Introduction
Lung cancer is the most prevalent cancer found in
both men and women and is the leading cause of cancer-related death
worldwide (1). Radiotherapy and
chemotherapy have been the major options in the treatment of
locally advanced lung cancer. Radiotherapy mainly aims at local
tumor control, while chemotherapy reduces the risk of distant
metastasis. Despite recent advances in radiotherapy (image
guidance, adaptive treatment planning, hyperfractionation or
hypofractionation, dose escalation), and the discoveries of novel
chemotherapy and targeted agents, the prognosis for 5-year survival
in patients with locally advanced lung cancer is still poor
(~15–25%) (1). One of the factors
contributing to treatment failure is local recurrence, which is
believed to stem from radiation resistance by cancer cells.
Identifying agents that overcome radiation resistance is a
promising strategy by which to enhance the effect of radiation.
β-elemene is the active component of elemene
(1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane), a naturally
occurring compound that is isolated from the traditional Chinese
medicinal herb Curcuma wenyujin. A number of studies have
confirmed that this agent enhances the radiosensitivity of human
cancer cell lines (2–4), as well as rabbit VX2 carcinoma
(5). Some in vitro studies
have attributed the enhanced radiation response to increased
apoptosis and cell cycle arrest in the G2/M phase (2,3,6,7).
Our previous studies found that β-elemene radiosensitized the lung
cancer A549 cell line by enhancing DNA damage and inhibiting DNA
repair (8), as well as decreased
radiation-induced expression of survivin and HIF-1α in an A549 cell
xenograft model (9). Thus it is
clear that β-elemene enhances the response to radiation through
multiple pathways; however, the underlying mechanisms remain to be
elucidated and potential novel pathways are still to be
unveiled.
It is well known that radiation increases the level
of intracellular reactive oxygen species (ROS), mainly
H2O2, and evokes a series of intracellular
biochemical events including DNA mutation and DNA damage, which
lead to cell cycle arrest and apoptosis (10,11).
Upregulation of antioxidant capacity due to intrinsic oxidative
stress in cancer cells was thought to confer radioresistance;
therefore, targeted redox modulation has been suggested as a
promising novel approach in cancer therapy (12). Peroxiredoxin-1 (Prx-1) is a critical
member among the Prx family, and is a major
H2O2 scavenger and signaling regulator.
Phosphorylation of Prx-1 allows localized
H2O2 accumulation for cell signaling
(13). As a critical player in
redox regulation in cancer cells, inhibition of Prx-1 may be an
effective way to enhance tumor radioresponse. However, it has not
been confirmed whether β-elemene inhibits Prx-1 for enhancing a
radioresponse.
Currently, proteomics concerning comprehensive
protein profile changes caused by multi-gene alterations is
considered to be an effective tool for studying the mechanisms of
disease, and for finding potential biomarkers and new therapeutic
targets. The main objective of the present study was to discover
new molecular targets with enhancement of radiosensitivity by
β-elemene, in lung adenocarcinoma xenografts. This was achieved
using two-dimensional differential in-gel electrophoresis (2D-DIGE)
and matrix-assisted laser desorption/ionization time-of-flight
tandem mass spectrometry. Reverse transcription-polymerase chain
reaction (RT-PCR) and western blotting techniques were used to
confirm the findings of the 2D-DIGE analysis. Our analysis revealed
that β-elemene directly or indirectly inhibited Prx-1 expression to
enhance tumor radiosensitivity.
Materials and methods
Chemicals and cell culture
β-elemene was purchased from Jingang Pharmaceutical
Co. (Dalian, China). The human lung adenocarcinoma cell line A549
was purchased from the Cell Center of the Chinese Academy of
Medical Sciences. Cells were grown in Rosewell Park Memorial
Institute (RPMI)-1640 medium (Gibco-BRL/Invitrogen, Carlsbad, CA,
USA), supplemented with 10% fetal bovine serum (TBD Bio, Tianjin,
China) at 37°C in a humidified atmosphere with 5%
CO2.
Animals and tumor models
Female athymic BALB/c nu/nu mice aged 6–8 weeks were
purchased from the Animal Experiment Center of Dalian Medical
University and maintained under specific pathogen-free (SPF)
conditions. The facilities and the protocol for these experiments
were consistent with the regulations of animal use for biomedical
experiments as issued by the Ministry of Science and Technology of
China, and approved by the Animal Care Committee of Dalian Medical
University. A549 cells were subcutaneously injected into the right
hind leg (1×107 cells/animal). The tumor sizes were
measured every two days using vernier calipers. At 4–5 weeks, tumor
volumes reached the required size (0.8–1.0 cm3). Tumor
volumes were calculated using the following formula: (0.5 × largest
diameter × smallest diameter2) as previously reported
(9).
Xenograft treatment with radiation and/or
β-elemene
For radiation treatment, the mice were placed in a
specially designed polyvinylchloride box, and the right hind legs
bearing the xenograft tumors were exposed out of the box. The mice
were immobilized and tumors were positioned in the center of a 4×3
cm radiation field, with the rest of the body remaining outside of
the radiation field. Radiation was delivered with 6 MeV electron
beams from a linear accelerator (Varian) at a single dose of 5 Gy.
For drug alone treatment, a single dose of 45 mg/kg of β-elemene
was injected intraperitoneally. For drug and radiation combined
treatment, radiation was delivered 1 h after β-elemene was
injected. For the control group, 0.9% sodium chloride (NaCl) was
injected intraperitoneally and the volume was the same as 45 mg/kg
of β-elemene. The procedure was performed as previously reported
(9).
Sample preparation for
electrophoresis
Nude mice with tumors of 0.8–1.0 cm3 were
divided into 2 groups (3 mice/group): radiation alone and combined
treatment (β-elemene + radiation). The mice were sacrificed 24 h
after treatment (9) and the tumors
(three from each group) were excised and ground into powder in
liquid nitrogen with a precooled mortar and pestle. Samples were
then homogenized using a glass homogenizer on ice in 1 ml of lysis
buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 30 mM Tris-HCl
and 2% (v/v) protease inhibitor (Roche Diagnostics, Mannheim,
Germany). After sonication on ice for 10 sec using an ultrasonic
processor, the samples were centrifuged for 30 min at 12,000 rpm to
remove particulate materials. The supernatant was collected and the
protein concentration was determined using a Protein Assay kit
(Bio-Rad, Hercules, CA, USA). Proteins from six samples were stored
at −80°C for future use.
Two-dimensional differential in-gel
electrophoresis and imaging
The pH of the lysate was adjusted to 8.0–9.0 with 50
mM NaOH, and the concentration was adjusted to 5 mg/ml with lysis
buffer. Equal amounts of proteins from the six samples were pooled
together as the internal standard. According to the statistical
principle, 50 μg of proteins taken from every group were labeled
with 400 pmol of Cy3 or Cy5 respectively, whereas 50 μg of internal
standards were labeled with 400 pmol of Cy2. Combined samples (150
μg) were then diluted with rehydration buffer and the total volume
was made up to 450 μl. The combined samples were loaded on a pH
gradient strip for isoelectric focusing (IEF) on an Ettan IPGphor
system (Amersham Biosciences, Uppsala, Sweden): 500 V for 1 h;
1,000 V for 1 h; 8,000 V for 8 h; 500 V for 4 h. After IEF, the
strips were equilibrated twice and transferred onto 12% vertical
polyacrylamide gels cast in low fluorescence glass plates using a
Hoefer SE600 system (GE Healthcare, Uppsala, Sweden).
Electrophoresis was conducted until the bromophenol blue front
reached the bottom of the gels. The gels were scanned on a Typhoon
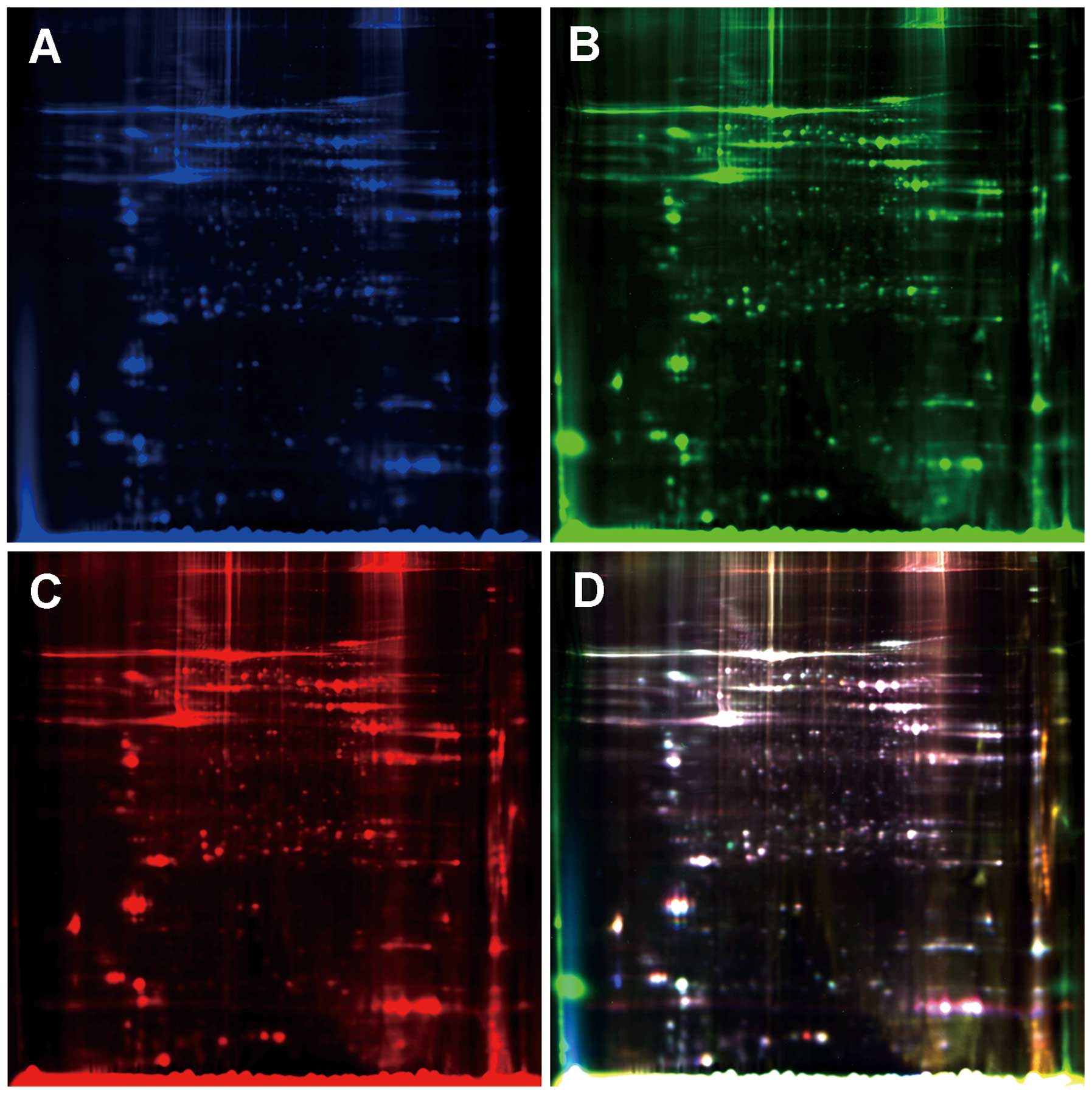
9410 scanner (Amersham Biosciences) using excitation/emission
wavelengths specific for Cy2 (488/520 nm), Cy3 (532/580 nm) and Cy5
(633/670 nm), respectively (Fig.
1). Intragel spot detection and quantification, as well as
intergel matching and quantification were performed using
Differential In-gel Analysis (DIA) and Biological Variation
Analysis (BVA) modules of DeCyder software version 6.5 (Amersham
Biosciences). The gel with the highest spot count was assigned as
the master gel. In DIA, the Cy2, Cy3 and Cy5 images for each gel
were merged, spot boundaries were automatically detected and
normalized spot volumes (protein abundance) were calculated. The
resulting spot maps were exported to BVA. Gel-to-gel matching of
the standard spot maps from each gel, followed by statistical
analysis of protein abundance change between samples was performed
in a BVA module. The standardized average spot volume ratios
exceeding 1.2 were considered statistically significant (Student’s
t-test, p<0.05).
Protein digestion and mass
spectrometry
For mass spectrometric analysis, three preparative
gels loaded with 1 mg of protein were run under the same conditions
and stained with Bio-Safe Colloidal Coomassie blue (Bio-Rad, San
Francisco, CA, USA). Differentially expressed protein spots of
interest were excised from both the DIGE analytic gels and
preparative gels with the help of Ettan Spot Picker (GE Healthcare)
and subjected to in-gel digestion with trypsin. Briefly, gel plugs
were destained with 30% acetonitrile in 0.1 M ammonium carbonate
(NH4HCO3) for 20 min and vacuum dried. Then, digestion
buffer (20 ng/ml trypsin in 20 mM NH4HCO3)
was added and the samples were digested at room temperature
overnight. Peptides were extracted twice with a solution containing
50% acetonitrile (ACN) and 0.1% trifluoroacetic acid (TFA). The
extracted peptides were removed, dried and re-suspended in 50% ACN
and 0.1% TFA. Equal volumes of sample and a-HCCA matrix (5 mg/ml)
were spotted and mixed on the MALDI-TOF target plate. Peptide
mixtures were analyzed with a 4800 Plus MALDI TOF/TOF™ Analyzer
(Applied Biosystems, USA) in a positive ion reflector mode. The
obtained peptide mass fingerprint spectra were analyzed by
searching the non-redundant protein database of the National Center
for Biotechnology Information.
Sample preparation for reverse
transcription-PCR and western blot analysis
Nude mice with tumors of 0.8–1.0 cm3 were
randomized to four groups (5 mice/group), that included a control
group and three treatment groups: β-elemene alone, radiation alone
and β-elemene combined with radiation. The mice were sacrificed and
the tumors were excised and cut into small pieces.
Reverse transcription-PCR analysis
Total RNA from the tumor tissues was isolated using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The reverse transcription-PCR analysis
was performed using a RT-PCR kit (Takara, Otsu, Japan). The
specific primer sequences were as follows: Prx-1 (forward primer,
5′-ATGTC TTCAGGAAATGCTAAAAT-3′ and reverse primer, 5′-TCAC
TTCTGCTTGGAGAAATATTC-3′); and β-actin (forward primer,
5′-CAAGAGATGGCCACGGCTGCT-3′ and reverse primer,
5′-TCCTTCTGCATCCTGTCGGCA-3′). The PCR protocol was as follows:
initial denaturation at 94°C for 2 min, followed by 35 cycles at
94°C for 30 sec, annealing at 59°C, and extension at 72°C for 1
min. The final extension was performed by an incubation step at
72°C for 7 min. The PCR products were subjected to electrophoresis
on agarose gel and visualized with ethidium bromide. The bands were
analyzed with Quantity One software (Bio-Rad).
Western blot analysis
The tumor pieces were mixed in RIPA buffer and
homogenized with polytron. Protein concentrations from tumor
lysates were determined by Bio-Rad assays. Equal amounts of
proteins were separated on SDS-10% PAGE, transferred to
polyvinylidene difluoride membrane, and probed with the indicated
rabbit anti-Prx-1 polyclonal antibody (Lab Frontier, Korea) at a
1:1,000 dilution. This was followed by polyclonal goat anti-rabbit
IgG antibody conjugated with horseradish peroxidase at a 1:2,000
dilution (Abcam, UK). The protein bands were detected by LabWorks
software (UVP, Upland, CA, USA).
Statistical analysis
Statistical analyses were performed with the
two-tailed Student’s t-test or analysis of variance (ANOVA) to
compare the experimental groups. Differences were considered
significant when p-values were ≤0.05. Results are expressed as mean
± SD as indicated. All calculations were performed using SPSS
version 11.0 (SPSS, Inc., Chicago, IL, USA).
Results
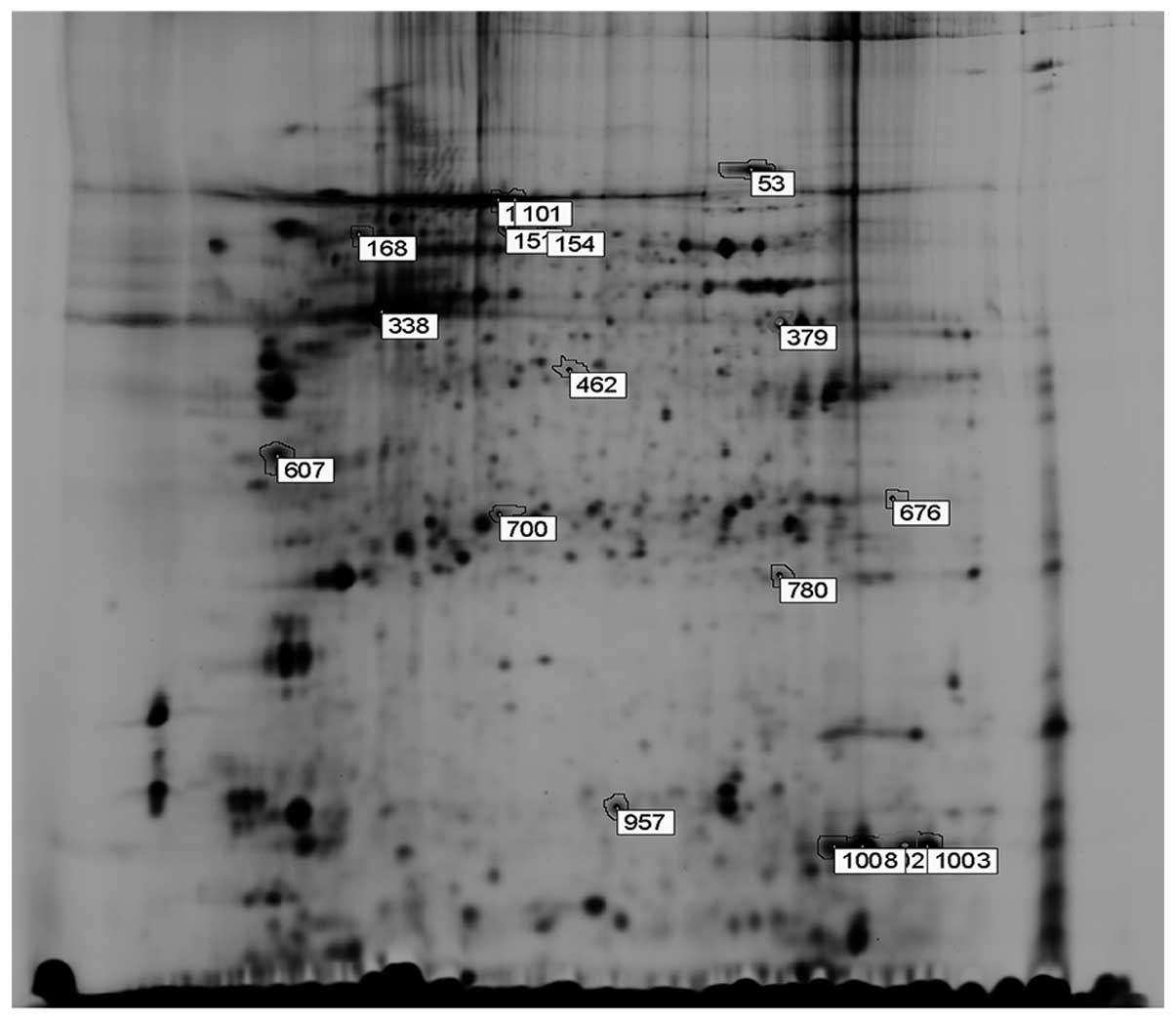
Analysis of 2-DE images
Results of the gel images were analyzed by DeCyder
Differential Analysis software and a total number of 17
significantly differentiated spots were found between the radiation
and the combined treatment group (Fig.
2). Among the 17 spots, 8 spots represented proteins that were
downregulated, whereas 9 spots were that of upregulated proteins in
the combined group vs. radiation alone group. The confidence level
and difference degree of 17 differentiated spots are shown in
Table I.
 | Table IThe confidence level and difference
degree of the 17 differentiated protein spots (radiation/combined
group). |
Table I
The confidence level and difference
degree of the 17 differentiated protein spots (radiation/combined
group).
| Pos | Differentiated
protein spots (radiation/combined group) | Average ratio |
|---|
|
|---|
| Master no. | t-test |
|---|
| 1 | 1002 | 0.0043 | 3.75 |
| 2 | 1003 | 0.0039 | 3.23 |
| 3 | 1008 | 0.0033 | 2.62 |
| 4 | 379 | 0.012 | 1.46 |
| 5 | 676 | 0.0053 | 1.33 |
| 6 | 338 | 0.0089 | 1.26 |
| 7 | 780 | 0.042 | 1.22 |
| 8 | 462 | 0.0061 | 1.21 |
| 9 | 151 | 0.025 | −1.20 |
| 10 | 700 | 0.0075 | −1.21 |
| 11 | 168 | 0.0041 | −1.22 |
| 12 | 607 | 0.027 | −1.31 |
| 13 | 53 | 0.034 | −1.48 |
| 14 | 154 | 0.00081 | −1.49 |
| 15 | 100 | 0.0018 | −1.54 |
| 16 | 101 | 0.026 | −1.55 |
| 17 | 957 | 8.9e0–0.05 | −1.90 |
Analysis of the differentially expressed
proteins by mass spectrometry
All 17 differentially expressed spots were selected
for analysis by mass spectrometry. The peptide mass finger-printing
(PMF) of every protein was obtained by MALDI-TOF MS and the
SWISS-PROT and the NCBI database search engine were used to
identify these proteins (Table
II). There were 13 proteins of human origin and 4 proteins of
mouse origin. Among the identified human proteins, No. 780 protein
called Prx-1, a radiation-related protein, was found to be
downregulated in the combined treatment group (Fig. 3).
 | Table IIThe results of peptide mass
fingerprinting (PMF) of every differentially expressed protein
spot. |
Table II
The results of peptide mass
fingerprinting (PMF) of every differentially expressed protein
spot.
| Protein ID | Protein name | Species | Accession no. | Mw | PI | Score | Confidence
level | Matched peptide
fragments |
|---|
| 53 |
Serotransferrin | Mouse | gi|21363012 | 78840.5 | 6.94 | 619 | 100 | 30 |
| 168 | Unnamed protein
product | Human | gi|193787214 | 47061.7 | 4.94 | 290 | 100 | 23 |
| 100 | Serum albumin
precursor | Mouse | gi|163310765 | 70700.5 | 5.75 | 846 | 100 | 18 |
| 101 | Serum albumin
precursor | Mouse | gi|163310765 | 70700.5 | 5.75 | 1.100 | 100 | 24 |
| 151 | Chain A,
TapasinERP57 HETERODIMER | Human | gi|220702506 | 54541.4 | 5.61 | 635 | 100 | 23 |
| 154 | Chain A,
TapasinERP57 HETERODIMER | Human | gi|220702506 | 54541.4 | 5.61 | 573 | 100 | 23 |
| 338 | Full-asctin, α
skeletal muscle | Human | gi|61218043 | 42366 | 5.23 | 770 | 100 | 12 |
| 379 | Creatine
kinase | Human | gi|180588 | 43302 | 6.77 | 661 | 100 | 25 |
| 607 | Tropomyosin α-4
chain isoform 2 | Human | gi|4507651 | 28618.5 | 4.67 | 161 | 100 | 10 |
| 462 | Unnamed protein
product | Human | gi|221040676 | 43022.8 | 4.99 | 103 | 99.997 | 7 |
| 700 | Antioxidant enzyme
AOE37-2 | Human | gi|799381 | 30748.9 | 5.86 | 462 | 100 | 12 |
| 676 | Chain S, structure
of the human Mcadetf E165βa complex | Human | gi|71042617 | 27996.2 | 8.57 | 342 | 100 | 11 |
| 780 | Peroxiredoxin 1,
isoform CRA_a | Human | gi|119627386 | 22324.4 | 8.27 | 794 | 100 | 16 |
| 957 | Transthyretin | Mouse | gi|136465 | 15880 | 5.77 | 484 | 100 | 7 |
| 1008 | Chain B, crystal
structure of human hemoglobin A2 (in R2 state) at 2.2 A
resolution | Human | gi|56553727 | 16028.3 | 7.97 | 170 | 100 | 4 |
| 1002 | Chain B, deoxy
hemoglobin | Human | gi|27574242 | 16090.3 | 6.75 | 149 | 100 | 4 |
| 1003 | Chain A, deoxy
Rhb1.1 (recombinant hemoglobin) | Human | gi|9256887 | 30418.8 | 8.91 | 184 | 100 | 4 |
Expression of Prx-1 mRNA in each
group
Prx-1 mRNA expression levels were investigated by
RT-PCR in tumor samples in the control, β-elemene and radiation
alone, and combined treatment groups (Fig. 4A). The levels of Prx-1 mRNA
expression were compared and the results are shown in Fig. 4B. β-elemene alone at a dose of 45
mg/kg significantly inhibited the Prx-1 mRNA expression (2.12-fold
lower) compared with the control group (p<0.01). However,
radiation significantly increased the Prx-1 mRNA expression
(1.36-fold higher) compared with the expression level in the
control group (p<0.01). Notably, Prx-1 mRNA expression was
significantly decreased in the β-elemene/radiation co-treatment
group (7.18-fold lower) compared with the basal expression in the
control group (p<0.01).
Expression of Prx-1 protein in each
group
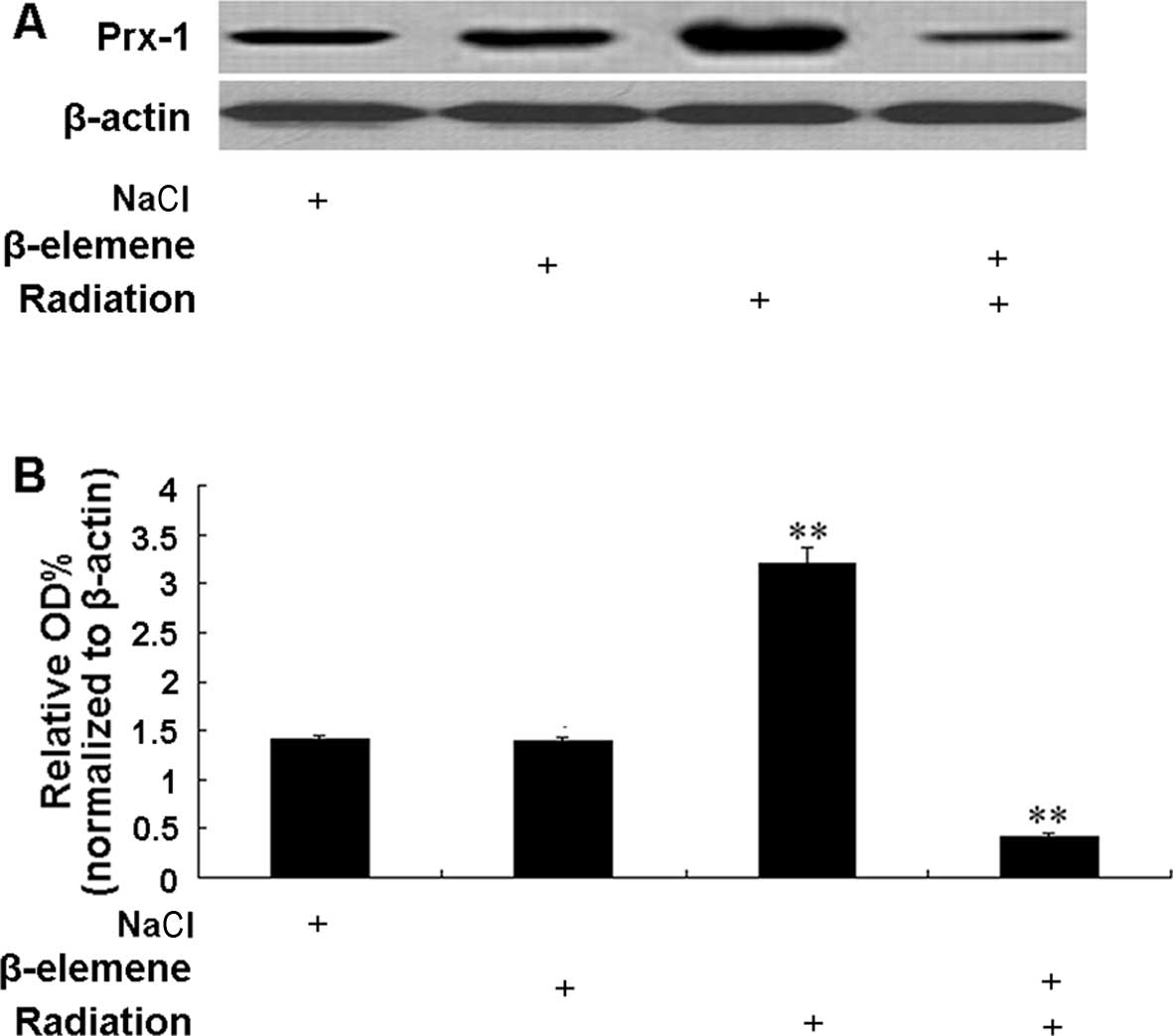
Prx-1 protein expression levels were investigated by
western blotting in tumor samples from the control, β-elemene and
radiation alone and co-treatment groups (Fig. 5A). The level of Prx-1 protein
expression was compared between the groups and the results are
shown in Fig. 5B. β-elemene alone
at the dose of 45 mg/kg had little effect on the Prx-1 protein
expression compared with control group. However, radiation
significantly increased the Prx-1 protein expression (2.29-fold
higher) compared with the expression level in the control group
(p<0.01). Notably, Prx-1 protein expression was significantly
decreased (3.33-fold lower) in the β-elemene/radiation co-treatment
group compared with the basal expression in the control group
(p<0.01).
Discussion
2D-PAGE has become one of the most widely used
methods in proteomics as the separation of a large number of
proteins is necessary in complex biological samples (14). Compared to conventional 2D-PAGE,
difference gel electrophoresis (DIGE)-based proteomics with
fluorescence labeling has many advantages such as higher
sensitivity, reproducibility and less technical variations due to a
pooled control as internal standard (15–17).
In the present study, we used 2D-DIGE and MALDI-TOF/TOF tandem mass
spectrometry to profile the different proteins between xenograft
models of the radiation group and β-elemene + radiation group.
Prx-1 is a unique protein that was differentially expressed in the
two treatment groups and downregulated in the combination group.
Prx-1 has been found to be elevated in numerous types of cancers,
including lung cancer (18–22). Several studies have demonstrated
that Prx-1 is an independent prognostic factor and a novel plasma
biomarker for lung cancer (23–25).
In addition to being a novel tumor marker, Prx-1 was also found to
be induced by radiation in various types of cancer cells in
vitro (26,27). Many cancer cells including lung
cancer exhibited decreased growth and increased radiation response
following suppression or knockdown of Prx-1 mRNA expression with
RNA interference strategy (28–31).
A549 cells also displayed increased radiosensitivity through
enhanced intracellular reactive oxygen species (ROS) level
following decreased Prx-1 protein expression with a targeted Prx-1
antibody (32). Our results
acquired from 2D-DIGE and spectrometry revealed that β-elemene
decreased the expression of Prx-1 to enhance the radioresponse in
the A549 cell xenograft model.
We further tested the expression of Prx-1 in the
radiation, β-elemene or combined treatment groups at the
transcription and translation levels. Our results showed that
radiation markedly induced the enhancement of Prx-1 expression,
which was similar to the results of the studies mentioned above. As
a major H2O2 scavenger and signaling
regulator, elevated Prx-1 expression in post-radiated cancer cells
can effectively reduce H2O2 levels induced by
radiation, resulting in the enhanced survival of cancer cells.
In our previous study, we treated A549 cell
xenografts with a 25, 45 or 100 mg/kg dose of β-elemene followed by
radiation. The enhancement factor (EF) following a dose of 25, 45
or 100 mg/kg dose was 0.84, 1.24 and 2.04, respectively (9). Since a drug is considered to exhibit a
synergistic effect with radiation only at a dose level where EF is
>1 (33), this suggests that
there was a synergistic effect when at least 45 mg/kg β-elemene was
used with radiation therapy. This dose level was subsequently
selected for later experiments including the present study. The 100
mg/kg dose level was not chosen in order to minimize the direct
cytotoxic effect of β-elemene. In the present study, β-elemene
alone at the dose of 45 mg/kg had little effect on the Prx-1
protein expression that was correlated with a moderate antitumor
effect. However, the finding that the 45 mg/kg dose of β-elemene
inhibited the Prx-1 mRNA expression suggests a possible influence
at the level of transcription.
The finding that the increased radiation-induced
Prx-1 mRNA/protein expression was significantly suppressed in the
combination treatment group suggests that β-elemene directly or
indirectly inhibited Prx-1 expression to enhance the
radio-sensitivity of the A549 cell xenograft model. Our previous
study showed that β-elemene induced increased levels of ROS in A549
cells (34). Here, we suggest that
the increasing ROS levels may be related to the inhibition of Prx-1
expression by β-elemene. The mechanisms underlying the correlation
between β-elemene and Prx-1 merit further investigation.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang H, Ma S and Feng J: In vitro study
of radiosensitization by β-Elemene in A549 cell line from
adenocarcinoma of lung. Chinese-German J Clin Oncol. 8:12–15. 2009.
View Article : Google Scholar
|
|
3
|
Cheng W, Qiao Z, Shi T, Huang C and Wang
Y: In vitro study on increase in radio sensitivity of renal cell
carcinoma induced by β-Elemene. J Xi’an Jiaotong Univ: Med Sci
(Chinese). 25:182–185. 2004.
|
|
4
|
Wu D, Li X, Zhao J, Wang H and Zhao D: A
study of radio-sensitivity of β-elemene to squamous cell carcinoma
of tongue Tca-8113 cell line in vitro. Zhong Liu Ji Chu Yu Lin
Chuang. 19:116–117. 2006.(In Chinese).
|
|
5
|
She J, Wang Z, Che X and Pan C:
Radiosensitization of β-elemene on VX2 carcinoma transplanted on
kidney in rabbits in vivo. Zhong Xi Yi Jie He Xue Bao. 4:392–396.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu DP, Jiang RB and Zhao DQ: Effects of
β-elemene on radiosensitivity, cell cycle, apoptosis, Bcl2 and Bax
expression of Tca8113 cells in vitro. Zheng Zou Da Xue Xue Bao.
44:414–417. 2009.(In Chinese).
|
|
7
|
She J, Wang Z and Che X: Expressions of
caspase-3 and Bcl-2 in radiosensitization of β-elemene on rabbit
VX2 renal carcinoma. Zhong Xi Yi Jie He Xue Bao. 27:2285–2287.
2006.(In Chinese).
|
|
8
|
Li LJ, Zhong LF, Jiang LP, Geng CY and Zou
LJ: β-Elemene radiosensitizes lung cancer A549 cells by enhancing
DNA damage and inhibiting DNA repair. Phytother Res. 25:1095–1097.
2011. View
Article : Google Scholar
|
|
9
|
Li G, Xie B, Li X, et al: Down-regulation
of survivin and hypoxia-inducible factor-1α by β-elemene enhances
the radiosensitivity of lung adenocarcinoma xenograft. Cancer
Biother Radiopharm. 27:56–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang T, Diaz AJ and Yen Y: The role of
peroxiredoxin II in chemoresistance of breast cancer cells. Breast
Cancer. 6:73–80. 2014.PubMed/NCBI
|
|
11
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Intracellular redox status and oxidative stress: implications
for cell proliferation, apoptosis, and carcinogenesis. Arch
Toxicol. 82:273–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: a radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woo HA, Yim SH, Shin DH, Kang D, Yu DY and
Rhee SG: Inactivation of peroxiredoxin I by phosphorylation allows
localized H2O2 accumulation for cell
signaling. Cell. 140:517–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbert BR, Harry JL, Packer NH, Gooley
AA, Pedersen SK and Williams KL: What place for polyacrylamide in
proteomics? Trends Biotechnol. 19(Suppl 10): S3–S9. 2001.
View Article : Google Scholar
|
|
15
|
Tonge R, Shaw J, Middleton B, et al:
Validation and development of fluorescence two-dimensional
differential gel electrophoresis proteomics technology. Proteomics.
1:377–396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gharbi S, Gaffney P, Yang A, et al:
Evaluation of two-dimensional differential gel electrophoresis for
proteomic expression analysis of a model breast cancer cell system.
Molecular Cell Proteomics. 1:91–98. 2002. View Article : Google Scholar
|
|
17
|
Yan JX, Devenish AT, Wait R, Stone T,
Lewis S and Fowler S: Fluorescence two-dimensional difference gel
electrophoresis and mass spectrometry based proteomic analysis of
Escherichia coli. Proteomics. 2:1682–1698. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang JW, Jeon HB, Lee JH, et al:
Augmented expression of peroxiredoxin I in lung cancer. Biochem
Biophys Res Commun. 289:507–512. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HJ, Chae HZ, Kim YJ, et al:
Preferential elevation of Prx I and Trx expression in lung cancer
cells following hypoxia and in human lung cancer tissues. Cell Biol
Toxicol. 19:285–298. 2003. View Article : Google Scholar
|
|
20
|
Lehtonen ST, Svensk AM, Soini Y, et al:
Peroxiredoxins, a novel protein family in lung cancer. Int J
Cancer. 111:514–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alfonso P, Catalá M, Rico-Morales ML, et
al: Proteomic analysis of lung biopsies: differential protein
expression profile between peritumoral and tumoral tissue.
Proteomics. 4:442–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Kim YS, Lee HL, et al: Expression
of peroxiredoxin and thioredoxin in human lung cancer and paired
normal lung. Respirology. 11:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JH, Bogner PN, Ramnath N, Park Y, Yu J
and Park YM: Elevated peroxiredoxin 1, but not NF-E2-related factor
2, is an independent prognostic factor for disease recurrence and
reduced survival in stage I non-small cell lung cancer. Clin Cancer
Res. 13:3875–3882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JH, Bogner PN, Baek SH, et al:
Up-regulation of peroxiredoxin 1 in lung cancer and its implication
as a prognostic and therapeutic target. Clin Cancer Res.
14:2326–2333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rostila A, Puustinen A, Toljamo T, et al:
Peroxiredoxins and tropomyosins as plasma biomarkers for lung
cancer and asbestos exposure. Lung Cancer. 77:450–459. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WC, McBride WH, Iwamoto KS, et al:
Induction of radio-protective peroxiredoxin-I by ionizing
irradiation. J Neurosci Res. 70:794–798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang B, Su Y, Ai G, Wang Y, Wang T and
Wang F: Involvement of peroxiredoxin I in protecting cells from
radiation-induced death. J Radiat Res. 46:305–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MF, Keng PC, Shau H, et al:
Inhibition of lung tumor growth and augmentation of
radiosensitivity by decreasing peroxiredoxin I expression. Int J
Radiat Oncol Biol Phys. 64:581–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang B, Wang Y, Liu K, et al:
Adenovirus-mediated transfer of siRNA against peroxiredoxin I
enhances the radiosensitivity of human intestinal cancer. Biochem
Pharmacol. 75:660–667. 2008. View Article : Google Scholar
|
|
30
|
Gao MC, Jia XD, Wu QF, Cheng Y, Chen FR
and Zhang J: Silencing Prx1 and/or Prx5 sensitizes human esophageal
cancer cells to ionizing radiation and increases apoptosis via
intracellular ROS accumulation. Acta Pharmacol Sin. 32:528–536.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dittmann LM, Danner A, Gronych J, et al:
Downregulation of PRDX1 by promoter hypermethylation is frequent in
1p/19q-deleted oligodendroglial tumours and increases radio- and
chemosensitivity of Hs683 glioma cells in vitro. Oncogene.
31:3409–3418. 2012. View Article : Google Scholar
|
|
32
|
Guo Q, Huang X, Zhang J, Luo Y, Peng Z and
Li S: Down-regulation of peroxiredoxin I by a novel fully human
phage display recombinant antibody induces apoptosis and enhances
radiation sensitization in A549 lung carcinoma cells. Cancer
Biother Radiopharm. 27:307–316. 2012. View Article : Google Scholar
|
|
33
|
Milas L, Fujii T, Hunter N, et al:
Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer
Res. 59:107–114. 1999.PubMed/NCBI
|
|
34
|
Li LJ, Zhong LF, Jiang LP, et al:
Lysosomal membrane permea-bilization contributes to elemene
emulsion-induced apoptosis in A549 cells. Free Radic Res.
45:1232–1240. 2011. View Article : Google Scholar : PubMed/NCBI
|