Introduction
Hepatocellular carcinoma (HCC) is a common malignant
neoplasm and the fourth leading cause of cancer-related mortality
in China (1). HCC is the dominant
form of primary liver cancer in patients with chronic liver disease
and cirrhosis (2). Patients with
early-stage HCC can be treated with surgery to remove part of the
liver can undergo or transplantation, which contributes toward a
5-year survival rate of >70% (3). However, for the majority of patients
with advanced-stage HCC whose disease is too advanced for them to
undergo surgical treatment, the 5-year survival rate is extremely
low at ~15% (4). Therefore,
improving understanding of the molecular mechanism involved in HCC
progression and identifying novel approaches to inhibit HCC
carcinogenesis are important.
Over recent decades, natural products have served
significant roles in the development of anticancer agents (5,6).
Resveratrol (trans-3,4′,5-trihydroxystilbene) was originally
isolated from the roots of white hellebore at 1940 (7). Resveratrol is often used in
traditional Chinese medicine to treat skin inflammations,
cardiovascular and liver diseases (8,9).
Resveratrol exerted a variety of biological effects, including
antioxidation, antiproliferation and chemopreventative effects
(10,11). Recently, accumulating evidence has
indicated that resveratrol also exerts antitumor effects by
inhibiting proliferation while inducing apoptosis and autophagy in
tumor cells (12,13). For instance, Szekeres et al
(9) reported that resveratrol
induced cell cycle arrest, apoptosis and autophagy in T-cell acute
lymphoblastic leukemia cells through inhibiting the protein kinase
B (Akt)/mechanistic target of rapamycin (mTOR)/p70S6K/4E-BP1 and
activating p38-mitogen-activated protein kinase signaling pathways.
Fabre et al (10) indicated
that resveratrol could induce autophagic and apoptotic death in
drug-resistant oral cancer cells and may become a novel approach
for the treatment of oral cancer in the near future (14). However, the antitumor effect of
resveratrol in the tumor progression of HCC remains unclear and
requires further investigation.
Autophagy is a highly conserved catabolic mechanism
in eukaryotes in which intracellular contents, including large
protein complexes and dysfunctional organelles, are transported to
lysosomes for degradation and reuse (15). Autophagy can prolong the survival of
cancer cells and enhance their resistance to apoptosis, and
paradoxically, defective autophagy has been associated with
increased tumorigenesis, but the mechanism underpinning this
remains unclear (16).
The present study aimed to investigate the antitumor
effect of resveratrol in HCC. Resveratrol treatment inhibited cell
viability, proliferation, invasion and migration of HCC cells
through inducing autophagy via regulating p53, phosphoinositide
3-kinase (PI3K)/Akt. The results of the present study suggested
that combined treatment with resveratrol and autophagy inducer may
serve as effective agents against HCC.
Materials and methods
Reagents and antibodies
Resveratrol, 3-methyladenine (3-MA) and pifithrin-α
were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany),
and were dissolved in dimethyl sulfoxide. Insulin-like growth
factor-1 (IGF-1) was purchased from PeproTech, Inc. (Rocky Hill,
NJ, USA). Primary antibodies against Beclin1 (1:1,000; cat. no.
3738), LC3 I/II (1:1,000; cat. no. 12741), p62 (1:1,000; cat. no.
88588), p53 (1:1,000; cat. no. 2524), p-Akt (1:2,000; cat. no.
4060), Akt (1:1,000; cat. no. 4685) and GAPDH (1:1,000; cat. no.
5174), as well as horseradish-peroxidase (HRP)-conjugated secondary
anti-rabbit (1:1,000; cat. no. 4877) and anti-mouse antibodies
(1:1,000; cat. no. 4874) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell culture
The HCC MHCC97-H cell line with wild-type p53 gene
was purchased from Shanghai Institute of Biotechnology and Cell
Biology of the Chinese Academy of Sciences (Shanghai, China). Cells
were cultured in RPMI-1640 complete culture medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
FBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100
µg/ml streptomycin and 100 U/ml penicillin in a humidified
atmosphere of 5% CO2 at 37°C.
Cell viability assay
MHCC-97H cells (5×103) were seeded into
96-well plates and treated with different concentrations of
resveratrol (0–200 µM) for 24 or 48 h. The purple formazan was
dissolved in dimethyl sulfoxide. Cell viability was measured by MTT
assay at 450 nm, according to the manufacturer's protocol. All
experiments were performed in triplicate and repeated at least
twice.
Cell invasion assay
The Transwell invasion chambers coated with Matrigel
(1 mg/ml) (BD Biosciences, Franklin Lakes, NJ, USA) were used to
perform an invasion assay of MHCC-97H cells. Briefly, 100 µl
serum-free Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) containing 1×105 cells/well was
added into the upper chamber, while the lower chamber contained 600
µl DMEM supplemented with FBS (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS. After 24 h of incubation, the cells in
the upper chamber were removed and the cells that had invaded
through the Matrigel matrix were fixed in 95% ethanol overnight at
4°C and stained with hematoxylin at 4°C for 2 h. The cell numbers
were counted and images were captured under an inverted microscope
(magnification, ×400; Olympus Corporation, Tokyo, Japan) on 5
randomly selected fields in each well.
Wound healing assay
Cell migration ability was determined using a wound
healing assay. Cells (1.5×106 cells/well) were seeded
into 6-well plates and cultured overnight at 37°C until the cells
reached 90% confluence. Next, a sterile pipette tip was used to
create a straight scratch. The destroyed cells were gently rinsed
off with PBS 3 times and cultured in MCDB153 medium (Sigma-Aldrich;
Merck KGaA) for another 24 h. Cell migration was observed and
images were captured under a confocal microscope (magnification,
×200; NIKON A1; Nikon Corporation, Tokyo, Japan) at 0 and 24 h.
Western blot analysis
MHCC-97H cells in different groups were lysed and
the protein was extracted using radioimmunoprecipitation assay
buffer (10X; Cell Signaling Technology, Inc.), containing 1%
phenylmethylsulfonyl fluoride. The extracted protein was determined
using a bicinchoninic acid protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Equal amounts
of proteins (20 µg) from each sample were separated by 5% SDS-PAGE,
prior to being transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Following blocking with 5%
skimmed milk at room temperature for 2 h, the membranes were
incubated with the aforementioned primary antibodies [diluted in
0.1 mmol/l PBS (PH 7.4) + 1% BSA + 0.1%
Na2N3] at 4°C overnight and the corresponding
HRP-conjugated secondary antibodies at room temperature for 1 h.
The signal was detected using an enhanced chemiluminescence
commercial kit (GE Healthcare, Chicago, IL, USA) according to the
manufacturer's protocol. Analysis was performed using ImageJ
software (version 1.48; National Institutes of Health, Bethesda,
MD, USA).
Analysis of autophagy
MHCC-97H cells were transfected with green
fluorescent protein (GFP)-LC3 plasmid (Invitrogen; Thermo Fisher
Scientific, Inc.) using Lipofectamine 3000™ (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following transfection for 48 h, cells were treated with different
concentrations of resveratrol, the intensity of GFP-LC3
fluorescence in cells was determined under a fluorescence
microscope (Olympus Corporation). Cells with >5 puncta were
considered to have accumulated autophagosomes.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay was used to
perform a cell proliferation assay. Cells were seeded into a
96-well plate with 2×103 cells/well in triplicate. CCK-8
reagent was added to each well (5 mg/ml) at indicated time points
and then incubated in the dark at 37°C for 2 h. Absorbance was
determined at a wavelength of 450 nm. All the experiments were
repeated at least 3 times.
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used
for analysis. Paired Student's t-tests were used to compare means
of two groups and one-way analysis of variance, followed by
Bonferroni's post hoc test, was used to compare means of multiple
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
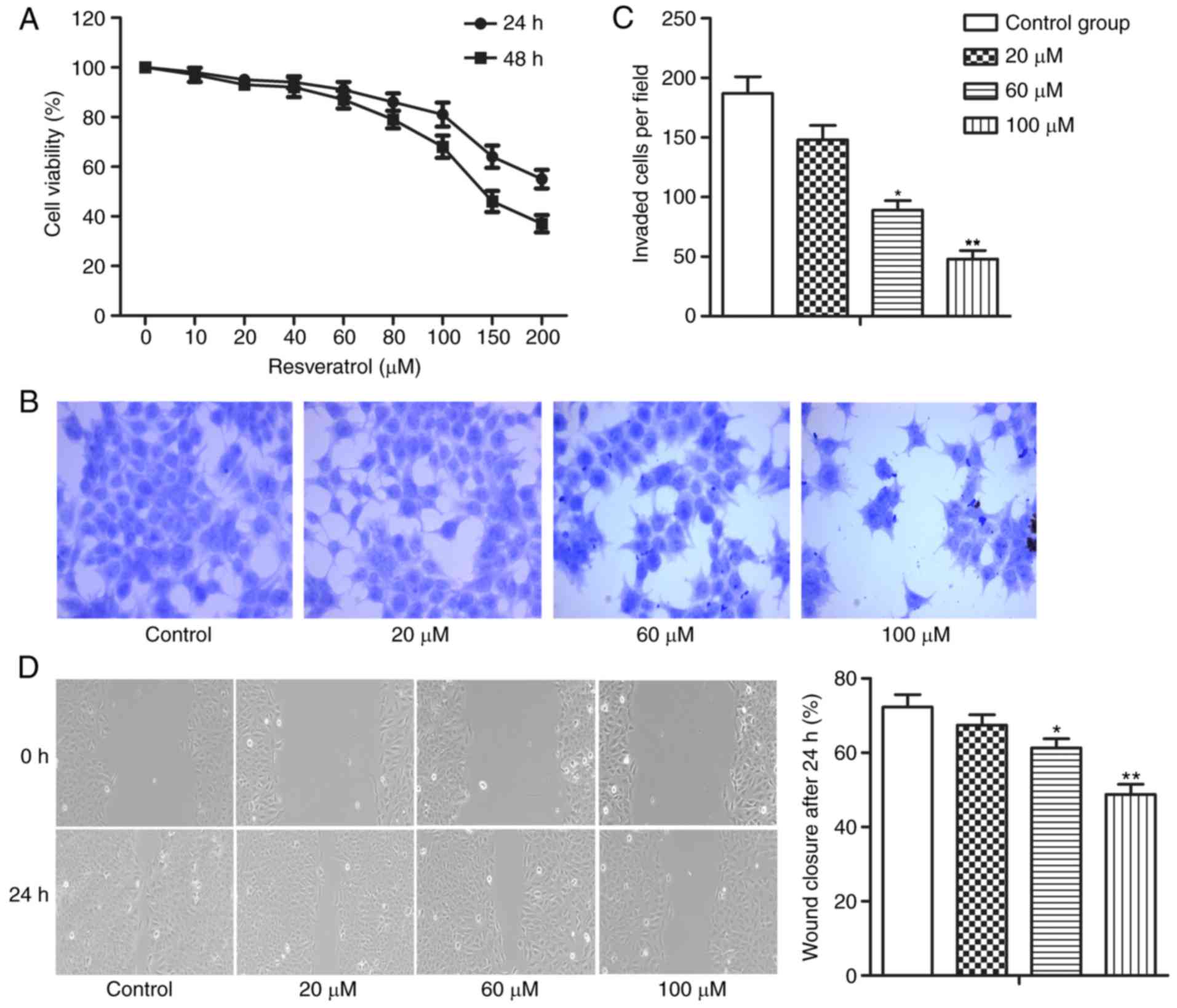
Resveratrol inhibits the viability,
invasion and migration of HCC cells
To begin with, the present study investigated the
potential therapeutic effect of resveratrol in the treatment of
HCC. It was revealed that resveratrol treatment suppressed the
viability of MHCC-97H cells in a dose- and time-dependent manner
(Fig. 1A). Next, the invasion and
migration abilities of MHCC-97H cells treated with different
concentrations of resveratrol (0, 20, 60 or 100 µM) were compared
in the present study. The results demonstrated that resveratrol
dose-dependently inhibited the invasion and migration abilities of
MHCC-97H cells (Fig. 1B-D;
*P<0.05 and **P<0.01). These results indicated that
resveratrol exerted antitumor effects in HCC through suppressing
the viability, invasion and migration of HCC cells.
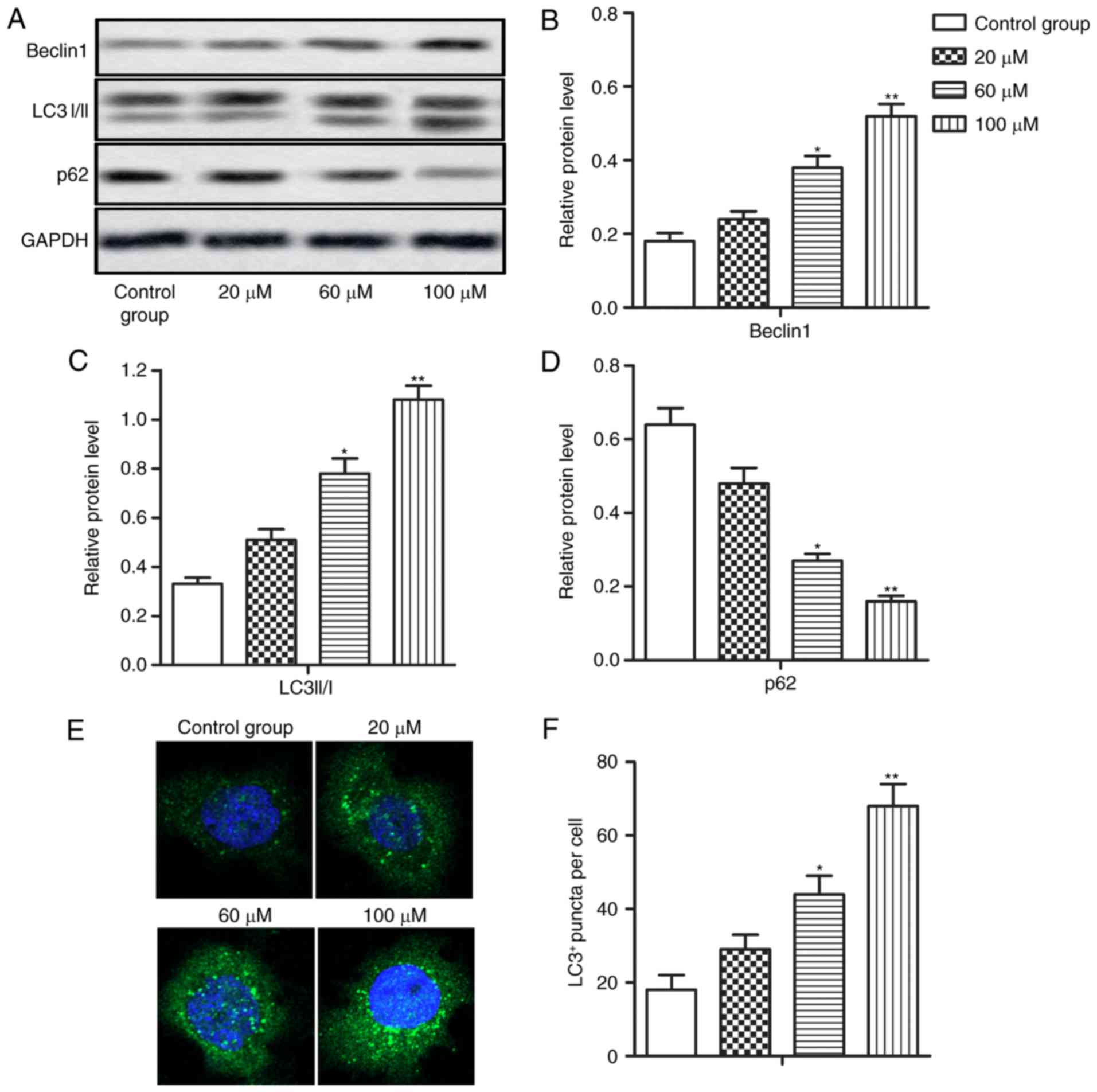
Resveratrol induces autophagy in HCC
cells
As resveratrol can act as an autophagy inducer
during tumor progression, the present study investigated whether
resveratrol induced autophagy in HCC cells. The relative expression
of Beclin1 and LC3 II/I, two well-validated biomarkers of
autophagy, was increased while the expression of p62, which can
clear autophagosomes, was significantly decreased by resveratrol in
a dose-dependent manner (Fig. 2A-D;
*P<0.05 and **P<0.01). Furthermore, LC3+ puncta
formation, which represented autophagosome formation, was markedly
dose-dependently upregulated by resveratrol treatment (Fig. 2E and F; *P<0.05 and **P<0.01).
The aforementioned results suggested that resveratrol can induce
autophagy in HCC cells.
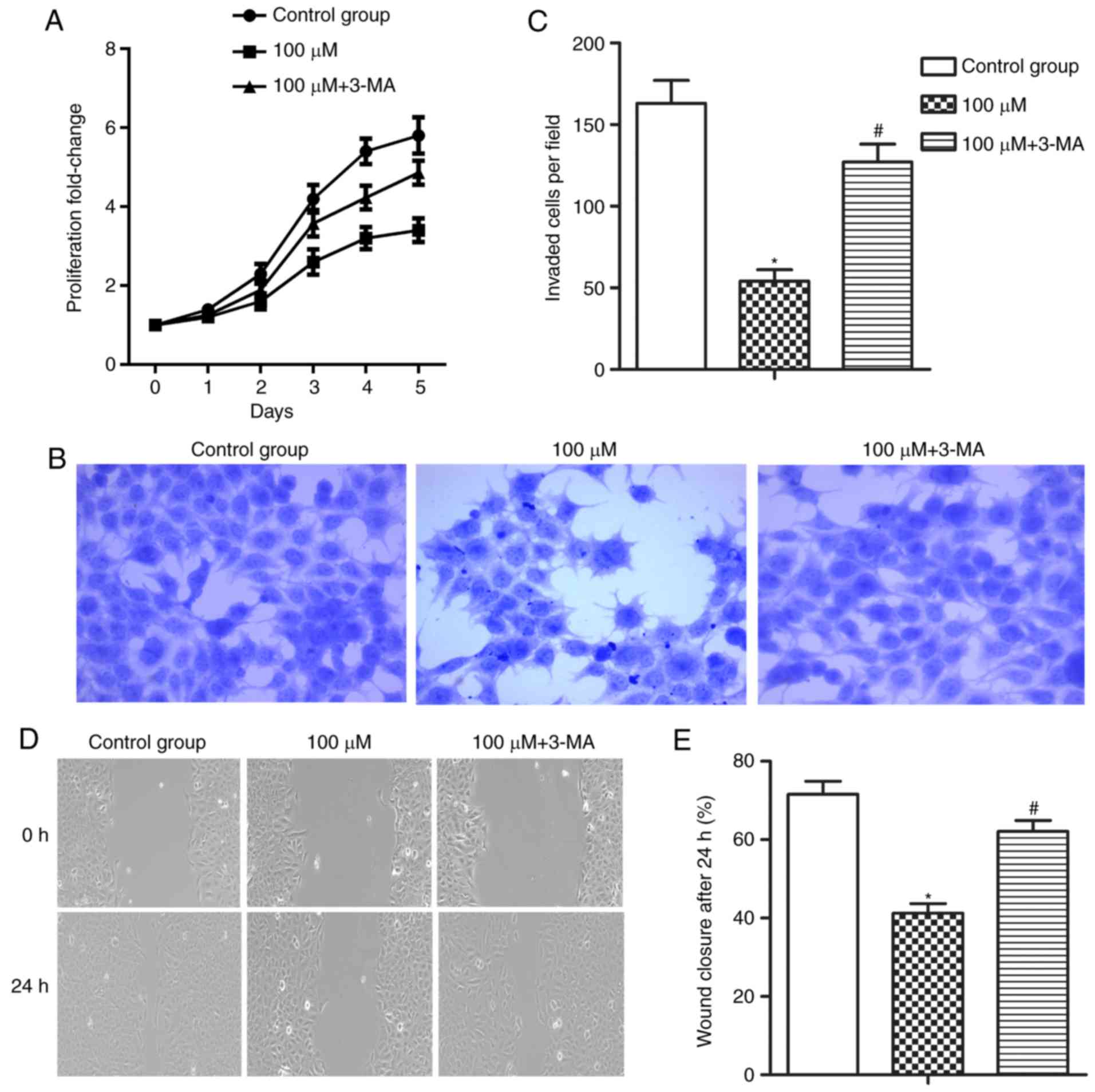
Autophagy inhibitor 3-MA counteracts
the effect of resveratrol
The present study further investigated the effect of
autophagy on the antitumor effect of resveratrol in HCC cells. 3-MA
(10 mmol/l) was used to inhibit resveratrol-induced autophagy in
MHCC-97H cells. The results demonstrated that suppressing
resveratrol-induced autophagy by 3-MA significantly promoted the
proliferation, invasion and migration abilities of MHCC-97H cells
compared with the 100 µM resveratrol group (Fig. 3A-E; *P<0.05 and
#P<0.05). The results elucidated that suppressing
autophagy counteracted the inhibitory effect of resveratrol on the
proliferation, invasion and migration abilities of HCC cells.
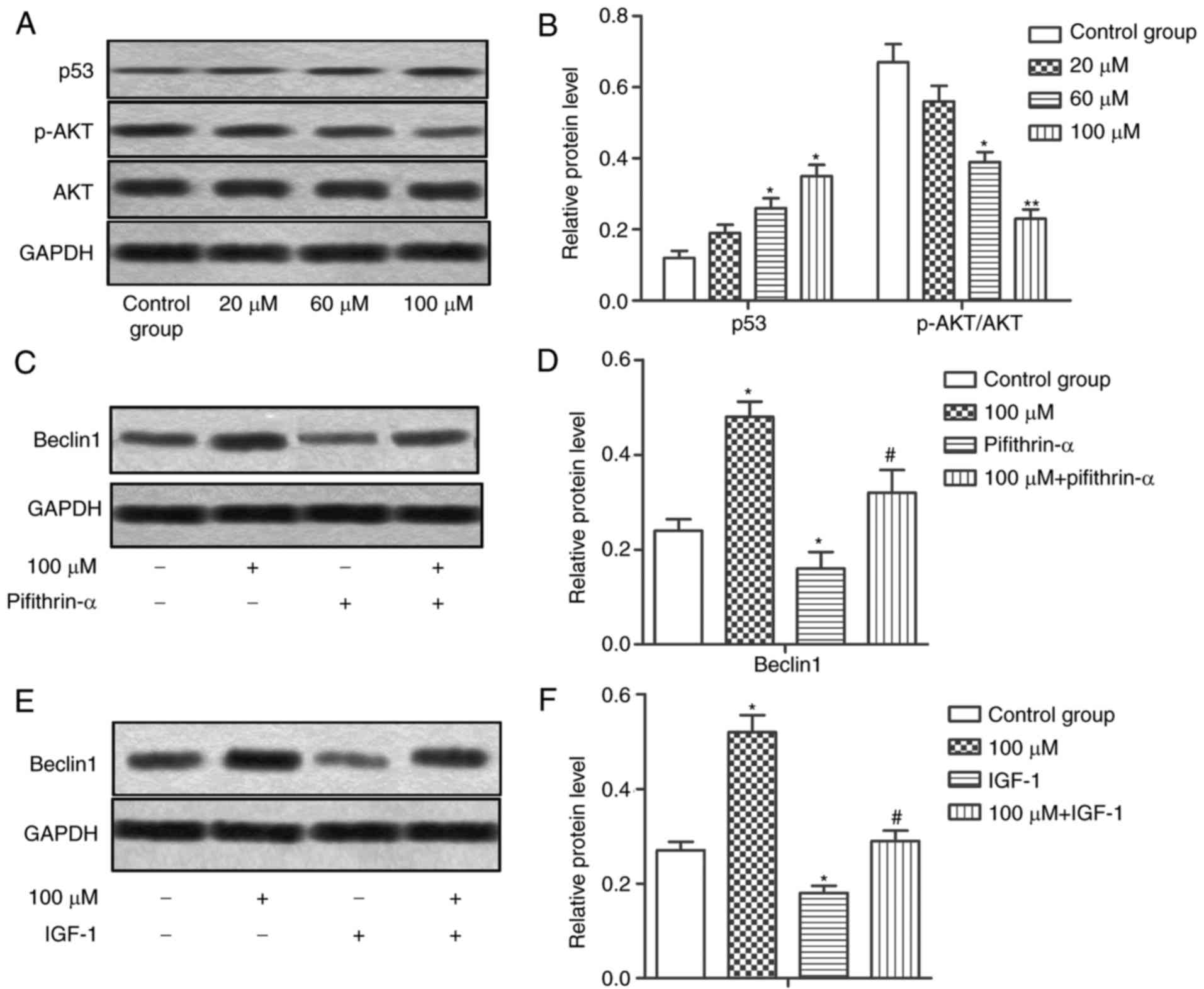
Resveratrol induces autophagy through
regulating the p53 and PI3K/Akt pathways
Resveratrol was reported to exert its antitumor
effects through the p53 or PI3K/Akt pathways (17,18).
To investigate whether these signaling pathways were also involved
in resveratrol-induced autophagy in HCC cells, western blot
analysis was performed to evaluate the expression of p53, p-Akt and
Akt. It was revealed that resveratrol upregulated the expression of
p53 while decreasing the ratio of p-Akt/Akt in HCC cells
dose-dependently, indicating that resveratrol activates p53 while
suppressing the PI3K/Akt pathway in HCC cells (Fig. 4A and B; *P<0.05 and **P<0.01).
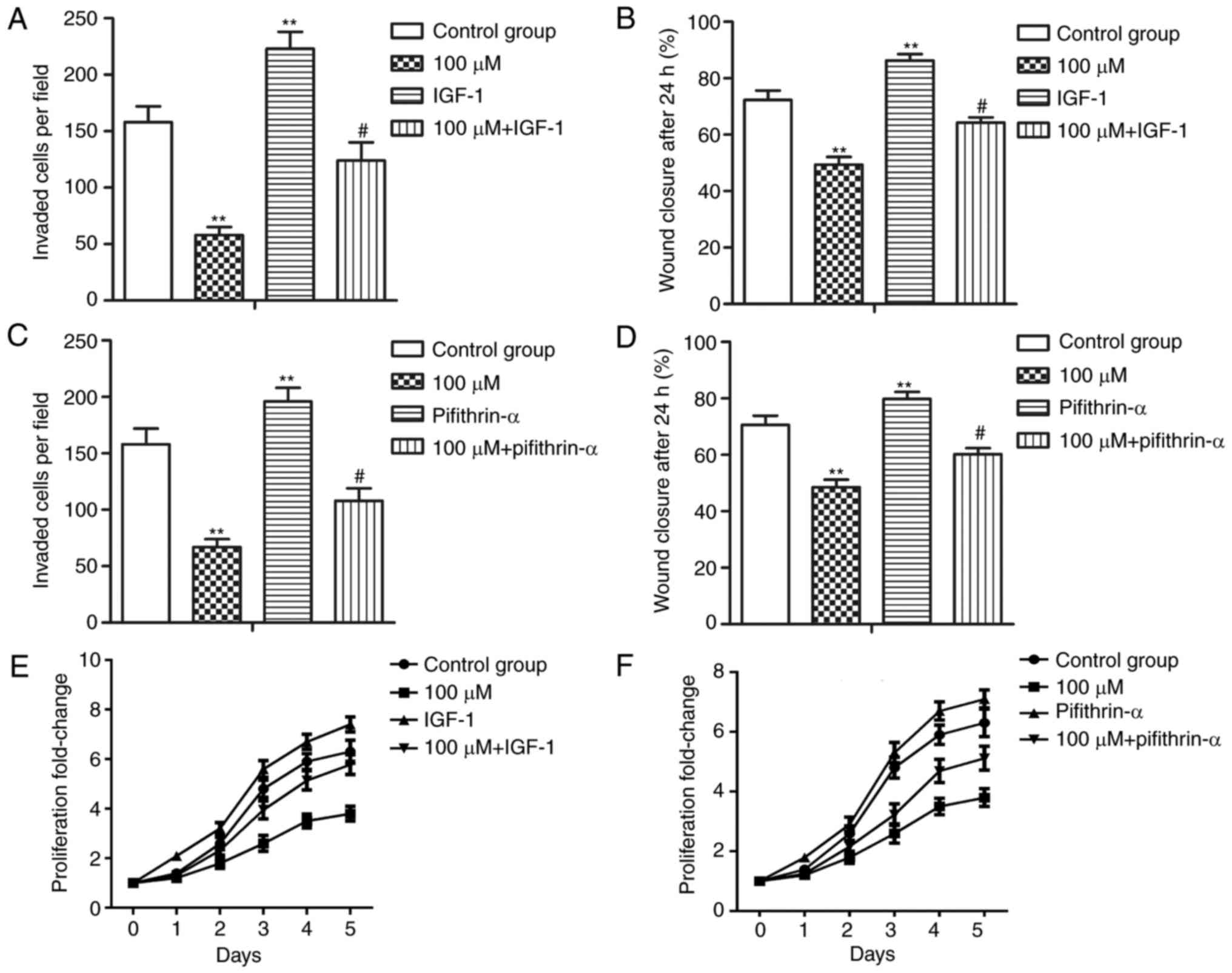
P53 inhibitor pifithrin-α (30 µM) and Akt activator IGF-1 (50
ng/ml) were used to treat MHCC-97H cells. The results demonstrated
that pifithrin-α and IGF-1 significantly decreased the expression
of Beclin1, suggesting that inhibiting p53 or activating PI3K/Akt
suppressed resveratrol-induced autophagy in HCC cells (Fig. 4C-F; *P<0.05 and
#P<0.05). In summary, the results of the present
study indicated that resveratrol induced autophagy through
activating p53 while suppressing the PI3K/Akt pathway in HCC
cells.
Pifithrin-α and IGF-1 counteract the
inhibitory effect of resveratrol on proliferation, invasion and
migration of HCC cells
The present study then investigated the effects of
pifithrin-α and IGF-1 on the motility and proliferation of HCC
cells. Pifithrin-α and IGF-1 treatment inhibited the invasion,
migration and proliferation abilities of MHCC-97H cells compared
with the 100 µM resveratrol group (Fig.
5A-F; **P<0.01 and #P<0.05). The results of
the present study suggested that pifithrin-α and IGF-1 counteracted
the inhibitory effect of resveratrol on the proliferation, invasion
and migration of HCC cells.
Discussion
Resveratrol, as a natural product compound, has been
demonstrated to have multiple potential chemoprotective activities
in various types of cells and animal models (19,20).
In addition to antioxidation and chemopreventive effects,
resveratrol has also been demonstrated to exert antitumor effects
through suppressing proliferation, and inducing apoptosis and
autophagy in a variety of cancer cells (21). However, the mechanism of the
antitumor effect of resveratrol in HCC remained unclear.
The present study was designed to investigate the
antitumor effect of resveratrol in HCC and its potential mechanism.
The results demonstrated that resveratrol treatment inhibited cell
viability, proliferation, invasion and migration through inducing
autophagy in HCC cells via activating p53 while suppressing
PI3K/Akt pathway in a time- and dose-dependent manner.
Numerous studies have reported that resveratrol
inhibited cell proliferation and suppressed cell mobility in
various types of cancer cells. For instance, Chai et al
(18) reported that resveratrol
induced mitochondrial apoptosis and decreased cell migration,
invasion and epithelial-mesenchymal transition (EMT)-inducing
transcription factor in oral squamous cell carcinoma cells.
Harikumar et al (19)
indicated that resveratrol suppressed invasion, colony forming
capacity and cell proliferation in colorectal cancer cells by
modulation of focal adhesion molecules. The inhibitory effects of
resveratrol on the adhesion, migration and invasion had also been
observed in human bladder cancer cells (22). In agreement with the results of
these studies, it was observed that resveratrol inhibited cell
proliferation, invasion and migration in HCC cells in a time- and
dose-dependent manner, suggesting that resveratrol exerted an
antitumor effect in HCC.
Autophagy is a major degradation system that
promotes the lysosomal digestion of organelles and cytoplasmic
components (23). The role of
autophagy in cancer cells is diverse: On the one hand, autophagy
can promote cancer progression through driving cell metabolism
(24); on the other hand, autophagy
itself can induce cell death, which is known as autophagic cell
death and induction of autophagy is also reported to facilitate the
activation of apoptosis (25,26).
Although the relevance of autophagy with therapeutic efficacies of
antitumor agents has been discussed, the conclusions are
contradictory. For instance, Lamy et al (25) indicated that autophagy promoted
cancer cell growth, which may counteract the effect of resveratrol
in Ishikawa endometrial cancer cells. However, Mariño et al
(26) determined that inhibition of
autophagy attenuated resveratrol-induced ovarian cancer cell death.
Autophagy-related proteins were detected through western blot
analysis and autophagosomes were quantified in the present study.
Beclin1 is a specific marker of autophagy and LC3 II, generated by
enzymatic cleavage, indicates the initiation of autophagy while p62
is reported to clear the autophagosome (27,28).
In agreement with the results of a study undertaken by Zhu et
al (29), the present study
observed dose-dependently enhanced autophagic activity in the
resveratrol treatment group. Furthermore, inhibiting autophagy with
3-MA counteracted the inhibitory effects of resveratrol on
proliferation, invasion and migration of HCC cells. The results
indicated that resveratrol inhibited HCC progression through
inducing autophagy, and enhancing autophagy can augment the
antitumor effect of resveratrol in HCC.
As resveratrol can induce apoptosis and autophagy in
cancer cells, the present study investigated the potential
signaling pathway involved in these processes. Nuclear
translocation of p53 is important for apoptotic induction. It was
reported that p53 was crucial for resveratrol-induced apoptotic
cell death in human ovarian cancer cells (30). The PI3K/Akt pathway is an important
signaling pathway associated with autophagy (31,32).
He et al (31) suggested
that resveratrol suppressed renal cell carcinoma viability and
migration while promoting apoptosis via the p53/AMPK/mTOR-induced
autophagy signaling pathway. Deng et al (32) also reported that resveratrol-induced
autophagy was regulated by the PI3K/Akt/mTOR pathway. Similarly,
the present study demonstrated that resveratrol treatment activated
p53 while inhibiting the PI3K/Akt pathway in HCC cells. In
addition, p53 inhibitor pifithrin-α (30 µM) and Akt activator IGF-1
treatment decreased the expression of Beclin1 and significantly
inhibited the proliferation, invasion and migration of HCC cells.
The aforementioned results demonstrated that resveratrol suppressed
the proliferation and mobility of HCC cells through inducing
autophagy via activating p53 while suppressing the PI3K/Akt
signaling pathways. As mutant p53 cancer cell lines were more
resistant to imiquimod-induced apoptosis than wild-type p53 cancer
cell lines (33), our future
studies will investigate the effect of resveratrol in HCC cells
containing p53 mutant genes. It is worth investigating the role of
resveratrol in converting mutant p53 protein to transcriptionally
active wild-type p53.
Taken together, the results of the present study
revealed that resveratrol inhibited the viability and mortality of
HCC cells through inducing autophagy via regulating the p53 and
PI3K/Akt pathways. Enhancing autophagy can augment the antitumor
effects of resveratrol in HCC. Therefore, combined treatment with
resveratrol and an autophagy inducer may be a viable option for
treating HCC. To the best of our knowledge, the present study was
the first to elucidated that autophagy was involved in the effect
of resveratrol on HCC cells. Specifically, resveratrol could
inhibit the progression of HCC through inducing autophagy via
regulating the p53 and PI3K/Akt pathways.
Acknowledgements
The authors would like to thank the members of The
Second Hospital of Dalian Medical University (Dalian, China), for
providing technical support concerning the present study.
Funding
Funding information is not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ analyzed and interpreted the data regarding
autophagy. XY was responsible for conducting western blot and
statistical analysis. SS was responsible for the design and
drafting of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
3-MA
|
3-methyladenine
|
|
IGF-1
|
insulin-like growth factor-1
|
|
SD
|
standard deviation
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dugo EB, Yedjou CG, Stevens JJ and
Tchounwou PB: Therapeutic potential of arsenic trioxide (ATO) in
treatment of hepatocellular carcinoma: Role of oxidative stress in
ATO-induced apoptosis. Ann Clin Pathol. 5:11012017.PubMed/NCBI
|
|
3
|
Oh IS, Sinn DH, Kang TW, Lee MW, Kang W,
Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, et al: Liver function
assessment using albumin-bilirubin grade for patients with very
early-stage hepatocellular carcinoma treated with radiofrequency
ablation. Dig Dis Sci. 62:3235–3242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanda T, Ogasawara S, Chiba T, Haga Y,
Omata M and Yokosuka O: Current management of patients with
hepatocellular carcinoma. World J Hepatol. 7:1913–1920. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomha SM, Edrees MM, Muhammad ZA and
El-Reedy AA: 5-(Thiophen-2-yl)-1,3,4-thiadiazole derivatives:
Synthesis, molecular docking and in vitro cytotoxicity evaluation
as potential anticancer agents. Drug Des Dev Ther. 12:1511–1523.
2018. View Article : Google Scholar
|
|
6
|
Rawat D, Shrivastava S, Naik RA, Chhonker
SK, Mehrotra A and Koiri RK: An overview of natural plant products
in the treatment of hepatocellular carcinoma. Anticancer Agents Med
Chem. Jun 3–2018.(Epub ahead of print). doi:
10.2174/1871520618666180604085612. View Article : Google Scholar
|
|
7
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson WD, Morrissey RL, Usborne AL,
Kapetanovic I, Crowell JA, Muzzio M and McCormick DL: Subchronic
oral toxicity and cardiovascular safety pharmacology studies of
resveratrol, a naturally occurring polyphenol with cancer
preventive activity. Food Chem Toxicol. 49:3319–3327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jager W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann NY Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabre KM, Saito K, DeGraff W, Sowers AL,
Thetford A, Cook JA, Krishna MC and Mitchell JB: The effects of
resveratrol and selected metabolites on the radiation and
antioxidant response. Cancer Biol Ther. 12:915–923. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunimasa K, Ohta T, Tani H, Kato E, Eguchi
R, Kaji K, Ikeda K, Mori H, Mori M, Tatefuji T, et al: Resveratrol
derivative-rich melinjo (Gnetum gnemon L.) seed extract
suppresses multiple angiogenesis-related endothelial cell functions
and tumor angiogenesis. Mol Nutri Food Res. 55:1730–1734. 2011.
View Article : Google Scholar
|
|
12
|
Li Q, Yue Y, Chen L, Xu C, Wang Y, Du L,
Xue X, Liu Q, Wang Y and Fan F: Resveratrol sensitizes
carfilzomib-induced apoptosis via promoting oxidative stress in
multiple myeloma cells. Front Pharmacol. 9:3342018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Chiu JF, Liu J, Deng Y, Xu C, Zhang
J and Li G: Resveratrol induces autophagy-dependent apoptosis in
HL-60 cells. BMC Cancer. 18:5812018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan TF, Bu LL, Wang WM, Ma SR, Liu JF,
Deng WW, Mao L, Yu GT, Huang CF, Liu B, et al: Tumor growth
suppression by inhibiting both autophagy and STAT3 signaling in
HNSCC. Oncotarget. 6:43581–43593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a
p53/p21WAF1/CIP1 and p27KIP1 pathway.
Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chai R, Fu H, Zheng Z, Liu T, Ji S and Li
G: Resveratrol inhibits proliferation and migration through SIRT1
mediated posttranslational modification of PI3K/AKT signaling in
hepatocellular carcinoma cells. Mol Med Rep. 16:8037–8044. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harikumar KB, Kunnumakkara AB, Sethi G,
Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S
and Aggarwal BB: Resveratrol, a multitargeted agent, can enhance
antitumor activity of gemcitabine in vitro and in orthotopic mouse
model of human pancreatic cancer. Int J Cancer. 127:257–268.
2010.PubMed/NCBI
|
|
20
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18:E25892017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai Y, Yang H, Zhang G, Hu L, Lei Y, Qin
Y, Yang Y, Wang Q, Li R and Mao Q: Inhibitory effects of
resveratrol on the adhesion, migration and invasion of human
bladder cancer cells. Mol Med Rep. 15:885–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo JY, Xia B and White E:
Autophagy-mediated tumor promotion. Cell. 155:1216–1219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamy L, Ngo VN, Emre NC, Shaffer AL III,
Yang Y, Tian E, Nair V, Kruhlak MJ, Zingone A, Landgren O, et al:
Control of autophagic cell death by caspase-10 in multiple myeloma.
Cancer Cell. 23:435–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kadowaki M and Karim MR: Cytosolic LC3
ratio as a quantitative index of macroautophagy. Methods Enzymol.
452:199–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Wang C, Yan F, Wang T, He Y, Li H,
Xia Z and Zhang Z: N-acetylcysteine attenuates diabetic myocardial
ischemia reperfusion injury through inhibiting excessive autophagy.
Mediators Inflamm. 2017:92572912017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H, Ding J, Wu J, Liu T, Liang J, Tang
Q and Jiao M: Resveratrol attenuates bone cancer pain through
regulating the expression levels of ASIC3 and activating cell
autophagy. Acta Biochim Biophys Sin. 49:1008–1014. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lang F, Qin Z, Li F, Zhang H, Fang Z and
Hao E: Apoptotic cell death induced by resveratrol is partially
mediated by the autophagy pathway in human ovarian cancer cells.
PLoS One. 10:e01291962015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He W and Cheng Y: Inhibition of miR-20
promotes proliferation and autophagy in articular chondrocytes by
PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 97:607–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng F, Ma YX, Liang L, Zhang P and Feng
J: The pro-apoptosis effect of sinomenine in renal carcinoma via
inducing autophagy through inactivating PI3K/AKT/mTOR pathway.
Biomed Pharmacother. 97:1269–1274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang SW, Chang SH, Mu SW, Jiang HY, Wang
ST, Kao JK, Huang JL, Wu CY, Chen YJ and Shieh JJ: Imiquimod
activates p53-dependent apoptosis in a human basal cell carcinoma
cell line. J Dermatol Sci. 81:182–191. 2016. View Article : Google Scholar : PubMed/NCBI
|