, Park MK, Lee EJ and Lee CH:
Resolvin D1 inhibits TGF-β1-induced epithelial mesenchymal
transition of A549 lung cancer cells via lipoxin A4 receptor/formyl
peptide receptor 2 and GPR32. Int J Biochem Cell Biol.
45:2801–2807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang R, Zhang J, Chen S, Lu M, Luo X, Yao
S, Liu S, Qin Y and Chen H: Tumor-associated macrophages provide a
suitable microenvironment for non-small lung cancer invasion and
progression. Lung Cancer. 74:188–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cardoso AP, Pinto ML, Pinto AT, Pinto MT,
Monteiro C, Oliveira MI, Santos SG, Relvas JB, Seruca R, Mantovani
A, et al: Matrix metalloproteases as maestros for the dual role of
LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC
Cancer. 15:4562015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim YH, Kwon HJ and Kim DS: Matrix
metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein
regulates cell migration, invasion, and adhesion. J Biol Chem.
287:38957–38969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang B, Shi L, Sun X, Wang L, Wang X and
Chen C: Production of CCL20 from lung cancer cells induces the cell
migration and proliferation through PI3K pathway. J Cell Mol Med.
20:920–929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi L, Zhang B, Sun X, Zhang X, Lv S, Li
H, Wang X, Zhao C, Zhang H, Xie X, et al: CC chemokine ligand
18(CCL18) promotes migration and invasion of lung cancer cells by
binding to Nir1 through Nir1-ELMO1/DOC180 signaling pathway. Mol
Carcinog. 55:2051–2062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang R, Wang S, Wang N, Zheng Y, Zhou J,
Yang B, Wang X, Zhang J, Guo L, Wang S, et al: CCL5 derived from
tumor-associated macrophages promotes prostate cancer stem cells
and metastasis via activating β-catenin/STAT3 signaling. Cell Death
Dis. 11:2342020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nakanishi T, Imaizumi K, Hasegawa Y,
Kawabe T, Hashimoto N, Okamoto M and Shimokata K: Expression of
macrophage-derived chemokine (MDC)/CCL22 in human lung cancer.
Cancer Immunol Immunother. 55:1320–1329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakamura ES, Koizumi K, Kobayashi M,
Saitoh Y, Arita Y, Nakayama T, Sakurai H, Yoshie O and Saiki I:
RANKL-induced CCL22/macrophage-derived chemokine produced from
osteoclasts potentially promotes the bone metastasis of lung cancer
expressing its receptor CCR4. Clin Exp Metastasis. 23:9–18. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H,
Zheng P and Zhao S: Gastric cancer-derived mesenchymal stromal
cells trigger M2 macrophage polarization that promotes metastasis
and EMT in gastric cancer. Cell Death Dis. 10:9182019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cao H, Huang Y, Wang L, Wang H, Pang X, Li
K, Dang W, Tang H, Wei L, Su M, et al: Leptin promotes migration
and invasion of breast cancer cells by stimulating IL-8 production
in M2 macrophages. Oncotarget. 7:65441–65453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Q, Li A, Yu S, Qin S, Han N, Pestell
RG, Han X and Wu K: DACH1 antagonizes CXCL8 to repress
tumorigenesis of lung adenocarcinoma and improve prognosis. J
Hematol Oncol. 11:532018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tataroğlu C, Kargi A, Ozkal S, Eşrefoğlu N
and Akkoçlu A: Association of macrophages, mast cells and
eosinophil leukocytes with angiogenesis and tumor stage in
non-small cell lung carcinomas (NSCLC). Lung Cancer. 43:47–54.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT,
Kuo ML, Lee YC and Yang PC: Up-regulation of tumor interleukin-8
expression by infiltrating macrophages: Its correlation with tumor
angiogenesis and patient survival in non-small cell lung cancer.
Clin Cancer Res. 9:729–737. 2003.PubMed/NCBI
|
|
59
|
Lewis JS, Landers RJ, Underwood JC, Harris
AL and Lewis CE: Expression of vascular endothelial growth factor
by macrophages is up-regulated in poorly vascularized areas of
breast carcinomas. J Pathol. 192:150–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Leek RD and Harris AL: Tumor-associated
macrophages in breast cancer. J Mammary Gland Biol Neoplasia.
7:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Talks K, Pezzella F, Gatter KC and Harris AL: Relation
of hypoxia inducible factor 1 alpha and 2 alpha in operable
non-small cell lung cancer to angiogenic/molecular profile of
tumours and survival. Br J Cancer. 85:881–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gordon SR, Maute RL, Dulken BW, Hutter G,
George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al:
PD-1 expression by tumour-associated macrophages inhibits
phagocytosis and tumour immunity. Nature. 545:495–499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peranzoni E, Lemoine J, Vimeux L, Feuillet
V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J,
Ouakrim H, et al: Macrophages impede CD8 T cells from reaching
tumor cells and limit the efficacy of anti-PD-1 treatment. Proc
Natl Acad Sci USA. 115:E4041–E4050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sharma SK, Chintala NK, Vadrevu SK, Patel
J, Karbowniczek M and Markiewski MM: Pulmonary alveolar macrophages
contribute to the premetastatic niche by suppressing antitumor T
cell responses in the lungs. J Immunol. 194:5529–5538. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Allavena P, Sica A, Vecchi A, Locati M,
Sozzani S and Mantovani A: The chemokine receptor switch paradigm
and dendritic cell migration: Its significance in tumor tissues.
Immunol Rev. 177:141–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiao J, Liu Z, Dong C, Luan Y, Zhang A,
Moore C, Fu K, Peng J, Wang Y, Ren Z, et al: Targeting tumors with
IL-10 prevents dendritic cell-mediated CD8+ T cell
apoptosis. Cancer Cell. 35:901–915.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang D, Yang L, Yue D, Cao L, Li L, Wang
D, Ping Y, Shen Z, Zheng Y, Wang L and Zhang Y: Macrophage-derived
CCL22 promotes an immunosuppressive tumor microenvironment via IL-8
in malignant pleural effusion. Cancer Lett. 452:244–253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Young MR, Endicott RA, Duffie GP and
Wepsic HT: Suppressor alveolar macrophages in mice bearing
metastatic Lewis lung carcinoma tumors. J Leukoc Biol. 42:682–688.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
De Palma M and Lewis CE: Cancer:
Macrophages limit chemotherapy. Nature. 472:303–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dijkgraaf EM, Heusinkveld M, Tummers B,
Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR
and van der Burg SH: Chemotherapy alters monocyte differentiation
to favor generation of cancer-supporting M2 macrophages in the
tumor microenvironment. Cancer Res. 73:2480–2492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mitchem JB, Brennan DJ, Knolhoff BL, Belt
BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L,
Piwnica-Worms D, et al: Targeting tumor-infiltrating macrophages
decreases tumor-initiating cells, relieves immunosuppression, and
improves chemotherapeutic responses. Cancer Res. 73:1128–1141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD,
Junaid SA, et al: Leukocyte complexity predicts breast cancer
survival and functionally regulates response to chemotherapy.
Cancer Discov. 1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang C, Yu X, Gao L, Zhao Y, Lai J, Lu D,
Bao R, Jia B, Zhong L, Wang F and Liu Z: Noninvasive imaging of
CD206-positive M2 macrophages as an early biomarker for
post-chemotherapy tumor relapse and lymph node metastasis.
Theranostics. 7:4276–4288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sugimura K, Miyata H, Tanaka K, Takahashi
T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: High infiltration of tumor-associated macrophages is associated
with a poor response to chemotherapy and poor prognosis of patients
undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg
Oncol. 111:752–759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Paulus P, Stanley ER, Schäfer R, Abraham D
and Aharinejad S: Colony-stimulating factor-1 antibody reverses
chemoresistance in human MCF-7 breast cancer xenografts. Cancer
Res. 66:4349–4356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Salvagno C, Ciampricotti M, Tuit S, Hau
CS, van Weverwijk A, Coffelt SB, Kersten K, Vrijland K, Kos K, Ulas
T, et al: Therapeutic targeting of macrophages enhances
chemotherapy efficacy by unleashing type I interferon response. Nat
Cell Biol. 21:511–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S,
Ma J, Ma L and You Z: The number and microlocalization of
tumor-associated immune cells are associated with patient's
survival time in non-small cell lung cancer. BMC Cancer.
10:2202010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Welsh TJ, Green RH, Richardson D, Waller
DA, O'Byrne KJ and Bradding P: Macrophage and mast-cell invasion of
tumor cell islets confers a marked survival advantage in
non-small-cell lung cancer. J Clin Oncol. 23:8959–8967. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pei BX, Sun BS, Zhang ZF, Wang AL and Ren
P: Interstitial tumor-associated macrophages combined with
tumor-derived colony-stimulating factor-1 and interleukin-6, a
novel prognostic biomarker in non-small cell lung cancer. J Thorac
Cardiovasc Surg. 148:1208–1216.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ma J, Liu L, Che G, Yu N, Dai F and You Z:
The M1 form of tumor-associated macrophages in non-small cell lung
cancer is positively associated with survival time. BMC Cancer.
10:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang B, Yao G, Zhang Y and Gao J, Yang B,
Rao Z and Gao J: M2-polarized tumor-associated macrophages are
associated with poor prognoses resulting from accelerated
lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo).
66:1879–1886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jung KY, Cho SW, Kim YA, Kim D, Oh BC,
Park DJ and Park YJ: Cancers with higher density of
tumor-associated macrophages were associated with poor survival
rates. J Pathol Transl Med. 49:318–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hirayama S, Ishii G, Nagai K, Ono S,
Kojima M, Yamauchi C, Aokage K, Hishida T, Yoshida J, Suzuki K and
Ochiai A: Prognostic impact of CD204-positive macrophages in lung
squamous cell carcinoma: Possible contribution of Cd204-positive
macrophages to the tumor-promoting microenvironment. J Thorac
Oncol. 7:1790–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Keklikoglou I and De Palma M: Cancer:
Metastasis risk after anti-macrophage therapy. Nature. 515:46–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li Y, Cao F, Li M, Li P, Yu Y, Xiang L, Xu
T, Lei J, Tai YY, Zhu J, et al: Hydroxychloroquine induced lung
cancer suppression by enhancing chemo-sensitization and promoting
the transition of M2-TAMs to M1-like macrophages. J Exp Clin Cancer
Res. 37:2592018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li
Q, Yang Z and Quan J: Identification of α-mangostin as an agonist
of human STING. Chemmedchem. 13:2057–2064. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Brandão RD, Veeck J, Van de Vijver KK,
Lindsey P, de Vries B, van Elssen CH, Blok MJ, Keymeulen K, Ayoubi
T, Smeets HJ, et al: A randomised controlled phase II trial of
pre-operative celecoxib treatment reveals anti-tumour
transcriptional response in primary breast cancer. Breast Cancer
Res. 15:R292013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhu B, Zou L, Cheng X, Lin Z, Duan Y, Wu
Y, Zhou F and Chen Z: Administration of MIP-3alpha gene to the
tumor following radiation therapy boosts anti-tumor immunity in a
murine model of lung carcinoma. Immunol Lett. 103:101–107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shiri S, Alizadeh AM, Baradaran B,
Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H and Tavassoli
A: Dendrosomal curcumin suppresses metastatic breast cancer in mice
by changing m1/m2 macrophage balance in the tumor microenvironment.
Asian Pac J Cancer Prev. 16:3917–3922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Han S, Wang W, Wang S, Wang S, Ju R, Pan
Z, Yang T, Zhang G, Wang H and Wang L: Multifunctional biomimetic
nanoparticles loading baicalin for polarizing tumor-associated
macrophages. Nanoscale. 11:20206–20220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cao M, Yan H, Han X, Weng L, Wei Q, Sun X,
Lu W, Wei Q, Ye J, Cai X, et al: Ginseng-derived nanoparticles
alter macrophage polarization to inhibit melanoma growth. J
Immunother Cancer. 7:3262019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang J, Song W, Guo J, Zhang J, Sun Z, Li
L, Ding F and Gao M: Cytotoxicity of different sized TiO2
nanoparticles in mouse macrophages. Toxicol Ind Health. 29:523–533.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Park J, Lim DH, Lim HJ, Kwon T, Choi JS,
Jeong S, Choi IH and Cheon J: Size dependent macrophage responses
and toxicological effects of Ag nanoparticles. Chem Commun (Camb).
47:4382–4384. 2011. View Article : Google Scholar : PubMed/NCBI
|