Introduction
Ovarian cancer (OC) is the gynecologic tumor with
the highest mortality rate, and the incidence of OC is increasing
each year (1). Usually, OC is
discovered at a late stage; thus, the clinical diagnosis is usually
advanced. At present, the primary clinical treatment method for
ovarian cancer is combined surgery, radiotherapy and chemotherapy.
However, the treatment effect is not ideal, and resistance and
relapse are common. The 5-year survival rate of patients is only
20–30 % (2). Therefore, if a
molecular biological pathway related to treatment resistance in
refractory ovarian epithelial cancer can be discovered, it may be
possible to identify new therapeutic targets and administer
individualized treatment to improve the prognosis of patients.
MicroRNAs (miRNAs) are noncoding single-stranded
small-molecule RNAs with regulatory functions that inhibit the
degradation or translation of target mRNAs by complementarily
binding to those mRNAs (3). miRNAs
have become a hotspot in medical research in recent years, and
these regulatory molecules have exhibited great potential as
therapeutic targets and regulatory factors of tumors (4–6). It is
estimated that up to 90% of human genes are regulated by miRNAs,
and >3,700 miRNAs have been identified in human cells (7,8).
MicroRNA-21 (miR-21) has been revealed to be overexpressed in most
epithelial cancers and is believed to play a pivotal role in the
progression of many malignancies, including lung, breast, stomach,
prostate, colon, brain, head and neck, esophagus and pancreatic
cancers (9). In addition, studies
have revealed that the knockdown of miR-21 impairs growth, induces
apoptosis and reduces the migration and invasion of cancer cells
(10).
The Wnt/β-catenin signaling pathway, which is one of
the most relevant pathways involved with cancer stem cells, has
been reported to be aberrantly activated in various types of
cancers, including ovarian cancer (11–14).
Among the Wnt signaling pathways, which include the classical
Wnt/β-catenin signaling pathway, the Wnt/PCP signaling pathway and
the Wnt/Ca2+ signaling pathway, the classical
Wnt/β-catenin signaling pathway is the most relevant to cancer
(15). In normal stem cells, the
function of the Wnt/β-catenin signaling pathway is to regulate cell
proliferation, self-renewal, migration and apoptosis. In cancer
stem cells, the Wnt/β-catenin signaling pathway plays a pivotal
role, as well. It is known that the Wnt/β-catenin pathway is one of
the major signaling pathways involved in the epithelial-mesenchymal
transition. Chau et al (16)
reported that c-Kit regulates ovarian cancer stem cells through the
activation of Wnt/β-catenin signaling. However, the mechanism
through which miR-21 and Wnt/β-catenin signaling regulate ovarian
cancer cells has not been thoroughly elucidated to date. Many
studies have confirmed that miRNAs activate Wnt/β-catenin signaling
in tumor tissues and cells. miR135a/b is an oncogenic miRNA that
suppresses APC directly to promote the activation of the β-catenin
pathway in colorectal cancer (17).
Luo et al (18) showed that
β-catenin may be an important downstream target gene of miR-21 and
Sox2 and is involved in the regulation of the migration and
invasion of human glioma. These findings raise the question of
whether miR21 also regulates the Wnt signaling pathway in ovarian
cancer. CD44 is a molecule that is located in the cell membrane and
consists of an extracellular domain, a transmembrane domain and a
cytoplasmic domain. The extracellular domain of CD44 contains an
N-terminal globular domain and a proximal stalk membrane region. A
subfamily of CD44 splice variants encompassing the variant domain 6
(CD44v6) has been implicated in the metastatic potential of tumors
(19–21). CD44 isoforms containing CD44v6
(isoforms v4-7 and v6-7) were revealed to confer metastatic
potential on nonmetastatic tumor cell lines in a syngeneic rat
tumor model (19). Numerous studies
have revealed that CD44v6 is highly expressed in OC tissues and is
associated with drug resistance in OC (22,23).
In addition, studies have shown that CD44v6 is a target gene of the
Wnt signaling pathway (24); thus,
we hypothesized that miRNA21 may influence the expression level of
CD44v6 in OC cells through the Wnt signaling pathway, thereby
affecting the proliferation, invasion and migration of OC cells; we
further hypothesized that miRNA21 may even affect the prognosis of
patients with OC. Our hypothesis was confirmed using miRNA21
analogs, inhibitors and agonists of the Wnt signaling pathway.
Materials and methods
Clinical sample collection
A total of 35 specimens of benign ovarian tumors and
40 specimens of malignant tumors were collected from January 2017
to December 2017 at Renmin Hospital of Wuhan University (Wuhan,
China). The age range of patients was 35–65 years of age and had no
other tumors. All patients reviewed the content and purpose of the
study and provided written informed consent. All patients knew and
agreed to participate in the study prior to specimen collection.
The project was approved by the Medical Ethics Committee of Renmin
Hospital of Wuhan University. All ovarian tumor tissues were
pathologically confirmed as epithelial ovarian cancer. Prior to the
acquisition of the tissue, the patients did not undergo any
tumor-related treatments, such as chemotherapy, radiation therapy
or immunotherapy.
Cell culture
SKOV3, A2780 and OVCAR3 cells are human serous OC
cell lines that were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA) cell bank. IOSE80, a normal
ovarian epithelial cell line, was also obtained from the ATCC cell
bank. The cell culture medium was DMEM/F12 (cat. no. SH30023.01;
HyClone; GE Healthcare Life Sciences) supplemented with 10% fetal
bovine serum (FBS; cat. no. 141215; Hangzhou Tianhang Biological
Technology Co., Ltd., Hangzhou, China) and 1% anti-cyanin. Cells
were grown in a cell culture flask in a humidified incubator at
37°C in an atmosphere composed of 95% air and 5% carbon dioxide.
The medium was changed every 2 days. Cells were passaged using
0.05% trypsin/ethylenediaminetetraacetic acid (cat. no. GNM25200;
Gino Biomedical Technology Co., Ltd., Hangzhou, China). A Wnt
inhibitor (XAV-939; cat. no. S1180; Selleck Chemicals, Houston, TX,
USA) and a Wnt agonist (AZD2858; cat. no. HY-15761; Medical
Chemical Corp., Torrance, CA, USA) were added at a concentration of
1 µM for 12 h.
Cell transfection
The cells were seeded at a density of
4×105 cells/well in 24-well culture plates containing
the appropriate amount of complete medium to achieve a cell density
of 30–50 % at the time of transfection. The specific miR-21
mimics/inhibitor and the negative control (NC) were designed and
purchased from GenePharma Co., Ltd. (Shanghai, China). The
sequences of the miRNA mimics and inhibitor are as follows:
hsa-miR-21-5p mimics: UAGCUUAUCAGACUGAUGUUGA,
AACAUCAGUCUGAUAAGCUAUU; hsa-miR-21-5p inhibitor
UCAACAUCAGUCUGAUAAGCUA; miRNA control
UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT. Cells were collected
for further analysis after 48 h of transfection.
Western blot analysis
Total protein was extracted from adherent cells as
follows: First, the adherent cells were washed 2–3 times with PBS
buffer. Then, the appropriate volume of total cell protein
extraction reagent (RIPA protein lysate; cat. no. AS1004; Aspen)
(with protease inhibitors added within minutes of use) was added to
the culture plates/vials for 3–5 min. Next, the cells were
collected into 1.5-ml centrifuge tubes and incubated in an ice bath
for 30 min with repeated pipetting to ensure the complete cell
lysis. After centrifugation at 13,000 × g for 5 min at 4°C, the
supernatant was collected, which contained the total protein
solution. The protein concentration of each sample was determined
using a BCA protein concentration assay kit (cat. no. AS1086;
Aspen). A total of 30 µg of protein was loaded onto 10%
SDS-polyacrylamide gels, separated and then transferred onto
polyvinylidene difluoride membranes (PVDF; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Then 5% milk blocking solution was
incubated for 2 h at room temperature. The membranes were probed
with primary antibodies against human CD44V6 (dilution 1:10,000;
cat. no. ab78960; Abcam, Cambridge, UK), β-catenin (dilution
1:10,000; cat. no. ab32572; Abcam) and GAPDH (dilution 1:2,000;
cat. no. ab37168; Abcam) at 4°C overnight. After several washes
with TBST, the membranes were incubated with HRP-conjugated goat
anti-mouse (dilution 1:10,000; cat. no. AS1106; Aspen) or
HRP-conjugated goat anti-rabbit (1:10,000; cat. no. AS1107; Aspen)
at room temperature for 2 h. The bound antibody was detected using
the SuperSignal West Pico ECL Chemiluminescent Kit (Thermo Fisher
Scientific, Inc., Rockford, IL, USA).
Quantitative real-time polymerase
chain reaction (qRT-PCR)
Total RNA from tissues and cells was isolated using
TRIzol reagent (cat. no. 15596-026; Invitrogen™; Thermo Fisher
Scientific, Inc., Watham, MA, USA) according to the manufacturer's
instructions. Next, 250 µl of chloroform was added, mixed well, and
incubated on ice for 5 min. Then, the samples were centrifuged at
10,000 × g for 10 min at 4°C. In an ultraclean workbench, 400 µl of
the supernatant was carefully absorbed into a 1.5-ml EP tube, and
an equal volume of 4°C precooled isopropanol was added. Then, the
samples were mixed by inversion and incubated at −20°C for 15 min.
Next, the samples were centrifuged at 10,000 × g for 10 min at 4°C,
after which the liquid was carefully decanted. Then, 1 ml of
precooled (4°C) 75% ethanol was added, and the tubes were inverted
several times to wash the RNA precipitate. Subsequently, the tubes
were centrifuged at 4°C at 10,000 × g for 5 min, after which the
liquid was decanted. Finally, the samples were dried on a clean
table for several minutes to fully evaporate the ethanol, and then
10 µl of RNase-Free Water was added to fully dissolve the RNA.
cDNA synthesis was performed using the M-MLV reverse
transcriptase kit (Invitrogen™; Thermo Fisher Scientific, Inc.).
Briefly, the following reagents were added into an EP tube: 1 µl of
the internal reference gene-specific reverse transcription primer
(10 µM), 1 µl of the target gene-specific reverse transcription
primer (10 µM), 1 µl of the dNTP mix, and 10 µl of RNA. The samples
were incubated at 65°C for 5 min and then on ice for 2 min. Next, 4
µl of the 5′ first strand synthesis buffer and 2 µl of 0.1 M DTT
were added into the EP tube, which was incubated in the PCR
instrument at 37°C for 2 min. Finally, 1 µl of M-MLV reverse
transcriptase was added, and the tubes were placed in the PCR
instrument and subjected to 37°C for 50 min, followed by 75°C for
15 min. Then, the samples were cooled to 4°C.
qRT-PCR analysis of the RNA was performed using the
Qiagen OneStep RT-PCR kit (Qiagen, Inc., Valencia, CA, USA). Total
RNA was polyadenylated and reverse-transcribed using the TaqMan
MicroRNA Reverse Transcription kit and TaqMan miRNA assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The expression of U6 (a small nuclear
RNA) was used as the internal control. The relative expression of
the tested genes was calculated and normalized using the
2−ΔΔCq method (25).
RT-PCR was performed on a Life Technologies StepOne™ Real-Time PCR
instrument using the SYBR® Premix Ex Taq™ kit (Takara
Bio Inc., Otsu, Japan). Each sample was tested in triplicate. The
amount of each reagent was as follows: 5 µl of 2X qPCR Mix, 1 µl of
primer working solution (2.5 µM), 1 µl of template, 2.8 µl of
ddH2O, and 0.2 µl of Rox dye. The following primers were
used: U6 RT primer, 5′-AACGCTTCACGAATTTGCGT-3′, forward,
5′-CTCGCTTCGGCAGCACAT-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
hsa-miR-21 RT primer,
5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAACATC-3′, forward,
5′-TGGGCTTATCAGACTGATGTTGA-3 and reverse
5′-CTCAACTGGTGTCGTGGAGTC-3′; H-GAPDH forward,
5′-CATCATCCCTGCCTCTACTGG-3′ and reverse,
5′-GTGGGTGTCGCTGTTGAAGTC-3′; H-β-catenin forward,
5′-GCCAAGTGGGTGGTATAGAGG-3′ and reverse,
5′-GGGATGGTGGGTGTAAGAGC-3′; H-CD44V6 forward,
5′-GCCTTTGATGGACCAATTACC-3′ and reverse,
5′-TCATTCCTATTGGTAGCAGGGA-3′.
Colony formation assay
Cells in the logarithmic growth phase were digested
with 0.25% trypsin. Then, the cells were suspended in DMEM medium
containing 10% fetal bovine serum. The cell suspension was diluted
and seeded at a density of 1,000 cells/well in a dish containing 10
ml of 37°C prewarmed culture medium. The dish was gently rotated to
uniformly disperse the cells. The cells were incubated at 37°C with
5% CO2 and saturated humidity for 2 to 3 weeks. When
macroscopic clones appeared in the culture dish, the culture was
terminated. The supernatant was discarded, and the cells were
carefully washed twice with PBS. Then, 4% paraformaldehyde was used
to fix the cells for 15 min. Subsequently, the appropriate amount
of GIEMSA application staining solution was added to the fixative
solution for 10 to 30 min. Subsequently, the staining solution was
slowly washed away with running water, and the plate was allowed to
air dry. The plate was inverted and overlaid with a grid of
transparencies, and the clones were counted directly with the naked
eye; alternatively, the number of clones >10 cells was counted
under a light microscope (low magnification). Finally, the clone
formation rate was calculated as follows: Clonal formation
rate=(no. of clones/no. of cells inoculated) ×100%.
Transwell migration and invasion
assay
The dyeing solution was prepared as follows: 0.5%
crystal violet solution diluted 1:5 with PBS solution was added
into the 0.01% crystal violet dye solution. The cells were prepared
into a suspension of 105 cells/ml, and 1 ml of the cell
suspension was centrifuged at 10,000 × g for 5 min, after which the
supernatant was discarded. Then, 1 ml of serum-free medium was
added and mixed evenly, after which 200 µl of the cell suspension
was added into the Transwell chamber. Next, 500 µl of complete
medium containing 10% FBS was added to the 24-well plate, and the
chamber was placed in the plate. The plate was incubated for 48 h
at 37°C in an incubator with 5% CO2. The chamber was
then removed, the medium was washed away with PBS, the glue and
cells in the upper chamber were wiped clean with a cotton swab, and
crystal violet stain was applied for 10 min. Then, the surface
crystal violet was washed away, and the side opposite to the
cell-inoculation side was photographed under an inverted
microscope. The cell invasion assay was performed in an identical
manner to the cell migration assay, except that a specific
proportion of Matrigel (Serum-free medium: matrigel=7:1) was added
to the Transwell chamber.
CCK-8
SKOV3 cells in the logarithmic growth phase were
digested with trypsin to prepare a cell suspension at a
concentration of 1×105 cells/ml, which was seeded in a
96-well plate at 8,000 cells/well and 100 µl/well. The plate was
incubated at 37°C with 5% CO2 for 24 h for the cells to
adhere. The medium was changed after 24 h. Five replicates were
used for each sample concentration. Next, 10 µl of CCK-8 solution
was added to all wells, and the plate was incubated for 2 h.
Finally, the absorbance at 450 nm was measured using a microplate
reader. Then, the cells that were treated with the solvent were
used as a control group, and the medium without cells served as the
blank group. The survival rate of the cells was calculated
according to the following formula:
Cell viability %=(experimental group-blank
group)/(control group-blank group) ×100%.
Immunohistochemistry
Following tissue embedding, sectioning, baking,
dewaxing, rehydration and antigen retrieval, the blocking solution
was added for 1 h. Then, the primary antibody, anti-CD44v6
(dilution 1:500; cat. no. ab78960; Abcam), was applied overnight at
4°C. Next, the sections were washed with PBS, and a secondary
antibody goat anti-mouse (dilution 1:200; cat. no. AS1106; Aspen)
was added at room temperature for 2 h. Then, the sections were
washed 3 times with PBS, and the color developer (DAB) was added.
Finally, the sections were rinsed, counterstained and sealed.
Immunofluorescence
The cell suspension was added to a coverslip and
incubated at 37°C in an incubator with 5% CO2 until the
cells were fixed (~2 h). The culture was continued for 2 h with the
addition of 2 ml of the cell culture solution. Then, the medium was
decanted, and the cells were washed 3 times with PBS. Next, 4%
paraformaldehyde was added for 30 min to fix the cells, and
subsequently the cells were washed three times with PBS. Next, the
slides were slightly dried, and 50–100 µl of the DAPI staining
solution (cat. no. AS1075; Aspen) was added and incubated for 10
min at room temperature, followed by 3 washes with PBS.
Subsequently, a 3% hydrogen peroxide solution was added, and the
slides were incubated at room temperature for 20 min in the dark,
and then the slides were placed in PBS (pH 7.4) 3 times on a
decolorizing shaker. After the slides were slightly dried, the
cells were covered with an anti-β-catenin primary antibody
(dilution 1:200; cat. no. ab16051; Abcam) diluted to a specific
concentration with 5% BSA and incubated in a humidified box at 4°C
overnight. Then, the slides were placed in PBS (pH 7.4) and washed
3 times on a bleaching shaker. After drying slightly, the cells
were covered with a secondary antibody of the corresponding species
(CY3-labeled goat anti-rabbit; dilution 1:50; cat. no. AS-1109;
Aspen) and incubated for 50 min at room temperature. Then, the
slides were washed 3 times with PBS, and 50–100 µl of DAPI staining
solution was added dropwise to each well, followed by incubation
for 5 min at room temperature. Subsequently, PBS was added to each
well for 3 washes. An appropriate amount of anti-fluorescence
quencher was added dropwise to the cells, the coverslips were
mounted, and the slides were observed under a fluorescence
microscope.
Statistical analysis
Data are expressed as the mean ± SEM. Data were
analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA) software. A
t-test was used to compare two groups, and one-way analysis of
variance (ANOVA) followed by Bonferroni post hoc test was used to
compare multiple groups. The Chi-square test was used to classify
the data. A P-value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-21 in benign and
malignant ovarian tissue samples
To investigate the expression level of miR-21 in
benign and malignant ovarian tissues, the expression level of
miR-21 was examined in 35 benign and 40 malignant ovarian tissue
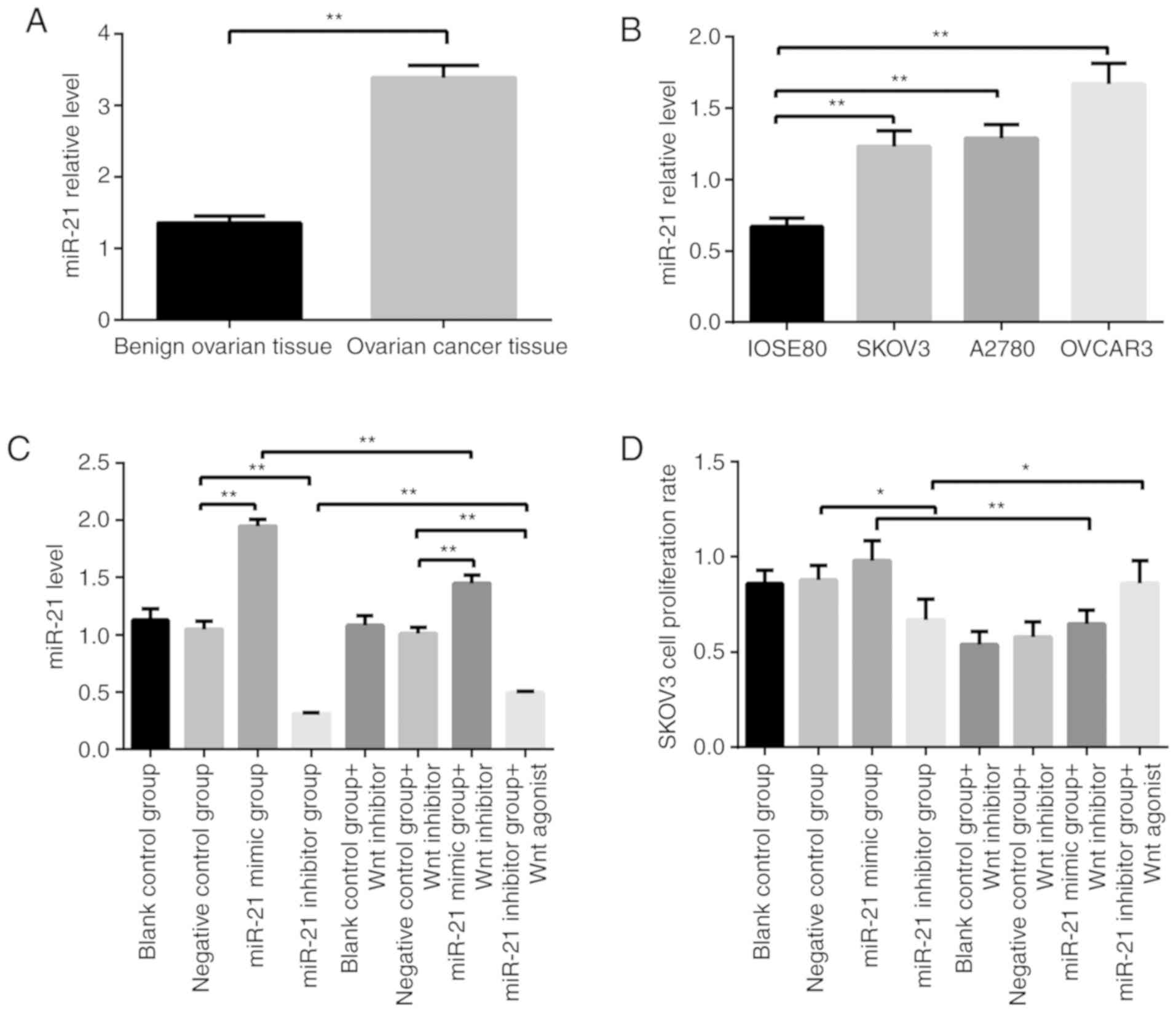
samples. The results are presented in Fig. 1A. As anticipated, the expression
level of miR-21 in malignant OC tissues was significantly higher
than that in benign ovarian tissues (Fig. 1A; P<0.01). This finding indicated
that miR-21 may play a role in the development of malignant ovarian
tumors.
Increased expression of miR-21 in
ovarian cancer cell lines
To further investigate the expression of miR-21 in
OC cell lines and normal ovarian epithelial cells, the expression
levels of miR-21 were examined in the OC malignant cell lines
SKOV3, OVCAR3, A2780 and the ovarian normal epithelial cell line
IOSE80. The results revealed that the expression level of miR-21 in
SKOV3 cells was significantly higher than that in the normal
ovarian epithelial cell line (Fig.
1B; P<0.01). This trend was consistent with the results of
the human tissue samples, indicating that miR-21 may in fact play
an important role in malignant OC.
Effect of miR-21 expression on the
biological behavior of ovarian cancer cells
To determine whether the expression level of miR-21
affects the biological behavior of OC cells, loss- and
gain-of-function experiments were performed in SKOV3 cells using
the transient transfection with miR-21 mimics or an inhibitor. The
transfection efficiency of RNA oligos was ascertained by qRT-PCR,
as revealed in Fig. 1C and the
proliferation rate of SKOV3 cells was determined by CCK-8 assay
(Fig. 1D). Compared with the
negative control group, the proliferation rate in the
miR-21-inhibitor group was lower Then, the cell clonal formation,
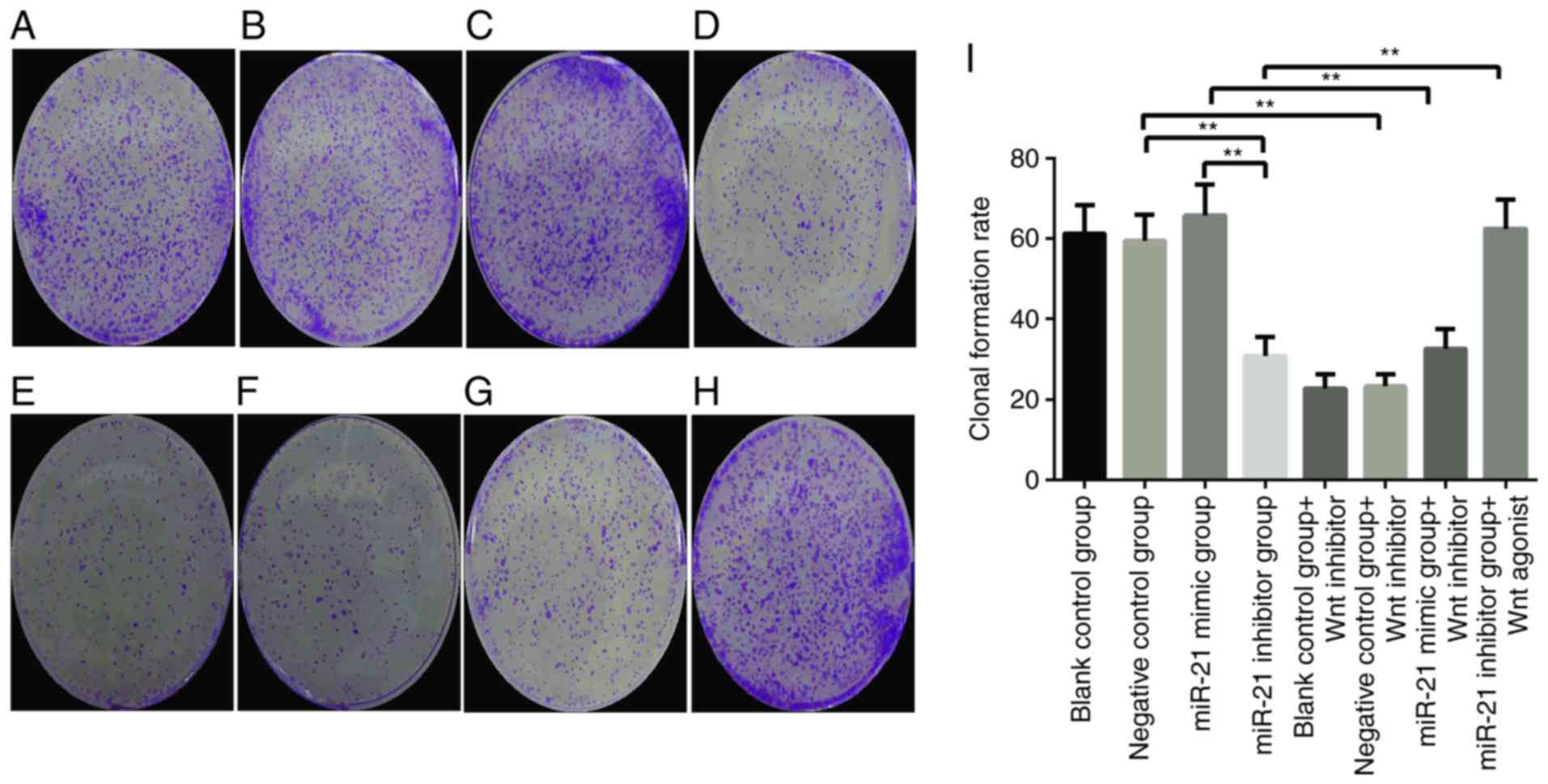
invasion and migration was examined in each group. As observed in
Fig. 2A and B, the SKOV3 cell
clonal formation rate was similar in the blank control group and
the negative group. Although there were no significant differences
between the negative control group and the miR-21-mimics group
(Fig. 2B, C, and I; P>0.05), the
colony formation rate of the SKOV3 cells in the miR-21-inhibitor
group was obviously lower than the negative control group (Fig. 2B, D and I; P<0.01). Compared with
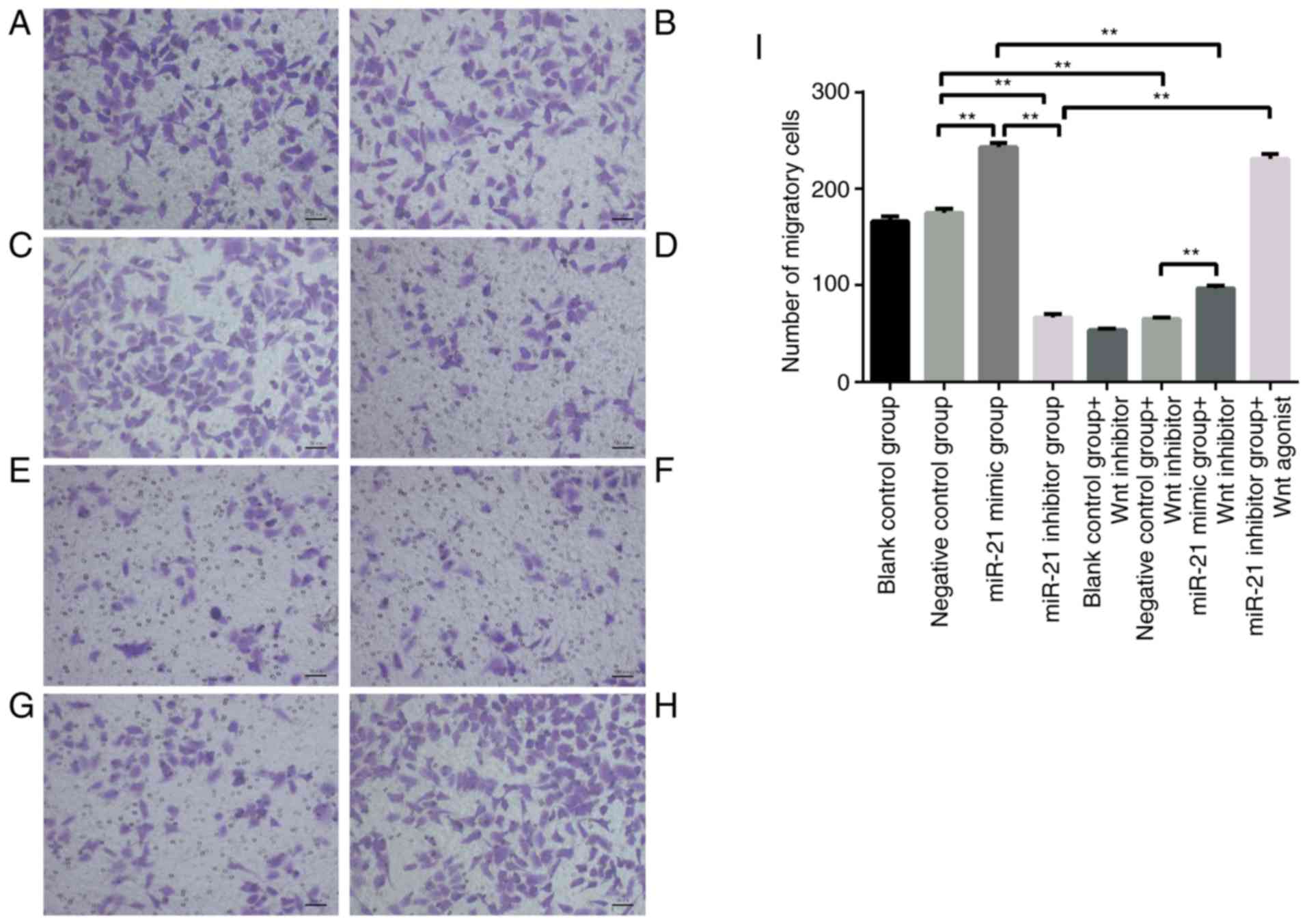
the negative control group, SKOV3 cell migration (Fig. 3B, C and I, P<0.01) and invasion
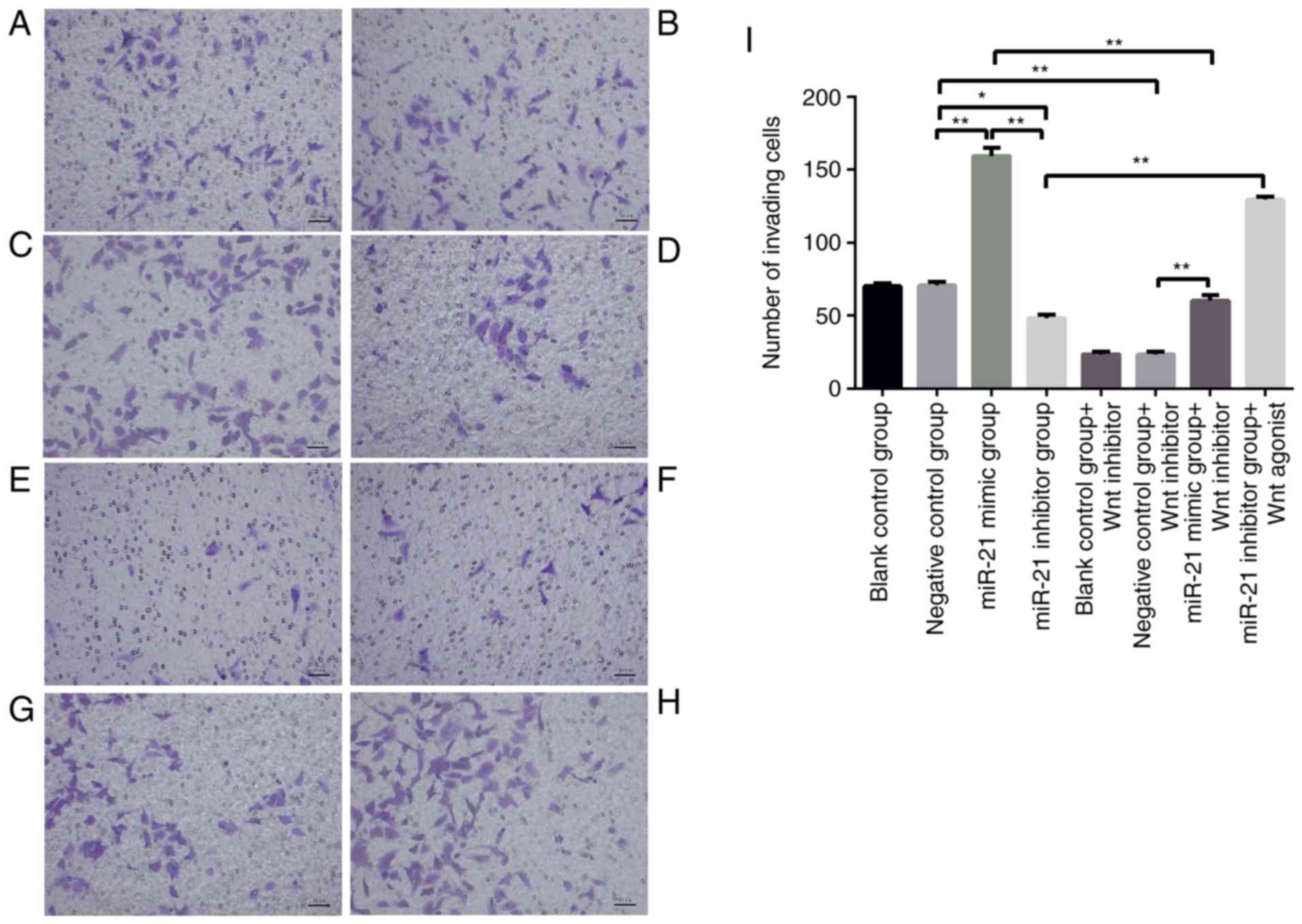
(Fig. 4B, C and I; P<0.01)
abilities in the miR-21-mimics group were significantly increased
and in the miR-21-inhibitor groups were significantly decreased.
These findings indicated that miR-21 affected the biological
behavior of OC.
miR-21 is involved in the activation
of the Wnt signaling pathway and the cytological behavior of
OC
To further explore the downstream signaling pathway
of miR-21, it was ascertained from literature that the Wnt
signaling pathway may be one of the primary downstream target genes
of miR-21. Therefore, a Wnt inhibitor (XAV-939) and a Wnt agonist
(AZD2858) were included in the experiment, and the results were
asceertained by qRT-PCR and western blot analysis. As revealed in
Fig. 1C, the results indicated that
the activation of the Wnt pathway could partly promote the
expression of miR-21. Concurrently, the results indicated that the
expression of Wnt/β-catenin was positively associated with the
expression of miR-21 (P<0.01). The corresponding cell clone
formation as well as cell invasion and migration rates were
revealed to be associated with the expression level of miR-21. High
expression levels of miR-21 promoted the proliferation, migration
and invasion of ovarian cancer cells. In the miR-21-mimics groups,
the rate of cell clone formation was significantly higher than that
of the miR-21-inhibitor group (Fig. 2C
and D, P<0.01), and similar results were obtained in the
invasion (Fig. 4C and D, P<0.01)
and migration experiments (Fig. 3C and
D, P<0.01). In addition, the cytological behavior was
influenced by Wnt/β-catenin pathway. When a Wnt inhibitor was added
to the miR-21-mimics group, proliferation, migration and invasion
of SKOV3 cells were observed to be decreased compared to the
miR-21-mimics group alone (Fig. 2C, G
and I, P<0.01; Fig. 3C, G and
I, P<0.01; Fig. 4C, G and I,
P<0.01). When a Wnt agonist was added in the miR-21-inhibitor
group, proliferation, migration and invasion of SKOV3 cells were
observed to be increased (Fig. 2D, H
and I, P<0.01; Fig. 3D, H and
I, P<0.01; Fig. 4D, H and I,
P<0.01). For the expression of β-catenin mRNA and protein, as
observed in Fig. 5, there was no
difference between the blank control group and the negative control
group. The β-catenin mRNA and protein expression was significantly
higher in the miR-21-mimics group compared with the negative
control group (Fig. 5, P<0.01).
After treatment with the Wnt inhibitor, the β-catenin mRNA and
protein expression was decreased compared to the miR-21-mimics
group alone (Fig. 5, P<0.01).
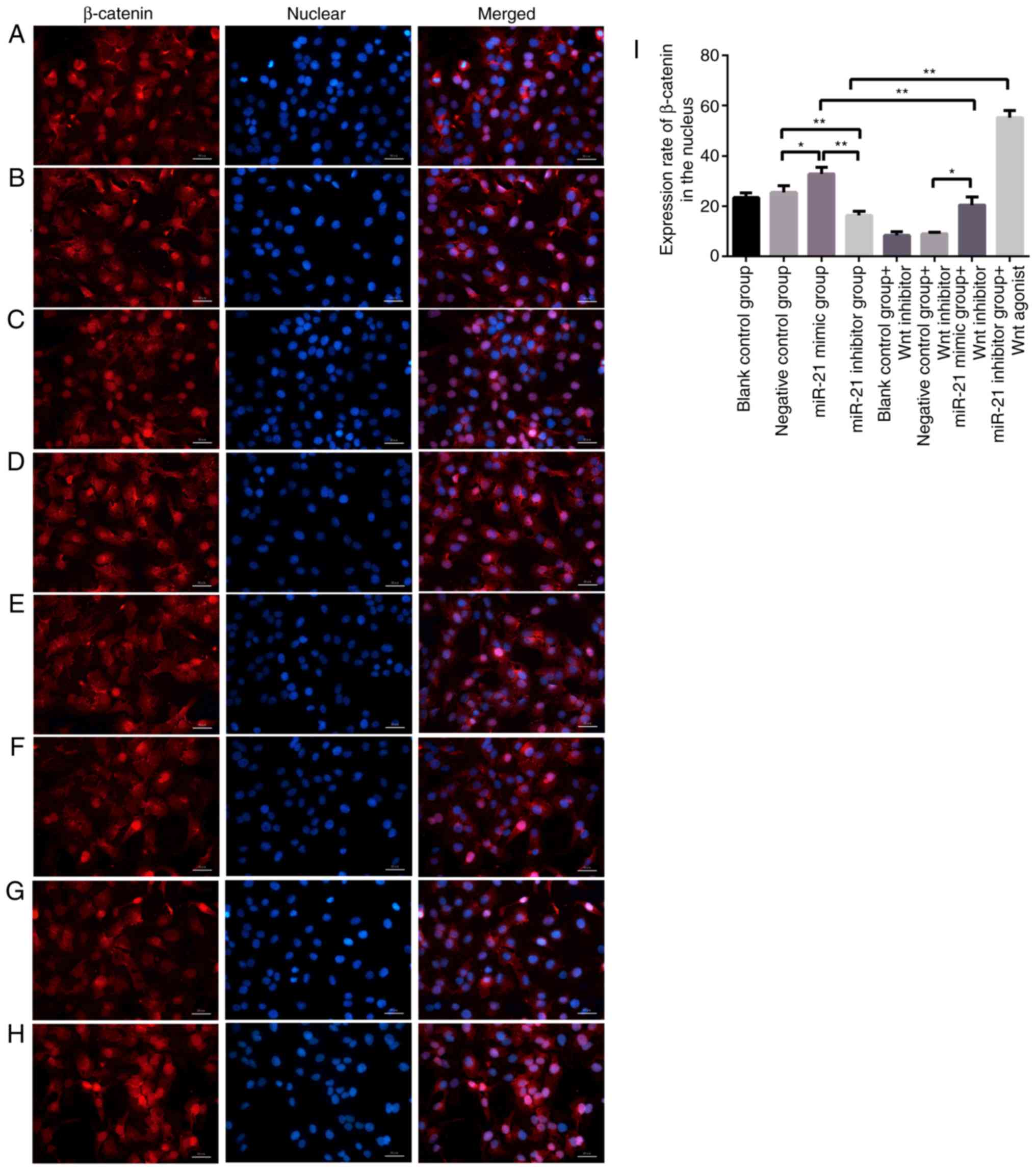
Since β-catenin is transferred into the nucleus to promote tumor
development, the enucleation of β-catenin in various groups of
miR-21 expression levels was further examined using
immunofluorescence. The results revealed that higher expression
levels of miR-21 led to the increased enucleation of β-catenin
(Fig. 6). These results indicated
that the Wnt signaling pathway could in fact be regulated by
miR-21, and moreover, high expression levels of miR-21 activated
the Wnt signaling pathway.
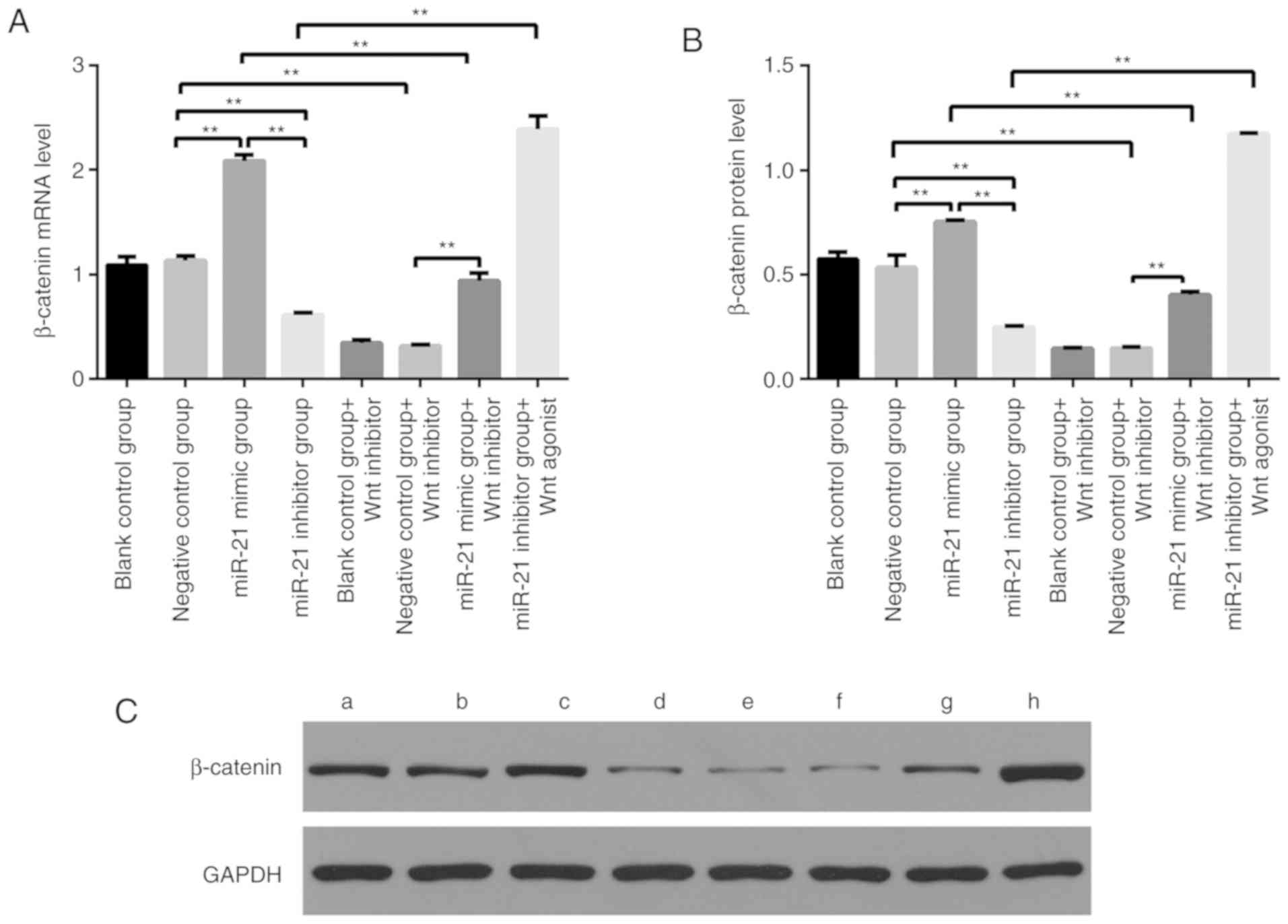 | Figure 5.(A) mRNA expression levels of
β-catenin in each group after different treatments (**P<0.01).
The mRNA expression level of β-catenin was significantly higher in
the miR-21-mimic group than that in the blank control group. In
addition, the mRNA expression level of β-catenin was significantly
lower in the miR-21 inhibitor group than that in the blank control
group. (B and C) Relative quantitative analysis of the expression
bands of each group of proteins and western blot analysis of
β-catenin protein expression in different treatment groups. The
protein expression level of β-catenin was significantly higher in
the miR-21-mimic group than that in the blank control group. In
addition, the protein expression level of β-catenin was
significantly lower in the miR-21-inhibitor group than that in the
blank control group. (**P<0.01). (C) a, blank control group; b,
negative control group; c, miR-21-mimic group; d, miR-21-inhibitor
group; e, blank control group+Wnt inhibitor; f, negative control
group+Wnt inhibitor; g, miR-21-mimic group+Wnt inhibitor; h,
miR-21-inhibitor group+Wnt agonist. |
The miR-21/Wnt/CD44V6 pathway is
involved in the biological behavior of OC
Previous studies have confirmed that CD44v6 is
highly expressed in OC tissues and cells and that CD44v6 is
involved in the development of OC; therefore, this molecule is
expected to be a molecular marker for the detection of OC (23). The immunohistochemical results of
CD44v6 in our human ovarian benign and malignant tissues were also
consistent with this conclusion (Fig.
7A). In addition, studies have reported that the Wnt pathway
affects the expression of CD44v6 in tumors (26). Due to the tissue-specific expression
of miR-21 and CD44, their molecular regulatory pathways in ovarian
cancer are still unclear; thus, the mRNA and protein expression of
CD44v6 in different treatment groups were analyzed. The mRNA and
protein expression levels of CD44v6 in the miR-21-mimic groups were
significantly higher than those in the negative control group
(P<0.01), whereas the mRNA and protein levels of CD44v6 in the
miR-21-inhibitor group were significantly lower than those in the
negative control group (P<0.01) (Fig. 7B). These findings indicated that
miR-21 affected the expression of CD44v6. To further confirm that
miR-21 affects the expression of CD44v6 through the activation of
the Wnt signaling pathway, the blank control group, the negative
control group and the miR-21 mimics group were treated with a Wnt
inhibitor, and a Wnt agonist was added to the miR-21-inhibitor
group and the mRNA and protein expression levels of CD44v6 were
reanalyzed. The expression of CD44v6 was revealed to be
significantly lower following the addition of the Wnt inhibitor,
whereas the expression of CD44v6 in the Wnt agonist group was
significantly increased compared to the miR-21-mimics group alone
(Fig. 7B and D). These findings
indicated that the miR-21/Wnt/CD44v6 axis plays an important role
in the development of OC.
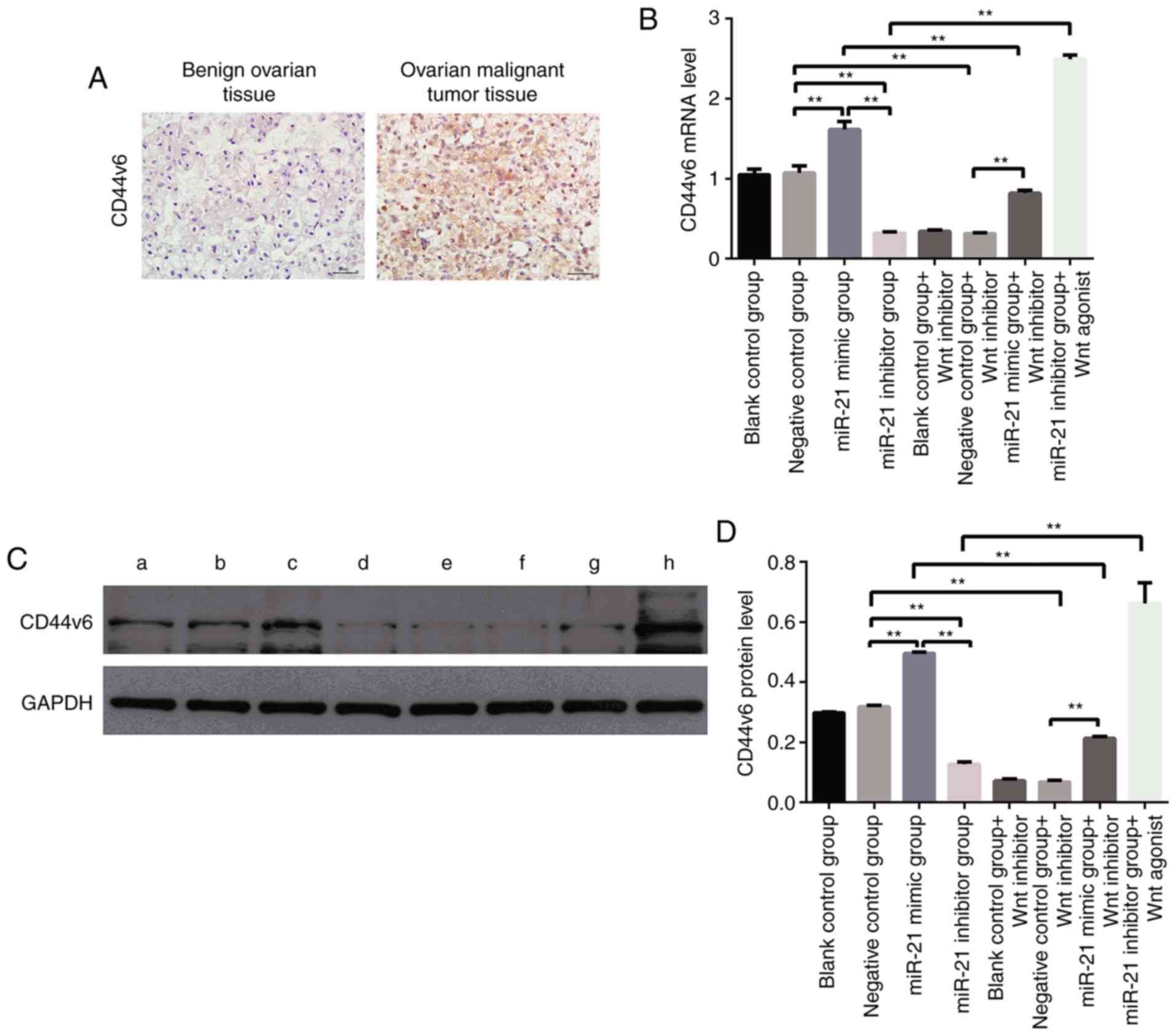 | Figure 7.(A) The protein expression of CD44v6
in benign ovarian tissues and ovarian cancer tissues was detected
by immunohistochemistry (×400, magnification). Yellow-brown
represents the positive expression of CD44v6, localized in the
envelope. (B) mRNA expression levels of CD44v6 in each group after
different treatments (**P<0.01). The mRNA expression level of
CD44v6 was significantly higher in the miR-21-mimic groups than
that in the blank control group. In addition, the mRNA expression
level of CD44v6 was significantly lower in the miR-21-inhibitor
group than that in the blank control group (**P<0.01). (C)
Western blot analysis of CD44v6 protein expression in different
treatment groups. a, blank control group; b, negative control
group; c, miR-21-mimic group; d, miR-21-inhibitor group; e, blank
control group+Wnt inhibitor; f, negative control group+Wnt
inhibitor; g, miR-21-mimic group+Wnt inhibitor; h, miR-21-inhibitor
group+Wnt agonist (D) Relative quantitative analysis of the
expression bands of each group of proteins. The protein expression
level of CD44v6 was significantly higher in the miR-21-mimic group
than that in the blank control group. In addition, the protein
expression level of CD44v6 was significantly lower in the
miR-21-inhibitor group than that in the blank control group
(**P<0.01). |
Discussion
miR-21 has been revealed to be involved in the
development of a variety of tumors, including liver, gastric,
non-small cell lung and breast cancer, as well as neurological
tumors and esophageal cancer (27,28).
It has been suggested that miR-21 may play an oncogenic role in
tumors (29). However, the role of
miR-21 in OC is still controversial, although this miRNA is
typically upregulated in OC (30).
In the present study, a significant increase in miR-21 expression
in malignant OC tissues was demonstrated. Furthermore, it was
confirmed in cell experiments that a high expression of miR-21
promoted the proliferation/invasion/migration of SKOV3 cells, and
the inhibition of miR-21 expression reduced the invasion/migration
and metastasis of SKOV3 cells (Figs.
2–4).
The Wnt/β-catenin signal transduction pathway has
been extremely conserved throughout biological evolution. In normal
somatic cells, β-catenin acts as a cytoskeletal protein at the cell
membrane to form a complex with E-cadherin to maintain adhesion
among cells of the same type and to prevent cell migration
(31). When the extracellular Wnt
signaling molecule binds to the specific receptor Frizzled protein
on the cell membrane to activate the intracellular disheveled
protein, GSK3B is inactivated, which prevents β-catenin from being
phosphorylated and degraded (32).
β-catenin can accumulate in the cytoplasm, and when the cytoplasmic
concentration of β-catenin reaches a certain level, β-catenin can
be transferred to the nucleus. In the nucleus, β-catenin binds to
the transcription factor family TCF/LEFs, which activates
protooncogenes such as cyclin D1 and c-Myc, leading to cell
proliferation, differentiation and maturation (32). Some researchers have studied the
effect of miR-21 on the Wnt signaling pathway by stably
transfecting miR-21-overexpressing HCT-116 cells. Their results
indicated that miR-21 overexpression leads to the activation of
Wnt/β-catenin signaling, such as decreased levels of axin (a
negative regulator of the Wnt signaling pathway), increased levels
of β-catenin, the induction of TCF/LEF (a group of transcription
factors that bind to DNA through a high-mobility group domain and
are involved in the Wnt signaling pathway) activity, and the
increased expression of the downstream target proteins c-Myc and
cyclin D1 (32–36). These conclusions are consistent with
our present results. Yu et al (37) demonstrated that miR-21 induces
stemness of colon cancer cells by activating the Wnt/β-catenin
pathway, which is mediated through the downregulation of TGFβR2. Wu
et al (38) studied miR-21
in lung cancer cells and revealed that in A549 human lung cancer
cells and Lewis lung carcinoma in mice, the key molecules β-catenin
and cyclin D1 and the Wnt/β-catenin signaling pathway were
positively correlated. In the present study, in OC cells, the
addition of a miR-21 mimics activated the Wnt signaling pathway,
whereas the addition of a miR-21 inhibitor blocked the Wnt
signaling pathway (Figs. 5 and
6). Unfortunately, we failed to
identify the target gene of miR-21 in ovarian cancer cells that
directly or indirectly activates the Wnt/β-catenin signaling
pathway. Studies have revealed that the TGF-β signaling pathway is
significantly activated in advanced epithelial ovarian cancer
(39), which is in contrast to the
inhibition of TGF-β receptors that occurs in colon cancer cells.
Therefore, in future studies, we will focus on identifying target
genes of miR-21 that specifically activate the Wnt/β-catenin
signaling pathway in ovarian cancer cells.
The primary role of CD44v6 is to promote cell
migration and invasion, and the expression of CD44v6 in malignant
ovarian tissue was revealed to be significantly increased (Fig. 7A). Studies have revealed that CD44v6
is a prominent target molecule of the Wnt signaling pathway
(40). Our experiments in the OC
cell line further demonstrated that the inhibition of the Wnt
signaling pathway leads to a decrease in the malignancy of OC
cells, a decrease in invasiveness, and a significant decrease in
the mRNA and protein expression of CD44v6 (Fig. 7B-D); after the inhibitory effects
wore off, the degree of malignancy of OC cells increased, the
invasiveness increased, and the mRNA and protein expression of
CD44v6 also significantly increased (Fig. 7B and C). Similar results have been
demonstrated in other tumor studies. Wielenga et al
(41) demonstrated that CD44v6
expression is downstream of Wnt signaling and was induced by the
β-catenin/Tcf-4 signaling pathway. The expression of CD44v6 was
increased in colorectal cancer stem cells by the activation of the
Wnt/β-catenin pathway, which promoted cell migration and
metastasis. Certain articles have reported that β-catenin can
regulate CD44v6 expression. Sun et al revealed that
Biejiajian Pills can significantly reduce the expression of
β-catenin by decreasing the phosphorylation of GSK-3β and blocking
the Wnt/β-catenin signaling pathway to cause downregulation of the
target genes CD44v6 and VEGF, which may be one of the molecular
mechanisms by which Biejiajian Pills suppress the proliferation and
invasiveness of hepatocellular carcinoma (26). In addition, Todaro et al
revealed that cytokine hepatocyte growth factor (HGF), osteopontin
(OPN), and stromal-derived factor 1α (SDF-1), secreted from
tumor-associated cells, increased CD44v6 expression in CR-CSCs by
activating the Wnt/β-catenin pathway, which promoted migration and
metastasis (42). In addition, Han
et al (43) determined that
the siRNA-mediated downregulation of β-catenin markedly inhibited
the invasion and migration of LoVo cells, which may be related to
the upregulation of E-cadherin and the reduction of CD44v6 and
MMP-7 in these cells. However, in the ‘miR-21 mimic+wnt inhibitor’
groups, the miR-21 mimics partially rescued CD44v6 expression.
There may exist Wnt-independent mechanisms that could also be
implicated in the miR-21-dependent regulation of CD44v6. CD44
progenitor cells do not confer metastatic capacity to the cells,
but when treated with cytokines to express CD44v6, the cells gain
metastatic potential. Moreover, a higher survival rate was detected
in patients with low expression of CD44v6 compared with patients
with high expression. Therefore, the authors surmised that the
metastatic process of colon cancer is initiated by the expression
of CD44v6 by cancer stem cells, indicating that CD44v6 is both a
functional biomarker and a therapeutic target (44). In ovarian cancer,
CD44+/CD117+ cancer stem cells exhibit a
strong proliferative capacity, a low degree of differentiation, and
increased resistance to chemotherapy drugs. These cells also
predict a poor prognosis (44).
However, CD44v6 is a membrane protein molecule that can transduce a
variety of molecular signaling pathways. This raises the question
of which molecules mediate the effects of CD44v6 on the biological
behavior of ovarian cancer. We studied this issue further, and it
was determined that CD44v6 affects the biological behavior of
ovarian cancer through the NF-κB pathway.
In conclusion, the present study revealed that
miR-21 regulates the expression of CD44v6 by activating the Wnt
signaling pathway, which plays an important role in the development
of ovarian cancer. These findings provide a potential new
therapeutic target for the clinical diagnosis and treatment of
ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Chinese Medical
Association Clinical Medical Research Special Fund Project (Award
no. 17020310700) and the Wuhan University Independent Research
Project (Award no. 413000117).
Availability of data and materials
All datasets on which the conclusions of the paper
depend are available to readers.
Authors' contributions
YC supervised the project. YW participated in the
fund raising, experimental design, data acquisition, and article
writing. MY performed the majority of the experiments and drafted
the manuscript. LZ and XY participated in the fund raising and
collected clinical specimens. DY designed and supervised the in
vitro functional study. SX supervised the results. YW performed
the preliminary experiments. YC was a major contributor in the
revision of the manuscript. All authors were involved in the
conception of the study, read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures performed and all clinical data
obtained in the present study involving human participants were
reviewed and approved by the Medical Ethics Committee of Renmin
Hospital of Wuhan University and were in accordance with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J, Sun H, Yang L, Deng Y, Yan Y, Wang
S, Yang G and Ma H: Improved survival in ovarian cancer, with
widening survival gaps of races and socioeconomic status: A period
analysis, 1983–2012. J Cancer. 9:3548–3556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang N, Kaur S, Volinia S, Greshock J,
Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, et al:
MicroRNA microarray identifies Let-7i as a novel biomarker and
therapeutic target in human epithelial ovarian cancer. Cancer Res.
68:10307–10314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu SL, Chen HY, Chang GC, Chen CY, Chen
HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weidhaas JB, Babar I, Nallur SM, Trang P,
Roush S, Boehm M, Gillespie E and Slack FJ: MicroRNAs as potential
agents to alter resistance to cytotoxic anticancer therapy. Cancer
Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Backes C and Keller A: Reanalysis of 3,707
novel human microRNA candidates. Proc Natl Acad Sci USA.
112:E2849–E2850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nusse R, Fuerer C, Ching W, Harnish K,
Logan C, Zeng A, ten Berge D and Kalani Y: Wnt signaling and stem
cell control. Cold Spring Harb Symp Quant Biol. 73:59–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang
T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al: Targeting
Wnt-driven cancer through the inhibition of Porcupine by LGK974.
Proc Natl Acad Sci USA. 110:20224–20229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai X, Yao Z, Li L and Huang J: Role of
DKK4 in Tumorigenesis and Tumor Progression. Int J Biol Sci.
14:616–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chau WK, Ip CK, Mak AS, Lai HC and Wong
AS: c-Kit mediates chemoresistance and tumor-initiating capacity of
ovarian cancer cells through activation of
Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene.
32:2767–2781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagel R, le Sage C, Diosdado B, van der
Waal M, Oude Vrielink JA, Bolijn A, Meijer GA and Agami R:
Regulation of the adenomatous polyposis coli gene by the miR-135
family in colorectal cancer. Cancer Res. 68:5795–5802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo G, Luo W, Sun X, Lin J, Wang M and
Zhang Y, Luo W and Zhang Y: MicroRNA21 promotes migration and
invasion of glioma cells via activation of Sox2 and β-catenin
signaling. Mol Med Rep. 15:187–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Günthert U, Hofmann M, Rudy W, Reber S,
Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H and Herrlich P:
A new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rudy W, Hofmann M, Schwartz-Albiez R,
Zöller M, Heider KH, Ponta H and Herrlich P: The two major CD44
proteins expressed on a metastatic rat tumor cell line are derived
from different splice variants: Each one individually suffices to
confer metastatic behavior. Cancer Res. 53:1262–1268.
1993.PubMed/NCBI
|
|
21
|
Seiter S, Arch R, Reber S, Komitowski D,
Hofmann M, Ponta H, Herrlich P, Matzku S and Zöller M: Prevention
of tumor metastasis formation by anti-variant CD44. J Exp Med.
177:443–455. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tjhay F, Motohara T, Tayama S, Narantuya
D, Fujimoto K, Guo J, Sakaguchi I, Honda R, Tashiro H and Katabuchi
H: CD44 variant 6 is correlated with peritoneal dissemination and
poor prognosis in patients with advanced epithelial ovarian cancer.
Cancer Sci. 106:1421–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motohara T, Fujimoto K, Tayama S,
Narantuya D, Sakaguchi I, Tashiro H and Katabuchi H: CD44 variant 6
as a predictive biomarker for distant metastasis in patients with
epithelial ovarian cancer. Obstet Gynecol. 127:1003–1011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katoh M: Multilayered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun H, He S, Wen B, Jia W, Fan E and Zheng
Y: Effect of Biejiajian Pills on Wnt signal pathway molecules
β-catenin and GSK-3β and the target genes CD44v6 and VEGF in
hepatocellular carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao.
34:1454–1458. 2014.(In Chinese). PubMed/NCBI
|
|
27
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonci D: MicroRNA-21 as therapeutic target
in cancer and cardiovascular disease. Recent Pat Cardiovasc Drug
Discov. 5:156–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB
and Cheng XC: MicroRNA-21 gene and cancer. Med Oncol. 30:3762013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaksman O, Tropé C, Davidson B and Reich
R: Exosome-derived miRNAs and ovarian carcinoma progression.
Carcinogenesis. 35:2113–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kimura M, Nakajima-Koyama M, Lee J and
Nishida E: Transient expression of WNT2 promotes somatic cell
reprogramming by inducing β-catenin nuclear accumulation. Stem Cell
Reports. 6:834–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Echevarría-Vargas IM, Valiyeva F and
Vivas-Mejía PE: Upregulation of miR-21 in cisplatin resistant
ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 9:e970942014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Li J, Li G and Wang S: Effects of
celastrol on enhancing apoptosis of ovarian cancer cells via the
downregulation of microRNA21 and the suppression of the
PI3K/AktNF-κB signaling pathway in an in vitro model of ovarian
carcinoma. Mol Med Rep. 14:5363–5368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eitan R, Kushnir M, Lithwick-Yanai G,
David MB, Hoshen M, Glezerman M, Hod M, Sabah G, Rosenwald S and
Levavi H: Tumor microRNA expression patterns associated with
resistance to platinum based chemotherapy and survival in ovarian
cancer patients. Gynecol Oncol. 114:253–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cevallos RR, Rodríguez-Martínez G and
Gazarian K: Wnt/β-catenin/TCF pathway is a phase-dependent promoter
of colony formation and mesendodermal differentiation during human
somatic cell reprogramming. Stem Cells. 36:683–695. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal
J, Sarkar FH and Majumdar AP: MicroRNA-21 induces stemness by
downregulating transforming growth factor beta receptor 2 (TGFβR2)
in colon cancer cells. Carcinogenesis. 33:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu D, Shi M and Fan XD: Mechanism of
miR-21 via Wnt/β-catenin signaling pathway in human A549 lung
cancer cells and Lewis lung carcinoma in mice. Asian Pac J Trop
Med. 8:479–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamura S, Matsumura N, Mandai M, Huang
Z, Oura T, Baba T, Hamanishi J, Yamaguchi K, Kang HS, Okamoto T, et
al: The activated transforming growth factor-beta signaling pathway
in peritoneal metastases is a potential therapeutic target in
ovarian cancer. Int J Cancer. 130:20–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schmitt M, Metzger M, Gradl D, Davidson G
and Orian-Rousseau V: CD44 functions in Wnt signaling by regulating
LRP6 localization and activation. Cell Death Differ. 22:677–689.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wielenga VJ, van der Neut R, Offerhaus GJ
and Pals ST: CD44 glycoproteins in colorectal cancer: Expression,
function, and prognostic value. Adv Cancer Res. 77:169–187. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han J, Gao B, Jin X, Xu Z, Li Z, Sun Y and
Song B: Small interfering RNA-mediated downregulation of
beta-catenin inhibits invasion and migration of colon cancer cells
in vitro. Med Sci Monit. 18:BR273–BR280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao Y, Foster R, Yang X, Feng Y, Shen JK,
Mankin HJ, Hornicek FJ, Amiji MM and Duan Z: Up-regulation of CD44
in the development of metastasis, recurrence and drug resistance of
ovarian cancer. Oncotarget. 6:9313–9326. 2015. View Article : Google Scholar : PubMed/NCBI
|