Introduction
The last several years have seen marked progress in
the understanding of the use of fibroblasts as an in vitro
model of cell aging and in skin tissue engineering therapies, such
as tissue repair and wound healing (1–3).
Furthermore, one of the most common uses of fibroblasts is
producing induced pluripotent stem cells (iPSCs) because of their
accessibility and relatively high reprogramming efficiency
(4). The iPSCs generated from
dermal fibroblasts can be differentiated into key disease-affected
cells in vitro. Generating patient-derived iPSCs has created
opportunities for rare disease modeling, such as in fibrosis and
osteoarthritis. Since these cells can simulate disease phenotypes,
they are useful high-throughput drug screening platforms that may
result in the reversal of these abnormal phenotypes (5,6).
Moreover, human dermal fibroblasts (HDFs) have been used to assess
skin fibrosis tendency in response to radiotherapy (7,8),
some oxidative phosphorylation disorders (9,10),
and to determine the presence or risk of metabolic diseases in
patients (11,12). Fibroblasts have also been used to
study neurodegenerative diseases, such as Parkinson's disease
(13,14) and Alzheimer's disease (15,16).
A further study has also shown the use of HDFs as a tool when
assessing the biological mechanisms of major depression and
antidepressant drug response (17). Fibroblast cultures have been
successfully used for a range of tests because fibroblasts are easy
to isolate, reliable and, most importantly, they grow rapidly and
continuously (18). Despite their
numerous advantages, primary cultures of fibroblasts are classified
as difficult to transfect cells (19,20).
The methods characterized by the highest transfection efficiency
(>70%) of fibroblasts are systems based on viral vectors;
however, due to the potential high toxicity of viral vectors, less
toxic techniques, i.e., non-viral systems, have been extensively
developed (21,22). Of all the non-viral systems,
nucleofection is considered to be the most effective method of
fibroblast transfection (20,23).
Its advantages are considered to be the high level of transgene
expression and cell viability post-transfection; however, the major
disadvantage of nucleofection is its high cost (24). Therefore, new non-viral
transfection systems that can match nucleofection efficiency are
constantly being tested. Among them, cationic lipids and cationic
polymers are strongly recommended for their capacity to form
particle-like complexes (called lipoplexes and polyplexes,
respectively), which are readily able to enter cells (25).
MicroRNAs (miRNA/miRs) are short non-coding RNAs
(~22 nucleotides) that post-transcriptionally regulate gene
expression via binding to the 3′ untranslated regions (3′ UTRs) of
target gene mRNA (26). Transient
transfection of chemically synthesized miRNA mimics (synthetic
double-stranded miRNA-like RNA molecules) or miRNA agomirs
(artificial double-stranded miRNA mimics) has broad applications in
genetic research (27). It is
widely known that the administration of miRNA mimics or miRNA
inhibitors leads to overexpressed or downregulated endogenous
miRNAs (28). Furthermore,
Paoletti et al (29)
demonstrated that adult human cardiac fibroblasts can be directly
reprogrammed into induced cardiomyocytes by transient transfection
with four miRNA mimics (miR-1, 133, 208 and 499, termed
‘miRcombo’). Due to their cytoplasmic activity, relatively small
size and ability to be injected systemically or locally by
nanoparticle-based supply systems, as well as avoiding the use of
viral vectors, both mimics and agomirs possess considerable
potential as therapeutic agents (30).
The present study used hsa-miR-302b-3p, a molecule
belonging to the miR-302b family and an important member of the
miR-302/367 cluster, which is crucial in cellular stemness and a
hallmark of diverse tumors (31,32).
He et al (33) showed that
miR-302b-3p is responsible for activation of the AKT pathway. As a
target gene to test the effectiveness of transfection using
miR-302b-3p, the carnitine O-octanoyltransferase (CROT) gene
was selected. CROT is an enzyme involved in the transport of
medium- and long-chain acyl-CoA out of peroxisomes (34). CROT has also been indicated as a
new factor in stimulating vascular calcification by promoting fatty
acid metabolism and mitochondrial dysfunction. Notably, inhibiting
CROT is considered to have potential as an anti-fibrotic therapy
(35).
The aim of the present study was to provide data to
support the selection of an appropriate transient transfection
method and experimental conditions within these methods for HDFs.
Therefore, various delivery systems were investigated for
miR-302b-3p transfection, including nucleofection and lipid-based
transfection reagents.
Materials and methods
Cell culture
Normal HDFs (NHDFs) were purchased from PromoCell
GmbH (NHDF-cryovial adult cell line, cat. no. C-12302) and were
cultured in Dulbecco's Modified Eagle's Medium (DMEM; Thermo Fisher
Scientific, Inc.) containing 10% heat-inactivated fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) and 1%
Gibco® Antibiotic-Antimycotic Solution (10,000 U/ml
penicillin, 10,000 µg/ml streptomycin and 25 µg/ml Gibco
Amphotericin B; Gibco; Thermo Fisher Scientific, Inc.) in an
incubator (5% CO2, 37°C).
Characteristics of miR-302b-3p
Hsa-miR-302b-3p (GenBank LM379226.1, chromosomal
location 4q25, approved symbol MIR302B) was used to transfect
NHDFs. The miRCURY LNA miRNA Mimic-5′ FAM (miR sequence
5′-UAAGUGCUUCCAUGUUUUAGUAG-3′) and its inhibitor (miRCURY LNA
Inhibitor 5′ TACTAAAACATGGAAGCACT-3′) were provided by Qiagen GmbH.
As a negative control, Negative Control 5 miRCURY LNA miRNA Mimic
(Qiagen GmbH; cat. no. YM00479904-ADB; product no. 339173) was
used. The guide strands of this control have no homology to any
known miRNA or mRNA sequences in mice, rats or humans, and have
been tested for adverse effects in multiple cell lines.
Transfection methods
Three non-viral transient transfection methods were
employed for the transfection of NHDFs: Transfection with i)
Nucleofector Amaxa™ 4D-Nucleofector™ System with X Unit apparatus
(hereafter referred to as nucleofection) (Lonza Group Ltd.); ii)
Viromer® Blue (Lipocalyx GmbH) and iii)
INTERFERin® (Polyplus-transfection SA). As a
physical/mechanical method of transfection, nucleofection is an
electroporation-based transfection method that allows nucleic
acids, such as DNA or RNA, to be transferred into cells by applying
a specific voltage and using specific reagents (36). Techniques using Viromer Blue and
INTERFERin are chemical transfection methods. According to the
manufacturer's protocol, Viromer Blue is a polymer-based
transfection reagent featuring a viral mechanism of membrane
fusion. The reagent is capable of forming a complex with miRNA and
transporting it into cells by endocytosis. By contrast, INTERFERin,
a non-liposomal cationic amphiphilic lipid-based transfection
reagent, is one of the most efficient reagents for small
interfering RNA delivery. The transfection protocols are described
hereafter. The reagent to nucleic acid ratio was consistent with
the manufacturers' protocols. Different cell densities were used
for the three transfection methods because all reagents were used
in the cell density range recommended by the manufacturer.
Transfection efficiency was assessed using a
fluorescence microscope (Leica DM IL LED; Leica Microsystems, Inc.)
by counting the total number of observed cells and the number of
cells that express fluorescence of a FAM™ dye-labeled synthetic
miRNA.
Nucleofection
Briefly, for nucleofection, cells were passaged 2
days before transfection. Cells (1×105) were seeded in
96-well plates. The cells were transfected upon reaching a
confluence of 90%. The cells were washed with phosphate-buffered
saline and dissociated with 0.25% Trypsin-EDTA solution at 37°C for
5 min. The enzyme was neutralized with DMEM containing 10% FBS.
Aliquots of the cells were taken and cell density was determined,
and then the required number of cells was resuspended in 20 µl
4D-Nucleofector™ Solution; 5 and 50 nM miRNA mimic and negative
control (10% of final sample volume) was added to each sample. For
transfection, the P2 Primary Cell 4D-Nucleofactor™ X Kit for Human
Dermal Fibroblasts Basic Nucleofector Kit (Lonza Group Ltd.) and
4D-Nucleofector System apparatus (Lonza Group Ltd.) were used
according to the manufacturer's protocol. After transfection, the
Nucleocuvette™ was incubated at room temperature for 10 min and
resuspended in pre-warmed medium to a total volume 100 µl. Next, 25
µl of each sample was transferred to a 96-well culture plate with
175 µl medium in each well (four wells for each sample and negative
control). The cells were then harvested after 4, 24, 48 and 72 h
and gene expression was measured.
Viromer blue
The standard complexation protocol was employed for
Viromer® Blue. Briefly, 8×104 cells were
seeded 1 day before transfection in one well of a 24-well plate in
0.5 ml DMEM. The medium was changed to fresh medium of the same
type immediately before transfection. Cells were transfected upon
reaching a confluence of 60–80%. Next, the transfection complex was
prepared: 45 µl Viromer solution mixed with 5 µl of 5 and 50 nM
miRNA mimic. Then, 50 µl of the transfection complex was added to
the cells in each well. Effects were monitored 24, 48 and 72 h
post-transfection.
INTERFERin
Briefly, ~2.5×104 cells were seeded 1 day
before transfection in a 24-well plate in 1 ml DMEM. The cells were
transfected upon reaching a confluence of 30–50%. Three different
miRNA mimic concentrations were tested: 1, 3 and 7 nM. Each
concentration of miRNA was diluted in 100 µl medium without FBS and
mixed with 2 µl INTERFERin. Afterwards, the DMEM was removed, and
0.5 ml fresh medium was added to each well, followed by 100 µl of
the transfection mix. CROT gene silencing was assessed
between 24 and 72 h.
Cell viability and cytotoxicity
assays
Metabolic activity and the viability of transfected
cells were measured via mitochondrial dehydrogenases, by means of
the colorimetric MTT assay (Invitrogen™ CyQUANT™ MTT Cell Viability
Assay; Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The absorbance was measured at a
wavelength of 570 nm using an Infinite M200 Pro Microplate Reader
(Tecan Group Ltd.).
To investigate the cytotoxic effects of
transfection, the CyQUANT lactate dehydrogenase (LDH) Cytotoxicity
Assay (Thermo Fisher Scientific, Inc.) was used. The assay measures
the LDH released from cells with a damaged membrane, whereby the
amount of LDH released into the medium is quantified via the level
of formazan formation. For this purpose, transfection with
INTERFERin® and Viromer® Blue was compared
with non-transfected NHDFs. A total of 24 h after transfection, the
CyQUANT LDH cytotoxicity assay was performed according to the
manufacturer's instructions. A sample of Triton-X-100 was used as
the positive control for 100% cytotoxicity.
Reverse transcription-quantitative PCR
(RT-qPCR) detection of CROT expression
RNA was isolated from NHDFs using the Total RNA Mini
Plus (A&A Biotechnology) according to the manufacturer's
protocol. The quality of isolated RNA was assessed via
electrophoresis using a 1.5% agarose gel (MilliporeSigma) and it
was quantified on a NanoDrop 2000c (Thermo Fisher Scientific,
Inc.). RT was performed using 0.5 µg total RNA for each sample
using a High-Capacity cDNA Reverse Transcription Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's recommendations. CROT (GenBank accession no.
NM_021151) was analyzed as a target gene of hsa-miR-302b-3p,
according to miRBase (https://mirbase.org). The expression of CROT
was normalized to the housekeeping genes GAPDH (GenBank
accession no. NM_002046.3) and 18S ribosomal RNA (18S;
GenBank accession no. X03205.1). Target and housekeeping gene
probes were provided by Applied Biosystems (Thermo Fisher
Scientific, Inc.) as ready to use assays: CROT TaqMan™ Gene
Expression Assay (FAM), Assay ID: Hs00221733_m1; GAPDH Human
GAPD (GAPDH) Endogenous Control (FAM™/MGB probe, non-primer
limited), Assay ID: Hs99999905_m1; 18S Eukaryotic 18S rRNA
Endogenous Control (FAM™/MGB probe, non-primer limited), Assay ID:
Hs99999901_s1. qPCR reaction was performed using TaqMan®
Gene Expression Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) as recommended by the manufacturer: 50°C for 5
min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 1 min. Gene expression was assessed using the
StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.).
Dual luciferase reporter (DLR)
assay
The prediction of downstream target gene sequences
of miR-302b-3p was performed using the miRDB website (https://mirdb.org). pmirGLO reporter vectors (Promega
Corporation) comprising CROT−3′UTR wild type (WT) and
CROT−3′UTR mutant (MUT) were transfected by nucleofection
into NHDF cells along with a miR-302b-3p inhibitor, miR-302b-3p
mimics or a miRNA negative control. The Dual-Luciferase®
Reporter Assay System (Promega Corporation) was used for the
luminescence intensity determination 48 h after transfection.
Comparison with Renilla luciferase activity was used as a
method of normalization.
Statistical analysis
The individual gene expression level was calculated
by relative quantitative analysis and the Pfaffl model, including
the reaction efficiency for individual genes (37). The normality of the gene expression
distribution of all analyzed variables was assessed using the
Shapiro-Wilk test. To compare experimental groups, one-way analysis
of variance (ANOVA) was used. The significant differences between
the analyzed groups were calculated using the Tukey-Kramer HSD
post-hoc comparison in Statistica version 10 (StatSoft, Inc.). The
results are presented as the mean ± standard deviation of three
experimental repeats. P<0.05 was considered to indicate a
statistically significant difference.
Results
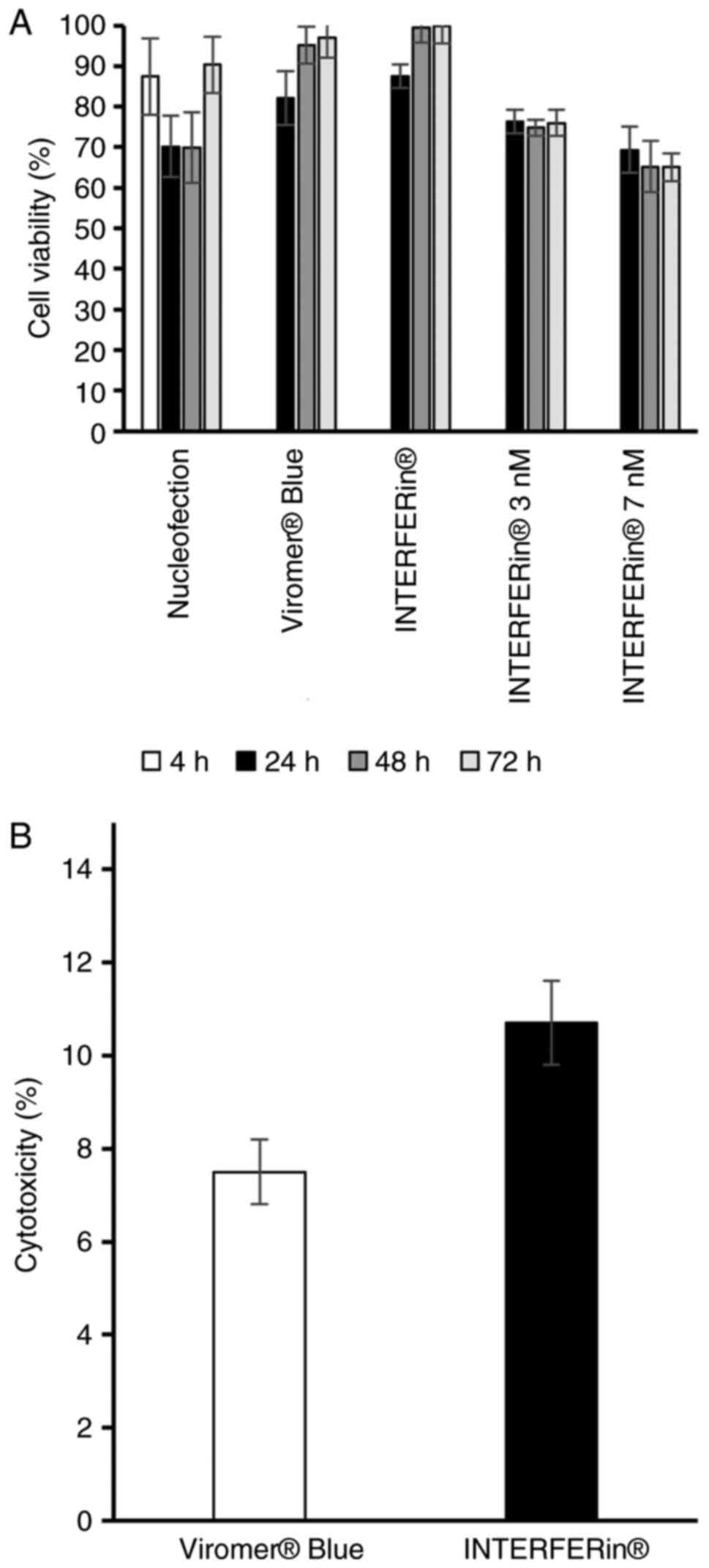
Cytotoxicity and cell viability
The MTT assay revealed that NHDFs transfected with
hsa-miR-302b-3p mimics delivered by INTERFERin, Viromer Blue or
nucleofection exhibited lower but not significantly reduced
viability when compared with non-transfected control cells
(Fig. 1A).
The cytotoxicity assay results demonstrated that,
after a 24-h incubation, both of the investigated miRNA delivery
agents (Viromer Blue and INTERFERin) had relatively low toxic
effects on NHDFs, i.e., 7.5 and 10.7% for Viromer Blue and
INTERFERin, respectively (Fig.
1B).
Transfection efficiency
In NHDFs, ~77.8 and 68.3% efficiencies were achieved
with nucleofection of 50 nM hsa-miR-302b-3p, after 4 and 24 h,
respectively (Fig. S1A). The
transfection efficiency with Viromer Blue after 48 h was ~67.8% (50
nM) and 57.9% (5 nM) (Fig. S1B).
Efficiencies of ~61.5% (7 nM), 58.2% (3 nM) and 53.3% (1 nM) were
achieved with INTERFERin 24 h after transfection. The percentage of
transfection was maintained up to 48 h after transfection but
decreased after 72 h (Fig.
S1C).
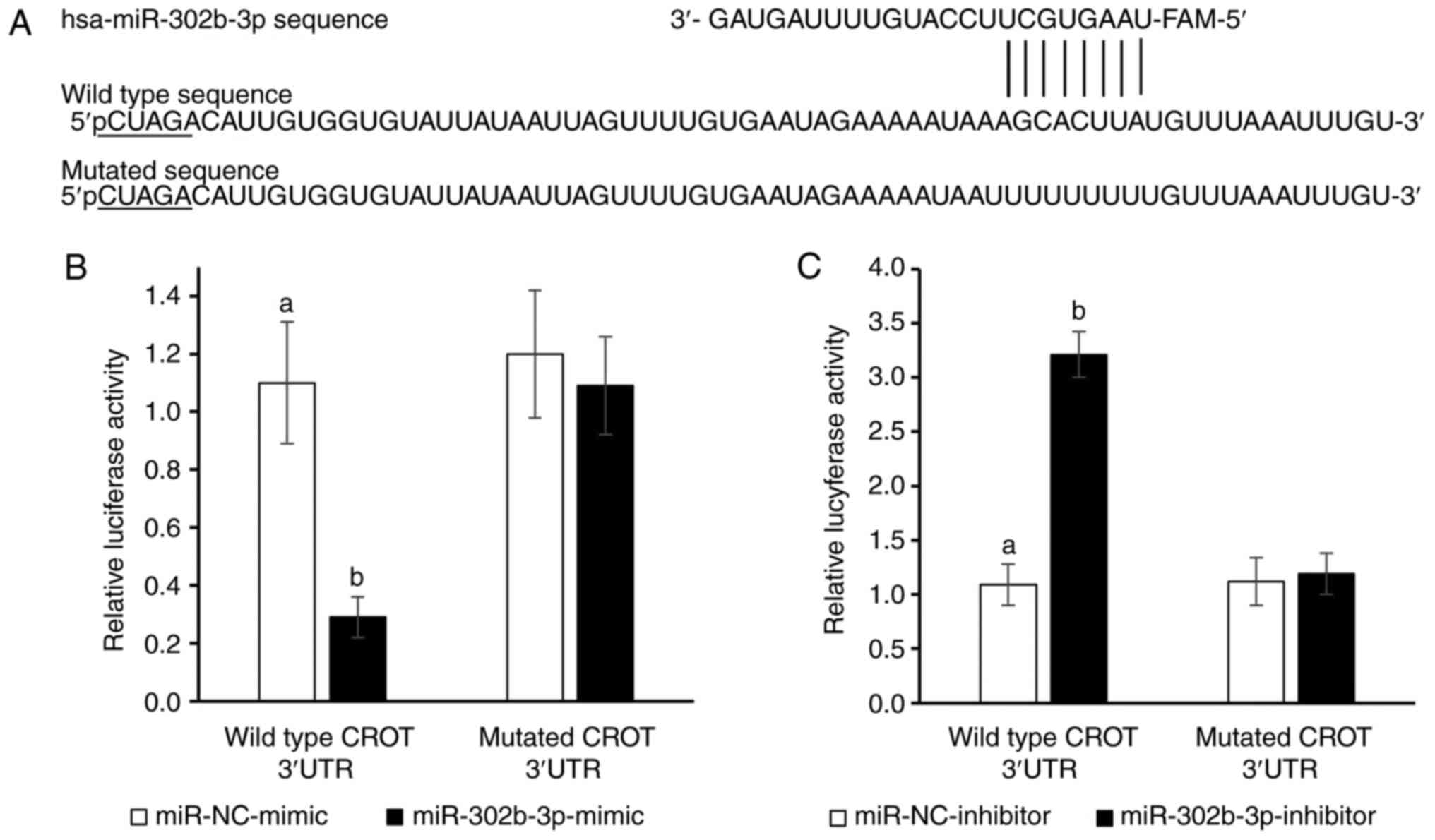
CROT is the direct target gene of
miR-302b-3p
To confirm that CROT is a target of
hsa-miR-302b-3p, CROT 3′UTR luciferase reporter plasmids
containing WT or MUT potential binding sites for miR-302b-3p were
designed (Fig. 2A) and a DLR assay
was performed (Fig. 2B and C).
Co-transfection of NHDFs with the WT CROT 3′-UTR construct
and hsa-miR-302b-3p mimic resulted in a significant decrease in
cellular luciferase activity compared with cells transfected with
the control mimic (P<0.05; Fig.
2B). Overexpression of miR-302b-3p did not affect the
luciferase activity of MUT CROT 3′-UTR (Fig. 2B). By contrast, transfection of
NHDFs with the miR-302b-3p inhibitor increased the luciferase
activity of the WT CROT 3′-UTR construct (P<0.05,
Fig. 2C), whereas it had no impact
on MUT CROT 3′-UTR. Overall, CROT was revealed to be a
target gene of miR-302b-3p.
Effect of hsa-miR-302b-3p on the
expression levels of the CROT gene
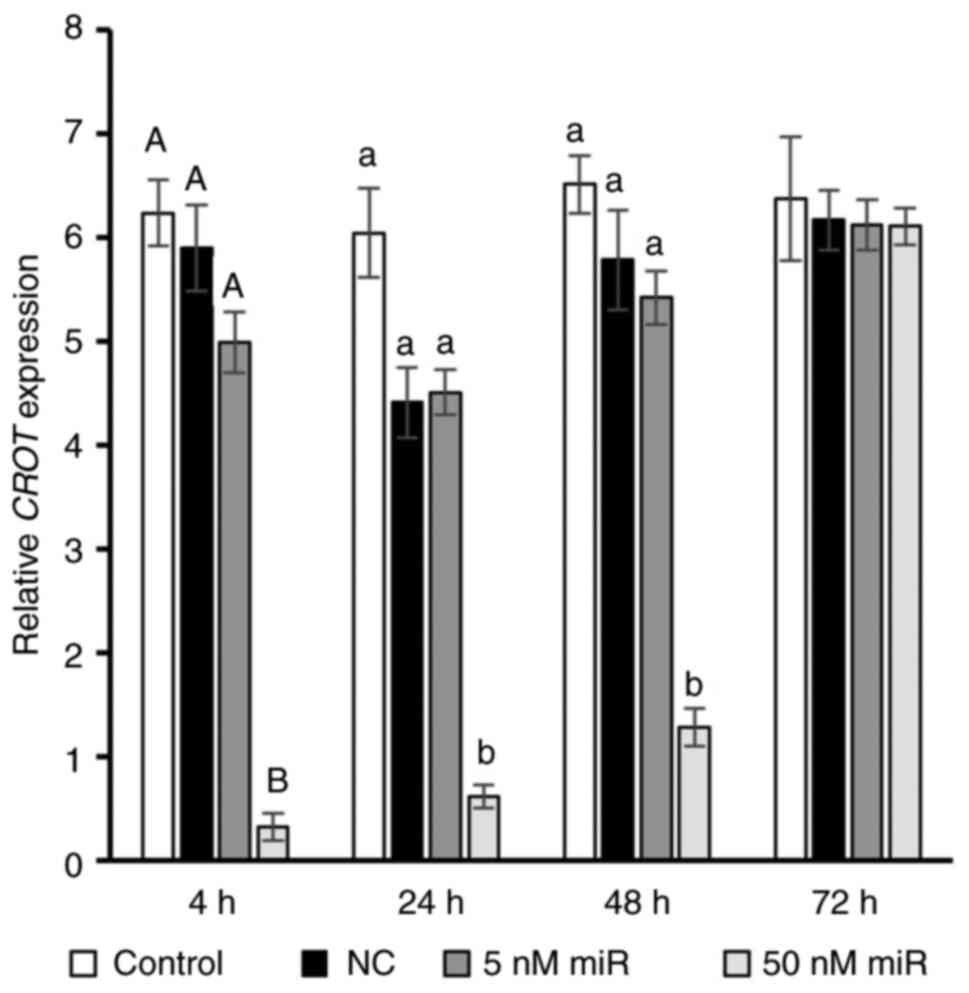
Using RT-qPCR, the mRNA expression levels of
CROT were detected following transfection with
hsa-miR-302b-3p. As expected, 50 nM hsa-miR-302b-3p nucleofection
caused a decrease in the expression levels of the CROT gene
in NHDFs; the greatest decline (21.4-fold; P<0.01) was observed
after only 4 h. Furthermore, 24 and 48 h after nucleofection,
CROT transcript expression was 7.5- and 4.7-fold lower
compared with the negative control (P<0.05). After 72 h, the
expression of the CROT gene reached the same level as the
negative control. However, transfection with 5 nM miRNA mimic had
no effect on CROT (Fig.
3).
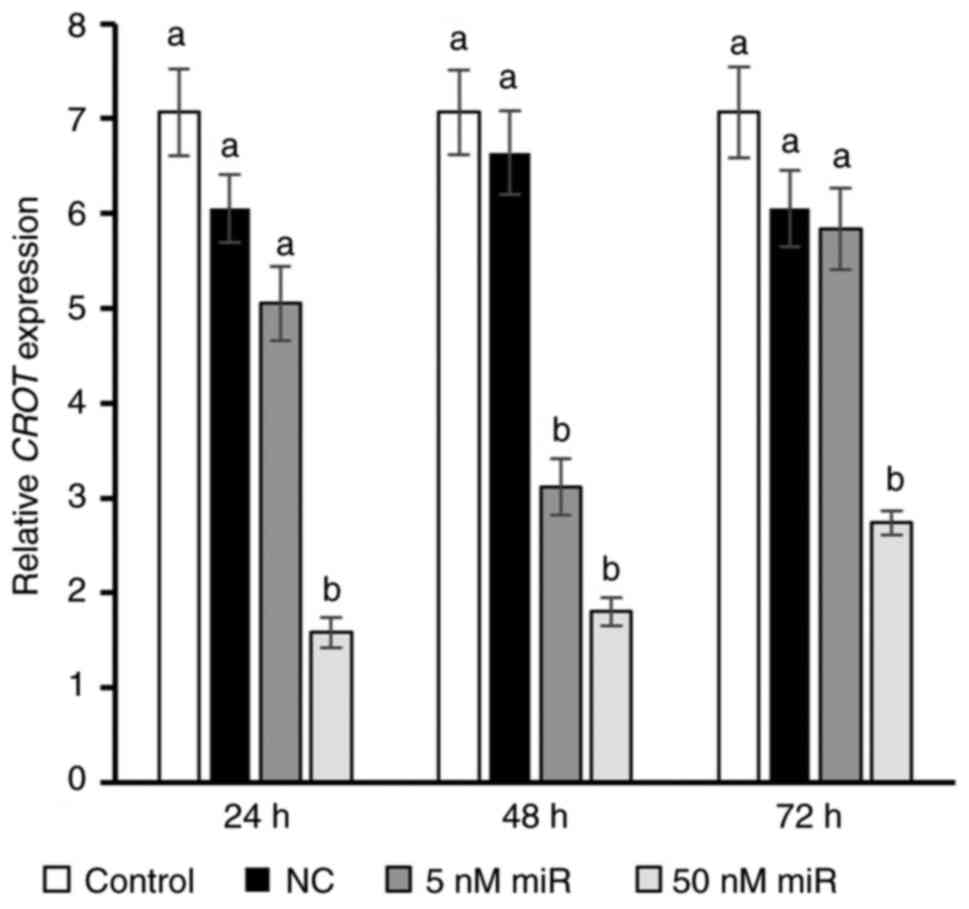
Viromer Blue-mediated transfection with 50 nM
hsa-miR-302b-3p also resulted in a significant decrease in
CROT gene expression, i.e., 3.8-, 3.7- and 2.2-fold at 24,
48 and 72 h, respectively. In the 5 nM group, a decrease in target
gene expression was only demonstrated after 48 h (P<0.05,
Fig. 4).
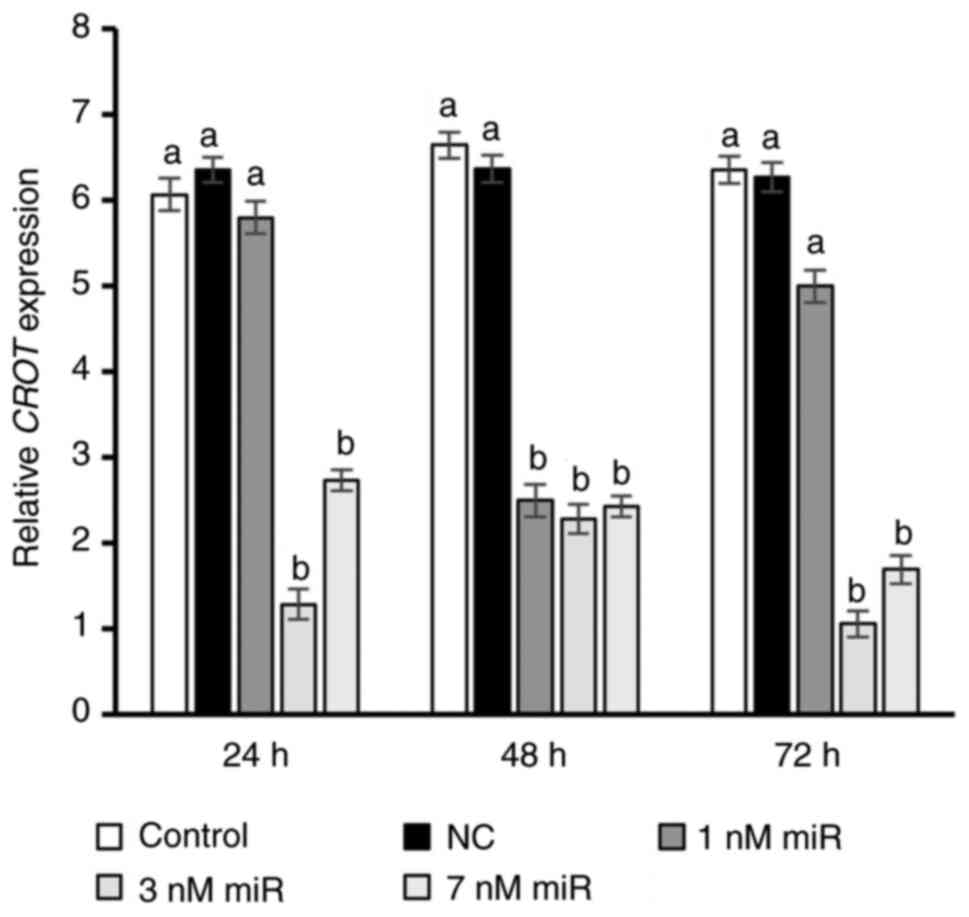
Transfection using INTERFERin was tested at three
different concentrations of hsa-miR-302b-3p (1, 3 and 7 nM).
Notably, a significant 2.7-fold decrease in CROT expression
was observed only after 48 h in the 1 nM group, whereas higher
concentrations of hsa-miR-302b-3p (3 and 7 nM) decreased target
gene expression at all measured time points compared with in the
control group (P<0.05; Fig. 5).
Moreover, 4.7- and 6-fold lower CROT transcript levels were
detected in the 3 nM group at 24 and 72 h post-transfection,
respectively (P<0.05). Unexpectedly, a weaker effect of 3 nM
hsa-miR-302b-3p on CROT expression (3-fold decrease) was
observed after 48 h (P<0.05). The impact of 3 nM hsa-miR-302b-3p
on CROT expression was strongest (6-fold decrease) after 72
h (P<0.05).
Discussion
Transient transfection can effectively deliver miRNA
mimics into in vitro cultured mammalian cells, and has been
adopted as a fast, easy and economical way to examine the functions
and mechanisms of action of endogenous miRNAs (38,39).
A range of transfection reagents have been employed to transfect
miRNAs into cells in order to achieve genetic manipulation.
Non-viral gene delivery methods can be generally divided into
chemical and physical approaches; however, there is no single
non-viral miRNA deliver strategy that is appropriate for all cell
types (24). Therefore, the aim of
the present study was to estimate the cytotoxic effects and
transfection efficiencies of nucleofection and two lipid-based
transfection reagents: Viromer Blue and INTERFERin, on NHDFs.
The present study demonstrated that all of the
chosen non-viral transfection systems decreased the expression of a
target gene of miR-302b-3p with good efficiencies. However,
nucleofection resulted in the highest silencing effect of
miR-302b-3p on CROT gene expression among all delivery
methods; however, its effect lasted for only 48 h. This result is
in line with the current state of knowledge. Nucleofection remains
the most effective non-viral system for transferring cargo
molecules into cells (24). This
method has been successfully employed in the transfection of
primary HDFs (20,23), rat dermal fibroblasts (23), and mouse and pig embryonic
fibroblasts (23,40).
If lipid-mediated transfection is the only method
available to alter the expression levels of the target gene, then
it is necessary to optimize the transfection conditions by
assessing the cytotoxicity of the cargo molecule, the miRNA
concentration and transfection time. The cytotoxicity results of
the present study indicated that INTERFERin caused a 1.4-fold
higher level of toxicity compared with Viromer Blue. These results
were also reflected in the viability assay. Carrying out a LDH
assay solely for chemical methods may be a limitation of the study.
A 1.5-fold higher, but not significant, level of toxicity has also
been observed in hepatocytes transfected with INTERFERin compared
with L-iMAX, which is based on the Lipofectamine chemical
transfection system (41).
Cationic lipids may interfere with membrane function, and the
integrity of the cell or subcellular compartments, and cause
toxicity (42). The most evident
difference between cationic lipids and cationic polymers is that
polymers do not possess a hydrophobic moiety and are completely
soluble in water. They form positively charged complexes with
negatively charged phosphate groups in nucleic acids, interact with
negatively charged proteoglycans on the cell surface, and enter the
cell by endocytosis (43). In
contrast to cationic liposomes, they pack the nucleotide molecule
into a relatively small space, which may be essential for
increasing transfection efficiency, as a smaller particle size may
be preferable (42).
Additionally, the concentration of miRNA mimics is
another parameter that should be examined with caution. Depending
on the transient transfection method, different miRNA mimic
concentrations were used, i.e., 5 and 50 nM for nucleofection, 5
and 50 nM for Viromer Blue, and 1, 3 or 7 nM for INTERFERin
according to the manufacturers' protocols. Jin et al
(44) reported that transient
transfection of miRNA mimics at high concentrations (>100 nM)
could alter gene expression in a non-specific manner. Furthermore,
this previous study suggested that miRNA mimics should be
introduced into cells at transfection concentrations much lower
than the 25 or 100 nM that are commonly used. In this context, the
best results were observed for INTERFERin. It is worth adding that
the ratio of reagent to nucleic acid may be important, particularly
in the case of chemical transfection with the use of cationic
lipids or cationic polymers. This is mainly due to the ratio of
positive charge contributed by the cationic lipid component of the
transfection reagent and negative charge contributed by the
phosphates of nucleic acid (45).
Another factor that may have an impact on
cytotoxicity or gene expression is cell density. However, such
analysis was not part of the present study as the protocols of the
three transfection systems were based on the cell density range
recommended by the individual reagent manufacturers.
Transfection is one of the procedures used in
medical research on fibroblasts. It is widely employed in
investigations related to gene therapies, wound healing, skin aging
or to obtain pluripotent cells through the reprogramming process,
for which fibroblasts are commonly used. Transfection takes
advantage of some desirable characteristics of fibroblasts, such as
the ease of isolation, rapid cell growth and a high degree of
robustness. On the other hand, primary fibroblasts are considered
difficult to transfect, mainly due to the low efficiency of
transporting cargo molecules into these cells (21,22).
Intensive efforts carried out in recent years have led to an
increase in the efficiency of the transfer of particles, mainly
DNA, to fibroblasts, both in the case of viral (transduction) and
non-viral (transfection) systems (24). However, techniques based on viral
systems remain the most effective way of transporting cargo
molecules into cells. With these methods, a high efficiency of
fibroblast transduction has been achieved. Unfortunately, the
safety risk of such vectors remains an obstacle.
In conclusion, despite the difficulty in
transfecting primary cells, progenitor cells and stem cells, there
has been considerable enthusiasm for further improvement of
chemical vectors in the hope of achieving efficacies and
efficiencies that could potentially mimic viral vectors. Based on
the observations of the present study, several recommendations can
be made for non-viral transfection systems. Firstly, nucleofection
remains the preferred method of choice, especially when using
smaller cargo molecules such as miRNA. However, lipid-based
transfection systems require lower miRNA concentrations and have
longer-lasting effects. The quality of these non-viral systems
continues to be improved and they will become an increasingly
competitive and low-cost option to consider.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Ministry of Science
and Higher Education to the University of Agriculture in Krakow,
Poland (grant no. SUB-020002-D015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EO, MK conceived and designed the experiments. MK,
PM and EO performed the experiments. EO and MK confirm the
authenticity of all the raw data. MK, PM, SB, HM, MM, AS, DZP and
EO analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The requirement for ethics approval was waived by
the Bioethics Committee of the Medical University of Silesia
(Katowice, Poland).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maier AB and Westendorp RGJ: Relation
between replicative senescence of human fibroblasts and life
history characteristics. Ageing Res Rev. 8:237–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lago JC and Puzzi MB: The effect of aging
in primary human dermal fibroblasts. PLoS One. 14:e02191652019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomes RN, Manuel F and Nascimento DS: The
bright side of fibroblasts: Molecular signature and regenerative
cues in major organs. NPJ Regen Med. 6:432021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raab S, Klingenstein M, Liebau S and Linta
L: A comparative view on human somatic cell sources for iPSC
generation. Stem Cells Int. 2014:7683912014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim Y, Nam Y, Rim YA and Ju JH:
Anti-fibrotic effect of a selective estrogen receptor modulator in
systemic sclerosis. Stem Cell Res Ther. 13:3032022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castro-Viñuelas R, Sanjurjo-Rodríguez C,
Piñeiro-Ramil M, Hermida-Gómez T, Rodríguez-Fernández S, Oreiro N,
de Toro J, Fuentes I, Blanco FJ and Díaz-Prado S: Generation and
characterization of human induced pluripotent stem cells (iPSCs)
from hand osteoarthritis patient-derived fibroblasts. Sci Rep.
10:42722020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nuta O, Somaiah N, Boyle S, Chua ML,
Gothard L, Yarnold J, Rothkamm K and Herskind C: Correlation
between the radiation responses of fibroblasts cultured from
individual patients and the risk of late reaction after breast
radiotherapy. Cancer Lett. 374:324–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Tu W, Tang Y and Zhang S:
Prevention and treatment for radiation-induced skin injury during
radiotherapy. Radiat Med Prot. 1:60–68. 2020. View Article : Google Scholar
|
|
9
|
de Paepe B, Smet J, Leroy JG, Seneca S,
George E, Matthys D, van Maldergem L, Scalais E, Lissens W, de
Meirleir L, et al: Diagnostic value of immunostaining in cultured
skin fibroblasts from patients with oxidative phosphorylation
defects. Pediatr Res. 59:2–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oblong JE, Bowman A, Rovito HA, Jarrold
BB, Sherrill JD, Black MR, Nelson G, Kimball AB and Birch-Machin
MA: Metabolic dysfunction in human skin: Restoration of
mitochondrial integrity and metabolic output by nicotinamide
(niacinamide) in primary dermal fibroblasts from older aged donors.
Aging Cell. 19:e132482020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Millioni R, Puricelli L, Iori E, Trevisan
R and Tessari P: Skin fibroblasts as a tool for identifying the
risk of nephropathy in the type 1 diabetic population. Diabetes
Metab Res Rev. 28:62–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Zhu Y, Lian N, Chen M, Bartke A and
Yuan R: Metabolic syndrome and skin diseases. Front Endocrinol
(Lausanne). 10:7882019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auburger G, Klinkenberg M, Drost J, Marcus
K, Morales-Gordo B, Kunz WS, Brandt U, Broccoli V, Reichmann H,
Gispert S and Jendrach M: Primary skin fibroblasts as a model of
Parkinson's disease. Mol Neurobiol. 46:20–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teves JMY, Bhargava V, Kirwan KR,
Corenblum MJ, Justiniano R, Wondrak GT, Anandhan A, Flores AJ,
Schipper DA, Khalpey Z, et al: Parkinson's disease skin fibroblasts
display signature alterations in growth, redox homeostasis,
mitochondrial function, and autophagy. Front Neurosci. 11:7372018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mocali A, Della Malva N, Abete C,
Mitidieri Costanza VA, Bavazzano A, Boddi V, Sanchez L, Dessì S,
Pani A and Paoletti F: Altered proteolysis in fibroblasts of
Alzheimer patients with predictive implications for subjects at
risk of disease. Int J Alzheimers Dis. 2014:5201522014.PubMed/NCBI
|
|
16
|
Drabik K, Malińska D, Piecyk K,
Dębska-Vielhaber G, Vielhaber S, Duszyński J and Szczepanowska J:
Effect of chronic stress present in fibroblasts derived from
patients with a sporadic form of AD on mitochondrial function and
mitochondrial turnover. Antioxidants (Basel). 10:9382021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mesdom P, Colle R, Lebigot E, Trabado S,
Deflesselle E, Fève B, Becquemont L, Corruble E and Verstuyft C:
Human dermal fibroblast: A promising cellular model to study
biological mechanisms of major depression and antidepressant drug
response. Curr Neuropharmacol. 18:301–318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong T, McGrath JA and Navsaria H: The
role of fibroblasts in tissue engineering and regeneration. Br J
Dermatol. 156:1149–1155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu CYM and Uludağ H: A simple and rapid
nonviral approach to efficiently transfect primary tissue-derived
cells using polyethylenimine. Nat Protoc. 7:935–945. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koster J and Waterham HR: Transfection of
primary human skin fibroblasts for peroxisomal studies. Methods Mol
Biol. 1595:63–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Xu Y, Wang B, Qiao W, Liu D and
Li Z: Cationic compounds used in lipoplexes and polyplexes for gene
delivery. J Control Release. 100:165–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kundu PP and Sharma V: Synthetic polymeric
vectors in gene therapy. Curr Opin Solid State Mater Sci.
12:89–102. 2008. View Article : Google Scholar
|
|
23
|
Zhang Z, Slobodianski A, Arnold A, Nehlsen
J, Hopfner U, Schilling AF, Perisic T and Machens HG: High
efficiency low cost fibroblast nucleofection for GMP compatible
cell-based gene therapy. Int J Med Sci. 14:798–803. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kucharski M, Mrowiec P and Ocłoń E:
Current standards and pitfalls associated with the transfection of
primary fibroblast cells. Biotechnol Prog. 37:e31522021.PubMed/NCBI
|
|
25
|
Pezzoli D, Kajaste-Rudnitski A, Chiesa R
and Candiani G: Lipid-based nanoparticles as nonviral gene delivery
vectors. Methods Mol Biol. 1025:269–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He L and Hannon GJ: Correction: MicroRNAs:
Small RNAs with a big role in gene regulation. Nat Rev Genet.
5:6312004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quévillon Huberdeau M and Simard MJ: A
guide to microRNA-mediated gene silencing. FEBS J. 286:642–652.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng B, Chen Y and Leong KW: MicroRNA
delivery for regenerative medicine. Adv Drug Deliv Rev. 88:108–122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paoletti C, Divieto C, Tarricone G, Di
Meglio F, Nurzynska D and Chiono V: MicroRNA-mediated direct
reprogramming of human adult fibroblasts toward cardiac phenotype.
Front Bioeng Biotechnol. 8:5292020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SWL, Paoletti C, Campisi M, Osaki T,
Adriani G, Kamm RD, Mattu C and Chiono V: MicroRNA delivery through
nanoparticles. J Control Release. 313:80–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Heikkinen L, Emily Knott K, Liang
Y and Wong G: Evolutionary conservation and function of the human
embryonic stem cell specific miR-302/367 cluster. Comp Biochem
Physiol Part D Genomics Proteomics. 16:83–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun S, Wang J, Liu J, Yin F, Xin C, Zeng
X, Li J and Chen Q: MiR-302b suppresses tumor metastasis by
targeting frizzled 6 in OSCC. J Dent Res. 100:739–745. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He R, Tang GL, Niu L, Ge C, Zhang XQ, Ji
XF, Fang H, Luo ZL, Chen M and Shang XF: Quietness Circ 0000962
promoted nerve cell inflammation through PIK3CA/Akt/NF-κB signaling
by miR-302b-3p in spinal cord injury. Ann Palliat Med. 9:190–198.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Basatemur GL, Jørgensen HF, Clarke MCH,
Bennett MR and Mallat Z: Vascular smooth muscle cells in
atherosclerosis. Nat Rev Cardiol. 16:727–744. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okui T, Iwashita M, Rogers MA, Halu A,
Atkins SK, Kuraoka S, Abdelhamid I, Higashi H, Ramsaroop A, Aikawa
M, et al: CROT (carnitine O-octanoyltransferase) is a novel
contributing factor in vascular calcification via promoting fatty
acid metabolism and mitochondrial dysfunction. Arterioscler Thromb
Vasc Biol. 41:755–768. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iversen N, Birkenes B, Torsdalen K and
Djurovic S: Electroporation by nucleofector is the best nonviral
transfection technique in human endothelial and smooth muscle
cells. Genet Vaccines Ther. 3:22005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Younger ST and Corey DR: Transcriptional
gene silencing in mammalian cells by miRNA mimics that target gene
promoters. Nucleic Acids Res. 39:5682–5691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z: The guideline of the design and
validation of MiRNA mimics. Methods Mol Biol. 676:211–223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCoy AM, Collins ML and Ugozzoli LA:
Using the gene pulser MXcell electroporation system to transfect
primary cells with high efficiency. J Vis Exp. 7:16622010.
|
|
41
|
Böttger J, Arnold K, Thiel C, Rennert C,
Aleithe S, Hofmann U, Vlaic S, Sales S, Shevchenko A and Matz-Soja
M: RNAi in murine hepatocytes: The agony of choice-a study of the
influence of lipid-based transfection reagents on hepatocyte
metabolism. Arch Toxicol. 89:1579–1588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kasai H, Inoue K, Imamura K, Yuvienco C,
Montclare JK and Yamano S: Efficient siRNA delivery and gene
silencing using a lipopolypeptide hybrid vector mediated by a
caveolae-mediated and temperature-dependent endocytic pathway. J
Nanobiotechnology. 17:112019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fan X, Yang J, Wu G, Wang M, Cheng X, Liu
C, Liu Q, Wen Y, Meng S, Wang Z, et al: Optimization of cationic
polymer-mediated transfection for RNA interference. Genet Mol Biol.
45:e202102372022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin HY, Gonzalez-Martin A, Miletic AV, Lai
M, Knight S, Sabouri-Ghomi M, Head SR, Macauley MS, Rickert RC and
Xiao C: Transfection of microRNA mimics should be used with
caution. Front Genet. 6:3402015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chan CL, Ewert KK, Majzoub RN, Hwu YK,
Liang KS, Leal C and Safinya CR: Optimizing cationic and neutral
lipids for efficient gene delivery at high serum content. J Gene
Med. 16:84–96. 2014. View Article : Google Scholar : PubMed/NCBI
|