Introduction
Head and neck squamous cell carcinoma (HNSCC)
affects ~500,000 new patients annually worldwide (1). HNSCC patients experience vital
dysfunctions, including difficulties in breathing, speech and
swallowing. Despite advances in the available therapies for HNSCC,
including surgery and radio-chemotherapy, the survival time has not
significantly improved. Lymph node metastasis is considered to be a
major cause of the poor survival times (2). Efforts to improve our understanding of
the molecular mechanisms of metastasis may aid in developing novel
therapeutic strategies for HNSCC.
Recent evidence has indicated that
microenvironmental constituents in the cancer stroma substantially
influence the propensity of cancer cells to metastasize (3). Oxygen is a key factor in the
microenvironment important for maintaining tissue homeostasis and
cellular metabolism by generation of adenosine-5′-triphosphate
(ATP). Notably, it has been estimated that regions of hypoxia
and/or anoxia account for ~60% of solid tumors arising as a result
of an imbalance between limited oxygen delivery and prolonged
consumption (4). Uncontrolled
growth of cancer cells results in hypoxia due to the increased
distance between the vasculature and certain regions of the tumor.
When cancer cells adapt to low oxygen stress, cellular pathways
controlling glucose uptake, metabolism, angiogenesis and
erythropoiesis are activated by hypoxia-inducible factor-1α
(HIF-1α) to facilitate cancer cell proliferation and progression
(5). Hypoxia, a hallmark of cancer,
activates hundreds of transcriptional activities by stabilizing
HIF-1α and inducing its translocation to hypoxia-response elements
(HREs) in target genes (6). Hypoxia
and HIF-1α signaling products influence multiple steps within the
metastatic cascade, including invasion, migration (Snail, Twist,
ZEB1/2, MMPs and CCR5), and establishment of the pre-metastatic
niche (LOX, SDF-1, CXCL12, CCL2 and exosomes) (7). In addition, HIF-1α-induced glycolytic
gene transcription drives hypoxic cells to produce pyruvate, which
is then converted into lactate rather than being oxidized via the
tricarboxylic acid (TCA) cycle and oxidative phosphorylation
(8). This metabolic shift (the
Warburg effect) can speed up energy generation, compensating for
increased ATP demand and enhancing biosynthesis, allowing tumor
cell proliferation and development even in a low-oxygen
microenvironment (9). Cancer cell
motility and cytoskeleton remodeling have been suggested to be
dependent on aerobic glycolysis (10). Previous investigations of tumor cell
metabolism have shown that strengthening cancer cell utilization of
the glycolytic pathway contributes to cell metastasis (10). Numerous immunochemical studies have
indicated that the elevated expression of HIF-1α and other
hypoxia-related proteins is correlated with lymph node metastasis
and poor prognosis in various tumor types, including lung cancer
and HNSCC (7).
Metadherin (MTDH), also known as AEG-1 or Lyric, is
located on human chromosome 8q22 and is recognized as an oncogene
that regulates numerous signaling pathways in cancer cells
(11). Our previous study indicated
that high expression of MTDH in HNSCC predicted an unfavorable
survival time in HNSCC patients (12), and promoted cell metastasis by
inducing angiogenesis and epithelial-mesenchymal transition (EMT)
in vitro (13,14). The PI3K/AKT pathway was demonstrated
to be involved in MTDH-induced angiogenesis and EMT in our previous
studies (13,14). Additionally, HIF-1α was reported to
regulate MTDH expression by activating the PI3K/AKT pathway in
glioma cells (15). However, the
potential regulatory mechanism associated with MTDH in HNSCC has
rarely been investigated. Our preliminary experiments indicated
that MTDH expression was elevated in hypoxic cell cultures.
However, whether MTDH is involved in hypoxia-induced metastasis and
glycolysis in HNSCC cells remains unclear.
In the present study, we confirmed that hypoxia
increased MTDH expression via HIF-1α expression. Knockdown of MTDH
expression in HNSCC cell lines interrupted hypoxia-induced
metastasis and glycolysis. Furthermore, reduced MTDH expression
decreased HIF-1α expression. The present study indicated that there
is a positive feedback loop between MTDH and HIF-1α in HNSCC.
Hypoxia promotes HNSCC metastasis and glycolysis via an MTDH-HIF-1α
loop pathway.
Materials and methods
Cell line culture and
transfection
The Tu686 cell line was provided by Dr Zhuo Chen of
Emory University's Winship Cancer Institute in Atlanta, Georgia and
was maintained as monolayer cultures in Dulbecco's modified Eagle's
medium (DMEM)/F12 medium (1:1) supplemented with 10% fetal bovine
serum (FBS) at 37°C under normoxia in a modular incubator chamber,
or under hypoxia with 5% CO2 and 1% O2
balanced with N2. The medium was changed every other
day. Exponentially growing cells were used for the following
experiments.
MTDH cDNA (GeneCopoeia, Guangzhou, China), shRNA
(sc-77797V; Santa Cruz Biotechnology, Santa Cruz, CA, USA, CA,
USA), HIF-1α cDNA (OriGene Technologies, Inc., Rockville, MD, USA),
siRNA (sc-35561; Santa Cruz Biotechnology), and their corresponding
control plasmids were transfected into the Tu686 cell line
according to the manufacturers instructions.
Western blotting
All western blot analysis was performed as
previously described (16). In
brief, loading protein was separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). The blotted membranes were incubated
with the primary antibody against MTDH (1:800; ProteinTech Group,
Inc., Chicago, IL, USA), HIF-1α (1:1,000; Santa Cruz
Biotechnology), E-cadherin (1:1,000), N-cadherin (1:800), vimentin
(1:800) (all from Cell Signaling Technology, Inc., Danvers, MA,
USA) and the secondary antibody (1:3,000; Beyotime, Shanghai,
China) in succession. A specific anti-β-actin protein (1:1,000;
Beyotime) was used to detect the quantity of the loading control
protein. Each experiment was repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Primers for β-actin, HIF-1α, E-cadherin, vimentin,
N-cadherin and VEGF mRNA were synthesized and validated by Beijing
Sunbiotech Co., Ltd. (Beijing, China). TRIzol® reagent
(cat no. 15596026; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used for RNA preparation. PowerUp™ SYBR®-Green
Master Mix (A25776; Thermo Fisher Scientific) was used in the PCR
amplification. The expression level was quantified by an Applied
Biosystems device using the 2−ΔΔCt method. Melting curve
analysis was performed at the end of the amplification cycle to
verify non-specific amplification. The detailed information of PCR
primers is listed as follows: VEGF-L, 5′-aggccagcacata ggagaga-3′
and VEGF-A, 5′-tttcttgcgctttcgttttt-3′; HIF-1α-L,
5′-ccacctatgacctgcttggt-3′ and HIF-1α-R, 5′-tatccaggctgtgt
cgactg-3′; β-actin-L, 5′-ctcttccagccttccttcct-3′ and β-actin-R,
5′-agcactgtgttggcgtacag-3′; E-cadherin-L, 5′-tgcccagaaaa
tgaaaaagg-3′ and E-cadherin-R, 5′-gtgtatgtggcaatgcgttc-3′;
vimentin-L, 5′-gagaactttgccgttgaagc-3′ and vimentin-R, 5′-tcca
gcagcttcctgtaggt-3′; N-cadherin-L, 5′-aggatcaaccccatacacca-3′ and
N-cadherin-R, 5′-tggtttgaccacggtgacta-3′.
Wound healing and Transwell
assays
Cells seeded into 6-well plates were allowed to
proliferate to near 100% confluence and were wounded by removing a
line of cells using a disinfected Eppendorf tip (100 µl). After
washing with FBS-free medium (0 h), the first image was
photographed under a microscope. The second image was captured in
the same way after 48 h. The closure of wound width in images was
measured three times by Photoshop software. The wound healing rate
= (average wound width at 0 h) - (average wound width at 48
h)/(average wound width at 0 h) × 100%.
Transfected cells were plated in Transwell cell
culture inserts (Corning Costar, Corning, NY, USA) at a density of
2×104 cells/well. The cells were maintained and allowed
to migrate for 48 h, after which the cells that had not invaded
were removed from the upper surface using a cotton swab. The cells
that had invaded to the lower surface were stained with crystal
violet solution and photographed under a microscope.
Glycolysis-related assays
Glucose Assay kit (ab65333), Deproteinizing Sample
Preparation kit-TCA (ab204708), Glucose Uptake Assay kit (ab136955)
and L-lactate assay kit (ab65330; all from Abcam, Cambridge, MA,
USA) were utilized in the glycolysis-related assays according to
the manufacturers protocols.
Statistical analysis
All statistical analyses were performed using IBM
SPSS statistical software, version 21.0 (SPSS, Inc., Chicago, IL,
USA). The differences between data derived from two groups in the
experiments were statistically analyzed by a Student's test.
P<0.05 was considered to indicate a statistical significant
result. All tests were two-sided.
Results
Hypoxia induces migration/invasion and
glycolysis in a HNSCC cell line
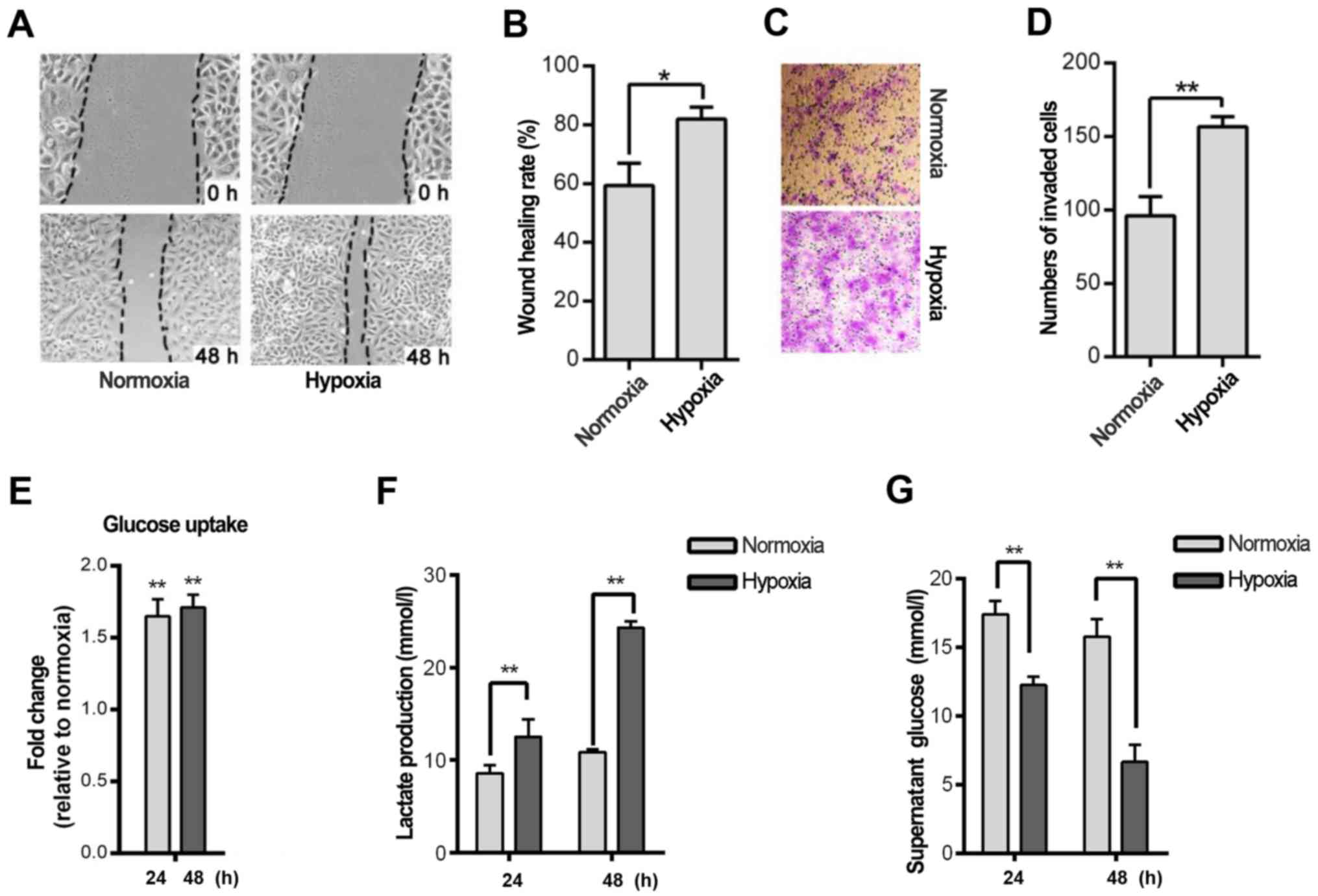
Hypoxia is a hallmark of cancer cells. Thus, we
investigated whether hypoxia induces cell migration and invasion in
the HNSCC Tu686 cell line. The wound-healing rate indicated that
hypoxia significantly promoted Tu686 cell healing after 48 h in
hypoxia compared to cells cultured in normoxia (P<0.05; Fig. 1A and B). A similar trend was
observed in the Transwell experiment (P<0.01; Fig. 1C and D). Considering that glycolysis
is a fundamental stress-adaption process in hypoxic cells,
glycolysis was analyzed in HNSCC cell lines at different time
points. Glucose uptake and lactate secretion were increased in the
Tu686 cells after 24 and 48 h of hypoxia treatment (P<0.01;
Fig. 1E and F). In addition, the
concentration of glucose in the cell culture supernatant was
reduced significantly (P<0.01; Fig.
1G). Furthermore, several glycolytic genes, including MCT1,
GLUT1 and PGI, were overexpressed in the hypoxic cells (data not
shown).
Hypoxia promotes MTDH expression and
induces EMT in an HNSCC cell line
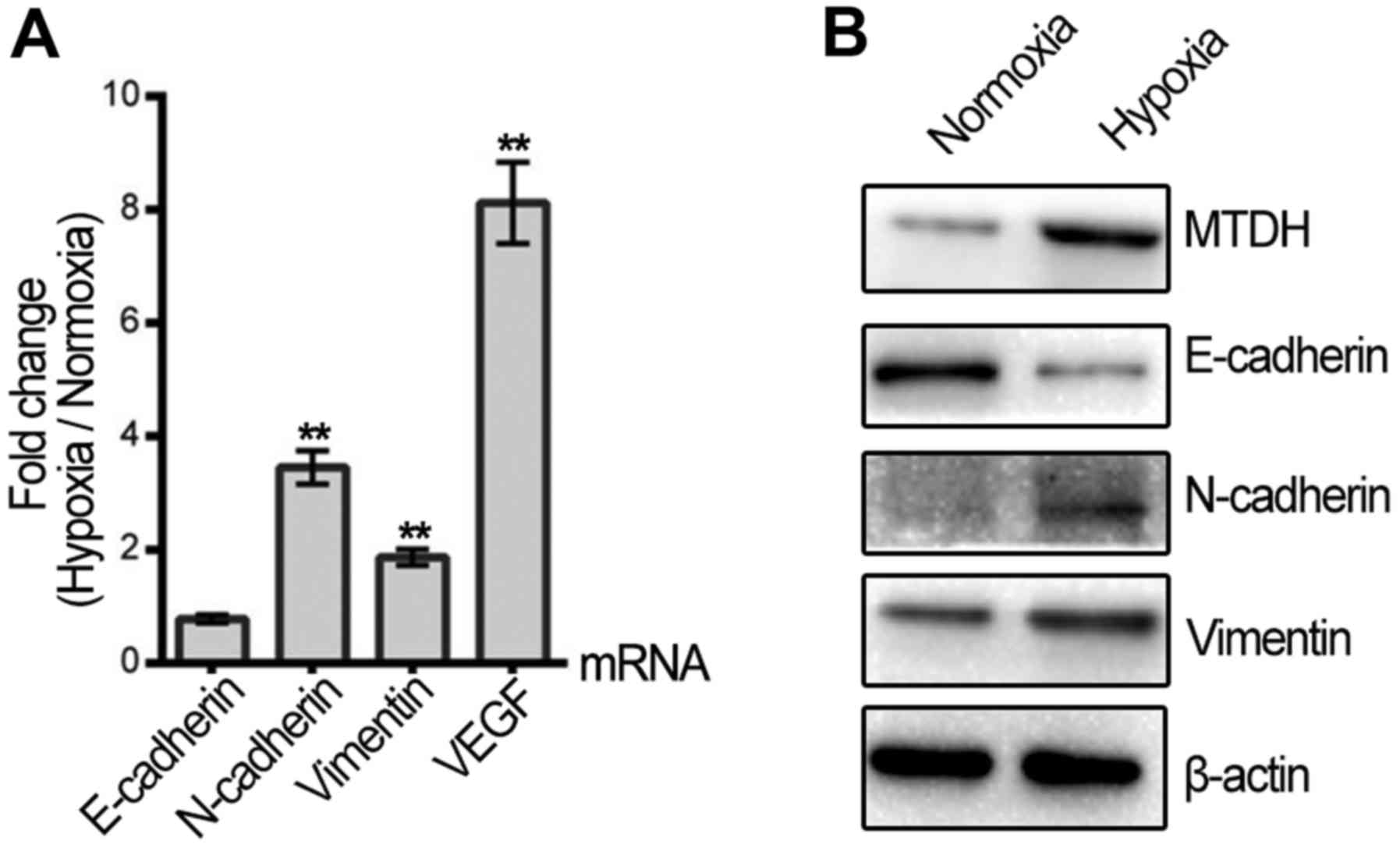
Since the migration and invasion of HNSCC cells were
increased by hypoxic treatment, qPCR and western blotting were
applied to assess whether hypoxia regulates the expression of VEGF
and EMT biomarkers, which are key molecules involved in cell
metastasis. VEGF mRNA was promoted in the hypoxic HNSCC cells. The
mRNA and protein expression levels of vimentin and N-cadherin were
upregulated, while E-cadherin protein was decreased following
hypoxic treatment (Fig. 2A and B).
Furthermore, MTDH expression was significantly elevated in the
Tu686 cells after 48 h of hypoxic treatment compared with normoxia
(Fig. 2B).
Hypoxia induces HNSCC cell metastasis
and glycolysis by regulation of MTDH
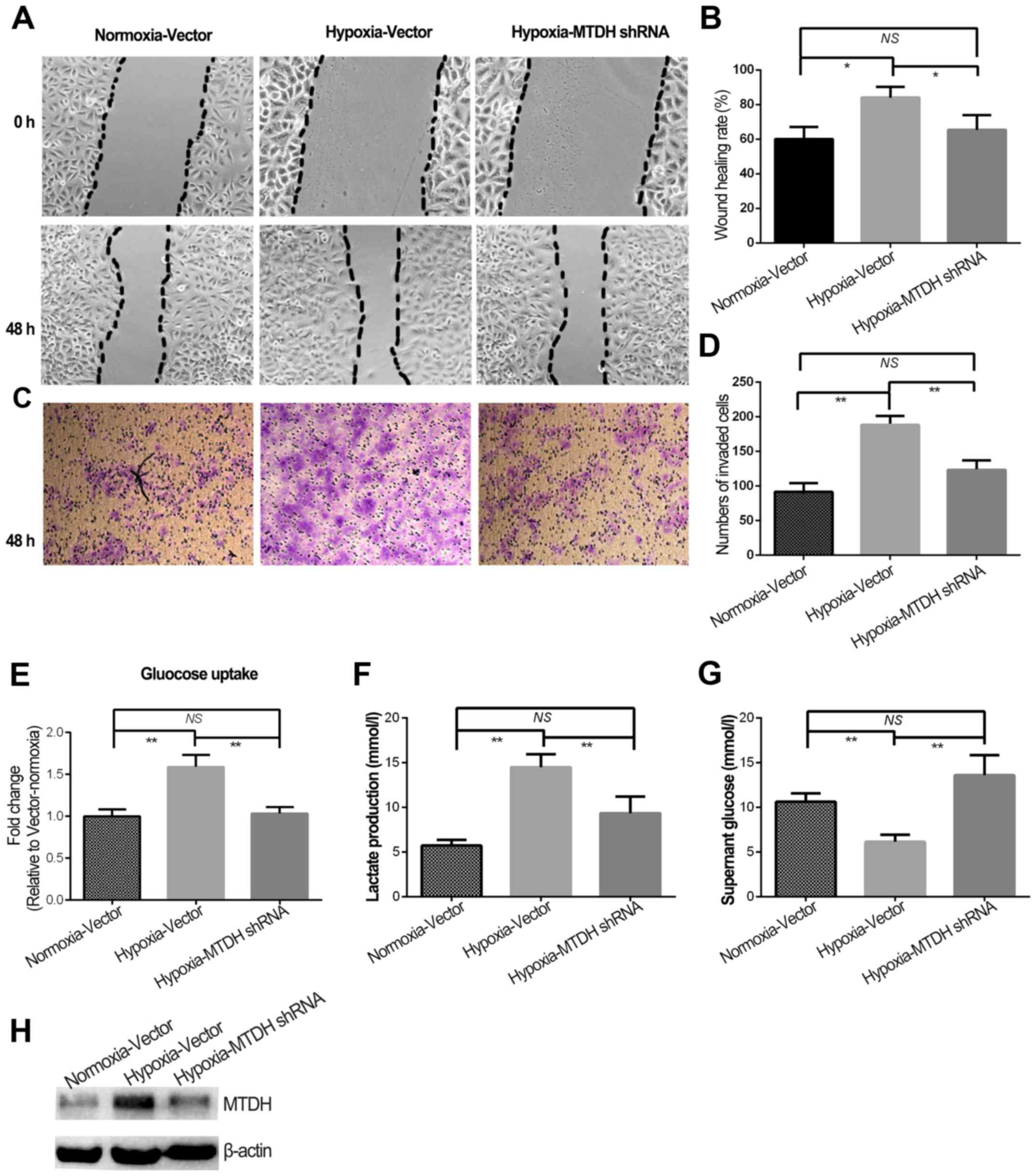
High expression of MTDH protein was previously shown
to predict poor prognosis in laryngeal squamous cell carcinoma
patients (12). Although MTDH
expression and migration/invasion were increased in HNSCC cells
treated with hypoxia, the role of MTDH in hypoxia-regulated HNSCC
metastasis remained to be uncovered. Therefore, MTDH expression was
knocked down in the HNSCC cells (Fig.
3H) and further experiments were performed. The wound healing
and invasion experiments indicated that knockdown of MTDH
expression in hypoxic cells significantly reduced cell migration
and invasion to the levels observed in normoxia (Fig. 3A-D). Additionally, glycolysis was
blocked in hypoxic cells when MTDH expression was knocked down
(P<0.01; Fig. 3E and G).
Furthermore, lactate production in hypoxic cells with MTDH
knockdown was almost reduced to the normoxic level (Fig. 3F).
Mutual regulation of MTDH and HIF-1α
in an HNSCC cell line
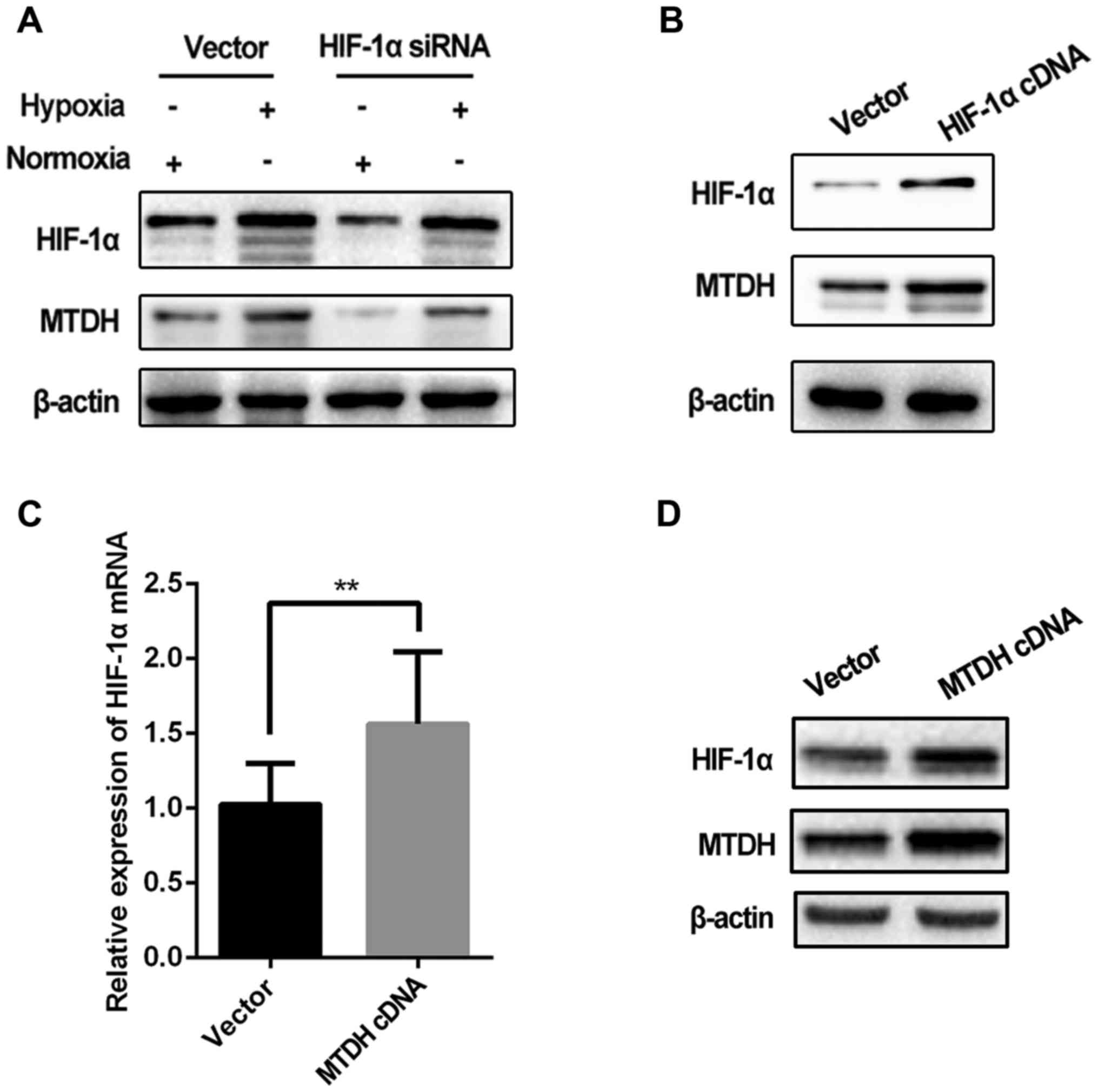
As the aforementioned results indicated that hypoxia
regulated MTDH expression, we subsequently investigated whether
HIF-1α, a classic indicator of hypoxia, regulated MTDH expression
in Tu686 cells. MTDH expression was assessed in cells transfected
with HIF-1α cDNA and siRNA.
The expression level of MTDH protein was markedly
decreased when HIF-1α was knocked down in the Tu686 cell line under
hypoxic and normoxic conditions (Fig.
4A). Furthermore, MTDH expression in hypoxic cells with HIF-1α
knockdown was reduced to a similar level as that observed in cells
cultured under normoxia (lane 1 vs. 4; Fig. 4A), suggesting that the
hypoxia-induced increase in the expression of MTDH in Tu686 cells
could be attenuated by HIF-1α knockdown. In addition, upregulation
of HIF-1α significantly increased MTDH protein expression (Fig. 4B). Notably, the expression of HIF-1α
mRNA was increased significantly in HNSCC Tu686 cells in which MTDH
was overexpressed (Fig. 4C and
D).
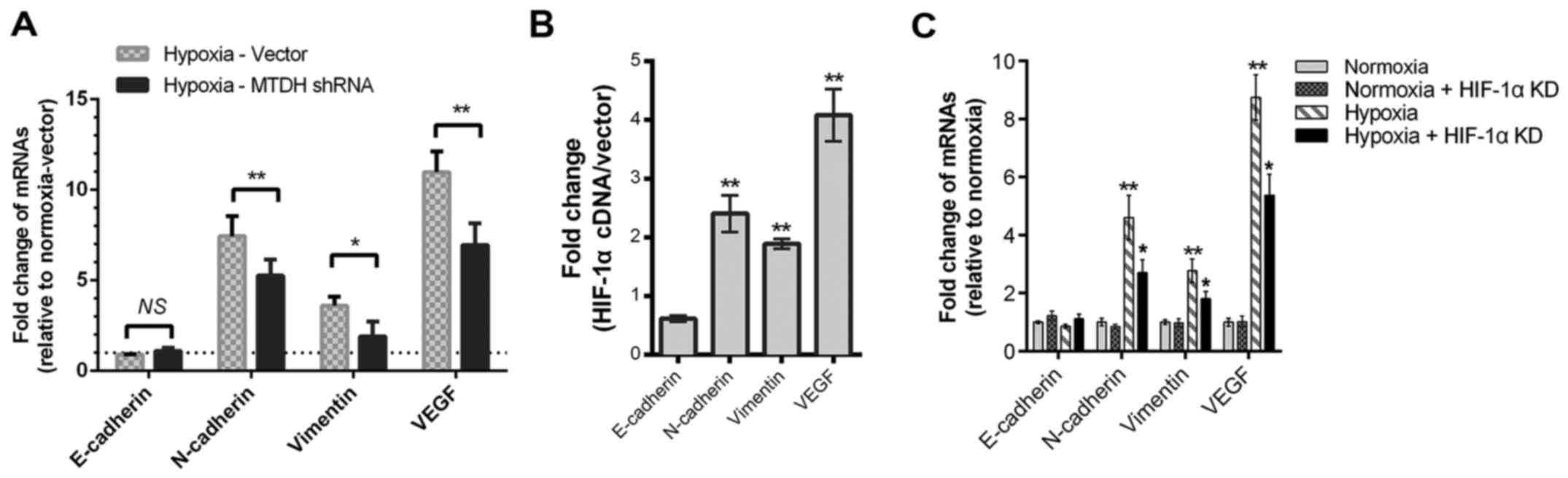
MTDH or HIF-1α knockdown partially
reverses the hypoxia-induced promotion of EMT and VEGF expression
in HNSCC cells
Our previous studies showed that MTDH regulated
HNSCC metastasis by altering the expression of EMT proteins and
VEGF in normoxic cell culture (13,14).
In the present study, compared with the plasmid vector group,
downregulation of MTDH decreases the mRNA expression levels of
vimentin, VEGF, and N-cadherin when cells were cultured under
hypoxic conditions (P<0.01; Fig.
5A). Considering the MTDH and HIF-1α loop in HNSCC, whether
HIF-1α regulated these EMT and angiogenesis biomarkers was
assessed. The mRNA levels of vimentin, N-cadherin and VEGF were
upregulated by HIF-1α cDNA overexpression (Fig. 5B). Meanwhile, decreased expression
levels of vimentin, N-cadherin and VEGF mRNA were detected when
HNSCC cells were transfected with HIF-1α siRNA (Fig. 5C).
Discussion
In the present study, we confirmed that hypoxia
induced cell metastasis, glycolysis and MTDH expression in an HNSCC
cell line. Additionally, downregulation of MTDH expression could
offset the hypoxia-induced increase in HNSCC cell metastasis and
glycolysis. We also verified that HIF-1α was crucial for the
regulation of MTDH by hypoxia. Importantly, MTDH was found to
regulate HIF-1α expression in turn. Furthermore, both MTDH and
HIF-1α could partially reverse the hypoxia-induced increases in EMT
biomarkers and VEGF expression in HNSCC cells.
A range of cellular signaling pathways, relating to
angiogenesis, autophagy, EMT and energy metabolism, are activated
in cells in order to adapt to hypoxic stress (17). Hypoxia is a hallmark of solid
cancers and promotes cell metastasis in several ways. The present
study indicated that hypoxic treatment of the Tu686 HNSCC cell line
induced migration and invasion in these cells, which is consistent
with the findings of previous studies in other cell lines (18). However, the detailed mechanism
underlying hypoxia-induced cell metastasis remains unclear. HIF-1α
is considered a representative biomarker of hypoxia, and glycolysis
is also associated with the hypoxic process. Therefore, hypoxia may
promote cell metastasis through HIF-1α-mediated transcriptional
changes and glycolysis-mediated energy changes.
When cells are cultured in an environment of 1%
O2, HIF-1α protein is stabilized by a reduction in
ubiquitination-mediated degradation (19). The present study confirmed that
HIF-1α protein, rather than mRNA, was increased in hypoxic HNSCC
cells (data not shown). HIF-1α, which is a sensitive indicator of
hypoxia, is translocated to the nucleus to regulate transcription.
Numerous target gene transcripts, including MMPs, EMT biomarkers
and VEGF, are increased in hypoxic microenvironments since they
contain hypoxia-response elements(HRE) (6).
The present study showed that hypoxia increased the
mRNA expression levels of glycolysis-related genes, such as MCT1,
MCT4, GLUT1, HK2, PGI, PGK1, ENO1, PFK2 and LDHA, in HNSCC cells
(data not shown). In addition, our data showed that hypoxia
promoted glucose uptake, lactate production and cell invasion in
HNSCC cells. In the clinical setting, glucose uptake has been used
in PET-CT imaging for assessing tumor metastasis and relapse
(20). Lactate was recently
investigated as a predictive biomarker for tumor recurrence and
poor survival time based on data from HNSCC obtained over a period
of 15 years (21). High level of
the lactate generator LDHA is an indicator of poor prognosis in
HNSCC patients (22). Glycolytic
enzymes, such as HK2, PGI, PGK1, ENO1 and PFK2, are involved in
cancer anaerobic glycolysis and metastasis (23). Elevated expression levels of MCT1/4,
a protein responsible for lactate shuttle, were detected in breast
and cervical cancer (24,25). Genetic depletion of MCT1/4 in breast
cancer impairs cell migration (26,27),
which may partially mediate the effect of glycolysis on HNSCC
metastasis.
Notably, the present study indicated that MTDH
protein expression was elevated in hypoxic HNSCC cells. MTDH is
well-documented as an oncoprotein in human malignancies, including
lung, colon, breast, live, glioma cancer and HNSCC (12,28).
Dysregulated MTDH expression levels in cancer may mediate tumor
proliferation, progression and sensitivity to chemoradiation
(11). Our previous data showed
that MTDH is an unfavorable factor for HNSCC patient survival time,
and promotes HNSCC cell metastasis and angiogenesis (12–14).
Therefore, whether MTDH is involved in hypoxia-induced metastasis
was studied. Knockdown of MTDH expression reversed the increase in
cell migration and invasion in hypoxic HNSCC cells. Considering
that hypoxia upregulates MTDH expression, this may indicate that
hypoxia-promoted HNSCC cell metastasis partially depends on MTDH
expression. Furthermore, the present study investigated the
regulation of MTDH in HNSCC cells under hypoxic conditions with or
without HIF-1α mediation. This demonstrated that the induction of
MTDH in HNSCC depends on HIF-1α protein expression, which is
consistent with a previous study in glioblastoma (15). The mechanism by which HIF-1α
regulates MTDH is not clear yet, although there are two hypotheses
to explain this regulation. The first is that, since MTDH contains
three HRE sites in its MTDH promoter (as identified through the web
tool), HIF-1α may promote MTDH transcription directly. The second
is that HIF-1α may increase MTDH expression indirectly via
activation of c-Myc transcription (29,30).
The multifaceted roles of MTDH in cancer are due to
the diverse downstream cellular signals activated, including the
PI3K/AKT, NF-κB, Wnt/β-catenin and MAPK pathways (31). Emerging reports have indicated that
genomic amplification (8q22 gain), transcriptional regulation
(Ha-Ras/PI3K/c-Myc), and post-transcriptional and translational
regulation (miRNA, CPEB1, Hbx and mono-ubiquitination) are involved
in the mechanism of MTDH regulation in cancer (11). Furthermore, PI3K activation is
crucial for protecting HIF-1α from degradation (32). Considering that elevated MTDH
expression has been shown to increase HNSCC cell metastasis through
activating the PI3K/AKT pathway (13), whether MTDH regulated HIF-1α in
HNSCC remained unclear. The present study indicated that knockdown
of MTDH in hypoxic HNSCC cells reduced cell invasion and migration
to a level similar to that of normoxia-cultured HNSCC cells,
implying that MTDH plays a vital role in hypoxic cell progression.
Our results also indicated that MTDH regulated HIF-1α expression in
HNSCC. When cells are subjected to hypoxic stress, HIF-1α may be
activated to allow adaption to a different mode of energy
generation by inducing the transcription of glycolytic genes. The
glucose uptake and lactate production assays revealed that
glycolysis was significantly decreased in hypoxic cells when MTDH
was downregulated. Thus, the present study indicated that MTDH may
regulate both HIF-1α and HIF-1α-induced glycolysis in HNSCC cells
with or without hypoxic stress. Our unpublished data showed that
the NF-κB pathway is also involved in MTDH-induced mRNA/protein
expression changes and cell metastasis. Given that HIF-1α is one of
the target genes transcriptionally regulated by NF-κB (33), MTDH may also regulate HIF-1α via the
NF-κB pathway in HNSCC. The possible involvement of NF-κB in MTDH
regulation of HIF-1α requires further studies to confirm.
The mutual regulation of MTDH and HIF-1α identified
in the present study may indicate a feedback loop connection
between MTDH and HIF-1α in HNSCC. Additionally, it is possible that
the intermodulation of MTDH and HIF-1α may affect cell metastasis
and EMT together. The present investigation indicated that EMT
biomarkers and cell metastasis were enhanced significantly in
hypoxic HNSCC cells. When MTDH shRNA was transfected into hypoxic
cells (and HIF-1α was also activated), EMT biomarker expression and
cell metastasis were reduced, suggesting that disruption of the
MTDH-HIF-1α loop connection inhibited HNSCC cell metastasis and
increased its resistance to EMT. There are two possible mechanisms:
HIF-1α may activate the EMT transcription factor Twist in hypoxic
HNSCC cells (34), or MTDH may
mediate the epigenetic regulation of Twist in cancer cells
(35). There may be multiple links
between MTDH, HIF-1α and Twist in HNSCC. The current results
indicated that decreased MTDH reversed the promotion of cell
metastasis by hypoxia, which may imply that hypoxia-regulated HNSCC
cell metastasis depends on MTDH expression. However, downregulation
of MTDH and HIF-1α partially reversed the induction of EMT
biomarkers by hypoxia, which may indicate that hypoxia promoted
HNSCC EMT via a complex pathway of mediators other than MTDH and
HIF-1α.
Considering the aforementioned findings in HNSCC,
there may be a loop connection between MTDH and HIF-1α in HNSCC
cells, and hypoxia may promote HNSCC cell glycolysis and metastasis
via this loop. If cells grow at a sufficient distance from the
oxygen-delivering vasculature within HNSCC tumors, adaption to
hypoxic stress occurs, resulting in the nuclear translocation of
HIF-1α to promote the transcription of glycolytic genes and
oncogenes, including LDHA and MTDH, to accelerate energy synthesis
and progression, respectively. MTDH activates the PI3K and NF-κB
pathways, which may increase HIF-1α expression in turn and further
enhance the aforementioned process. The present study also found
that HNSCC cell migration/invasion and glycolysis in hypoxia were
suppressed when this positive feedback was disrupted by knockdown
of MTDH expression. Thus, targeting MTDH in HNSCC may be a
promising direction for improving the survival time of HNSCC
patients, as MTDH reduced the malignant activity of HNSCC cells in
normoxia and hypoxia. In summary, hypoxia promotes HNSCC cell
migration/invasion and glycolysis by mediating a HIF-1α-MTDH
feedback loop. These findings implicate HIF-1α-MTDH as a promising
target for anticancer drugs in solid tumors, and help to explain
the pro-tumorigenic and unfavorable effect of MTDH in HNSCC as
reported in our previous study.
Acknowledgements
We thank all our laboratory members for their
insightful suggestions in discussion. The present study was funded
by grants from the National Natural Science Foundation of China
(nos. 81602389, 81472696, 81202128 and 81272974), and the Natural
Science Foundation of Hunan Province (nos. 2017JJ3456 and
2015JJ3137).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mamelle G, Pampurik J, Luboinski B, Lancar
R, Lusinchi A and Bosq J: Lymph node prognostic factors in head and
neck squamous cell carcinomas. Am J Surg. 168:494–498. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rankin EB and Giaccia AJ: Hypoxic control
of metastasis. Science. 352:175–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palazon A, Goldrath AW, Nizet V and
Johnson RS: HIF transcription factors, inflammation, and immunity.
Immunity. 41:518–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Shen SM, Zhao XY and Chen GQ:
Targeted genes and interacting proteins of hypoxia inducible
factor-1. Int J Biochem Mol Biol. 3:165–178. 2012.PubMed/NCBI
|
|
8
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, Tan M and Cai Q: The Warburg effect
in tumor progression: Mitochondrial oxidative metabolism as an
anti-metastasis mechanism. Cancer Lett. 356:156–164. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verdone JE, Zarif JC and Pienta KJ:
Aerobic glycolysis, motility, and cytoskeletal remodeling. Cell
Cycle. 14:169–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emdad L, Das SK, Hu B, Kegelman T, Kang
DC, Lee SG, Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC: A promiscuous
protein partner critical in cancer, obesity, and CNS diseases. Adv
Cancer Res. 131:97–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Su Z, Li G, Yu C, Ren S, Huang D,
Fan S, Tian Y, Zhang X and Qiu Y: Increased expression of
metadherin protein predicts worse disease-free and overall survival
in laryngeal squamous cell carcinoma. Int J Cancer. 133:671–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signalling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu GC, Yu CY, She L, Tan HL, Li G, Ren
SL, Su ZW, Wei M, Huang DH, Tian YQ, et al: Metadherin regulation
of vascular endothelial growth factor expression is dependent upon
the PI3K/Akt pathway in squamous cell carcinoma of the head and
neck. Medicine. 94:e5022015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noch E, Bookland M and Khalili K:
Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose
deprivation in glioblastoma. Cancer Biol Ther. 11:32–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu G, Cai G, Liu Y, Tan H, Yu C, Huang M,
Wei M, Li S, Cui X, Huang D, et al: Quantitative iTRAQ LC-MS/MS
proteomics reveals transcription factor crosstalk and regulatory
networks in hypopharyngeal squamous cell carcinoma. J Cancer.
5:525–536. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan Y, Li X, You B, Shi S, Zhang Q and
You Y: MicroRNA-338 inhibits migration and proliferation by
targeting hypoxia-induced factor 1α in nasopharyngeal carcinoma.
Oncol Rep. 34:1943–1952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yee Koh M, Spivak-Kroizman TR and Powis G:
HIF-1 regulation: Not so easy come, easy go. Trends Biochem Sci.
33:526–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta T, Master Z, Kannan S, Agarwal JP,
Ghsoh-Laskar S, Rangarajan V, Murthy V and Budrukkar A: Diagnostic
performance of post-treatment FDG PET or FDG PET/CT imaging in head
and neck cancer: A systematic review and meta-analysis. Eur J Nucl
Med Mol Imaging. 38:2083–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blatt S, Voelxen N, Sagheb K, Pabst AM,
Walenta S, Schroeder T, Mueller-Klieser W and Ziebart T: Lactate as
a predictive marker for tumor recurrence in patients with head and
neck squamous cell carcinoma (HNSCC) post radiation: A prospective
study over 15 years. Clin Oral Investig. 20:2097–2104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koukourakis MI, Giatromanolaki A, Winter
S, Leek R, Sivridis E and Harris AL: Lactate dehydrogenase 5
expression in squamous cell head and neck cancer relates to
prognosis following radical or postoperative radiotherapy.
Oncology. 77:285–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Payen VL, Porporato PE, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 1: Tumor pH, glycolysis and the pentose phosphate pathway.
Cell Mol Life Sci. 73:1333–1348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pinheiro C, Longatto-Filho A, Ferreira L,
Pereira SM, Etlinger D, Moreira MA, Jubé LF, Queiroz GS, Schmitt F
and Baltazar F: Increasing expression of monocarboxylate
transporters 1 and 4 along progression to invasive cervical
carcinoma. Int J Gynecol Pathol. 27:568–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levenson AS, Thurn KE, Simons LA,
Veliceasa D, Jarrett J, Osipo C, Jordan VC, Volpert OV, Satcher RL
Jr and Gartenhaus RB: MCT-1 oncogene contributes to increased in
vivo tumorigenicity of MCF7 cells by promotion of angiogenesis and
inhibition of apoptosis. Cancer Res. 65:10651–10656. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallagher SM, Castorino JJ, Wang D and
Philp NJ: Monocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231. Cancer Res. 67:4182–4189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee S-G, Kang D-C, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Podar K and Anderson KC: A therapeutic
role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell
Cycle. 9:1722–1728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SG, Su ZZ, Emdad L, Sarkar D and
Fisher PB: Astrocyte elevated gene-1 (AEG-1) is a target gene of
oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc.
Proc Natl Acad Sci USA. 103:pp. 17390–17395. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emdad L, Das SK, Dasgupta S, Hu B, Sarkar
D and Fisher PB: AEG-1/MTDH/LYRIC: Signaling pathways, downstream
genes, interacting proteins, and regulation of tumor angiogenesis.
Adv Cancer Res. 120:75–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mottet D, Dumont V, Deccache Y, Demazy C,
Ninane N, Raes M and Michiels C: Regulation of hypoxia-inducible
factor-1alpha protein level during hypoxic conditions by the
phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta
pathway in HepG2 cells. J Biol Chem. 278:31277–31285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rius J, Guma M, Schachtrup C, Akassoglou
K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG and Karin M:
NF-kappaB links innate immunity to the hypoxic response through
transcriptional regulation of HIF-1alpha. Nature. 453:807–811.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Hu J, Li J, Liu Y, Yu J, Zhuang
X, Mu L, Kong X, Hong D, Yang Q, et al: Epigenetic activation of
TWIST1 by MTDH promotes cancer stem-like cell traits in breast
cancer. Cancer Res. 75:3672–3680. 2015. View Article : Google Scholar : PubMed/NCBI
|