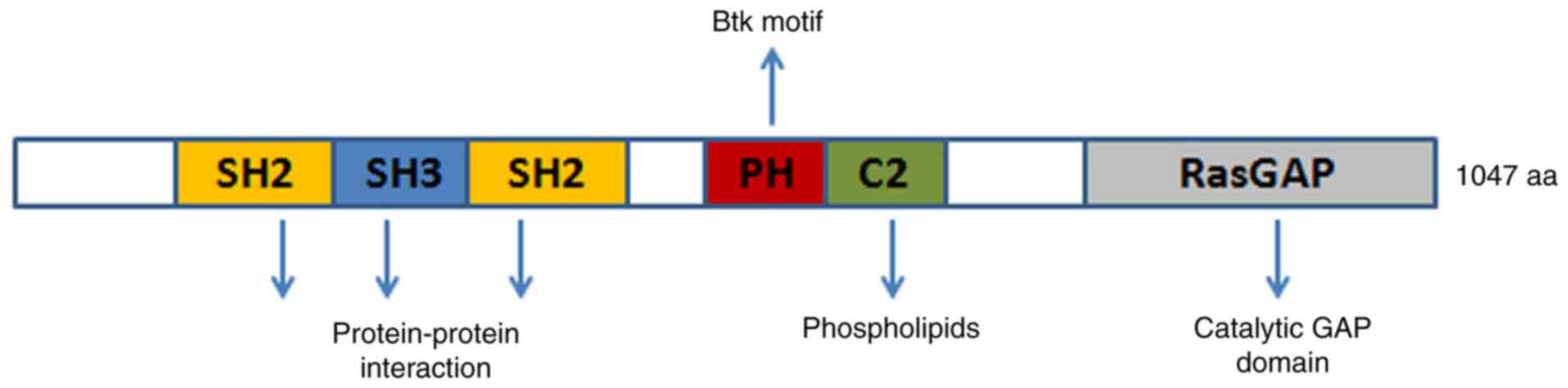
Ras p21 protein activator 1 (RASA1) is located on
chromosome 5q14.3 and is a member of the RasGAP family which
includes NF1, DAB2IP, and RASAL2 (1). RASA1 contains the following domains:
Src homology 2 and 3 (SH2 and SH3), N-terminal C2A and C2B,
GTPase-activating protein (GAP), and pleckstrin homology (PH),
which is attached to a Bruton's tyrosine kinase (Btk) motif. RASA1
is a GAP with dual-specificity that enhances and accelerates the
GTPase activity of Ras and Rap. Notably, intracellular
Ca2+ levels regulate the GAP activity of RASA1. When
Ca2+ concentrations are high, the C2 domains of Ras and
Rap allow for the binding of phospholipids while the PH domain
remains inactive and prevents lipid binding. RASA1 is normally
located in the cytoplasm as a soluble protein and is recruited to
the plasma membrane upon receptor-mediated increases in
intracellular Ca2+ concentrations (2). The RasGAP activity of RASA1 is
increased when RASA1 associates with the membrane since RasGAP
activity is limited in the soluble form of RASA1, although the
underlying mechanism is not known (3). SH2-pTyr interaction allows for RASA1
to interact with p190RhoGAP (p190RhoGAP-A, ARHGAP35), which is a
GAP for Rho (4). Due to its special
structure, RASA1 is involved in physiological functions such as
cell growth, proliferation, differentiation, and apoptosis
(Fig. 1). RASA1 mutation or
epigenetic inactivation has been revealed in numerous human
cancers, which has attracted interest for further
investigation.
The inhibition of Ras signal transduction by RASA1
has been revealed to negatively affect the maintenance of lymphatic
vasculature by VEGFR-3 in a steady-state situation (14). RASA1 has also been revealed to
directly interact with mitogen-activated protein kinase kinase
kinase kinase 4 (MAP4K4) for lymphatic vascular development
(15–17).
RASA1 is necessary for the normal development and
function of T cells and a study has revealed that RASA1-deficiency
in mice increased apoptosis of CD4+CD8+
double-positive thymocytes (18).
Motility of macrophages and cytoskeletal and adhesion structures
have been revealed to be modulated by RASA1-mediated translocation
of p190RhoGAP (GRLF1) induced by BCL6 (19). RASA1 has been discovered to play a
role in the development of neuron axons (20), dendritic cell differentiation
(21), follicular development
(22), epididymal development
(23), skin wound healing (24,25),
and stress (26) (Table I).
Since RASA1 is a central player of angiogenesis,
studies have focused on RASA1 mutation or loss-of-function in
vascular diseases such as capillary malformation-arteriovenous
malformation (CM-AVM) syndrome, Klippel-Trenaunay-Weber syndrome
(KTWS), Sturgeon-Weber syndrome (SWS), vein of Galen aneurysmal
malformation (VGAM), MEF2C-related disorders, and Parkes-Weber
syndrome (PKWS). Atypical capillary malformation (CM) is a key
characteristic of CM-AVM and is frequently paired with multiple
arteriovenous malformations (AVMs) and at least one of the
following: Rapid vascular malformation, arteriovenous fistulas
(AVFs), parks Weber syndrome (PWS), and hereditary haemorrhagic
telangiectasia (HHT) (27). CM
usually manifests as multifocal wine spots on the skin due to
inactivating mutations of RASA1 in 68% of cases (28). p120RASGAP is encoded by RASA1 and
directly affects EPHB4, which acts on capillaries, leading to HHT
and CM (29,30). KTWS may rarely occur in the affected
limb of patients with cutaneous haemangioma, venous varicosity, and
hypertrophy of osseous-soft tissue (31). Mutations in RAP1A have been
associated with KTWS and interact with RASA1 and Krev interaction
trapped protein 1 (KRIT1) (32,33).
Patients with SWS harbour vascular malformations in the face, eyes,
and brain due to a mutation in RASA1. The underlying mechanism is
thought to be Nrf2-mediated oxidative stress, although the causal
relationship has not been fully elucidated (34,35).
VGAM encompasses a rare vascular malformation in the brain of
children caused by RASA1, endoglin, and activin receptor-like
kinase 1 (ACVRL1) mutations, which encode for ALK1 and SMAD4
(36,37). Patients with MEF2C-related disorders
are severely intellectually impaired and have limited mobility and
speech. In addition, they show hypotonia, seizures, and multiple
minor anomalies of the brain. Moreover, RASA1 mutation and a
decrease in MECP2 and CDKL5 expression have been correlated with
this disease (38). PKWS is a rare
vascular malformation syndrome with extensive capillary
malformations that appear at birth or during early childhood and
patients exhibit mutation of RASA1 that leads to a loss of function
(39,40). ENG, ACVRL1, and SMAD4 are components
of transforming growth factor-beta (TGB-β) signalling and mutations
in these genes can cause HHT, which is the most commonly inherited
vascular disorder. HHT has also been correlated with RASA1, BMP9,
and GDF2 (32,35,41,42).
Cardiovascular and cerebrovascular diseases bring
great pressure on countries with an ageing population. A previous
study has revealed that RASA1 and its associated factors and
vascular smooth muscle cell dysfunction through angiotensin II are
central pathways involved in cardiovascular diseases. miR-132 was
revealed to prevent CREB activation by angiotensin II via RASA1
(43). Myocardial fibrosis is an
important process of cardiac hypertrophy and heart failure, which
has been correlated to an upregulation of RASA1 via miR-21
(44,45). Obesity is a risk factor for
cardiovascular disease and A study has demonstrated that
PPARγ/miR-223 participates in the deposition of adipocytes by
targeting RASA1 (46). Another
study has linked tricuspid atresia, a congenital heart defect with
fatal consequences, with the homozygous RASA1 germline mutation
c.1583A>G (p.Tyr528Cys) (47).
An animal model of cerebral ischemia has revealed that the
GAS5/miR-335/RASA1 axis is regulated to inhibit neuron apoptosis
and promote neuroprotection (48).
Chronic kidney disease is related to renal fibrosis
and miR-132 has been revealed to inhibit the development of renal
fibrosis by targeting RASA1, leading to reduced proliferation of
myofibroblasts (49). A leading
cause of blindness is age-related macular degeneration (AMD), where
studies have revealed abnormal expression of RASA1 in OXYS rats
(50). Myelodysplastic syndromes
(MDS) encompass a group of haematopoietic stem cell disorders that
are characterised by abnormal myeloid cell differentiation and
development, ineffective haematopoiesis, refractory hemocytopenia,
and haemopoietic failure. Notably, it has been revealed that MDS is
related to RASA1 downregulation (51). In a mouse model for CCI-induced
neuropathic pain, intrathecal injection of miR-144 mimics reduced
the mechanical and thermal pain experienced by mice through the
downregulation of RASA1 (52)
(Table II).
Investigation into changes in genomic DNA and RNA in
HL-60 cells revealed that RASA1 is related to tumorigenesis
(53). The Ras-GAP SH3 domain
inhibits Rho-GAP activity and inhibits tumour development (54). RASA1 has also been reported as an
oncogene through large-scale tumour sequencing studies that are
based on 3D mutation clusters in relation to the protein structure
(55).
In tumour development, mutations of Ras at residues
12, 13, or 61 affect the activity of intracellular guanosine
triohosphte (GTP), which alternates between GDP and GTP forms and
activates RasGAP proteins and Ras by Ras GTPase, regulating the
guanine nucleotide exchange factors (RasGEFs). The understanding of
RASA1 and its relationship with tumour formation is qualitative and
is limited to the abnormal expression of RASA1 and several other
genes from the sequencing of certain tumours. It has been revealed
that abnormal or downregulation of RASA1 expression affects
tumorigenesis and the continued technological development in the
field of molecular biology allows for more in-depth research, where
it has been found that the expression of RASA1 in most tumour cells
is associated with intracellular miRNA. Furthermore, a small
portion of tumorigenesis may be due to the coupling of RASA1 to its
associated protein or gene (56).
Nevertheless, the role of RASA1 in tumour formation requires
further study.
Lung cancer incidence and deaths continue to rise
and place high pressure on health care with its high incidence
(2.09 million new cases in 2018 vs. 1.8 million in 2012) and
mortality rate (1.76 million deaths in 2018 vs. 1.6 million in
2012) compared with other tumours (57). The occurrence of lung cancer has
been linked to RASA1 mutations by next-generation sequencing
(58,59). Zhu et al revealed that
hsa-miR-182 downregulated RASA1 to suppress proliferation of lung
squamous cell carcinoma through tissue microarray and quantitative
PCR (60). Recently, Shi et
al demonstrated that miR-30c and miR-21 were significantly
upregulated by two KRAS isoforms in small-cell lung cancer and
induced drug resistance by inhibiting key tumour suppressor genes
such as neurofibromatosis type one (NF1), RASA1, BID, and RASSF8
(61). Mutations of EGFR result in
drug resistance and relapse in patients with non-small cell lung
cancer (NSCLC) (62). Furthermore,
miR-31 directly inhibits the expression of RASA1 and FIH-1, leading
to at least partial activation of Raf/MEK/ERK and PI3K/Akt
signalling in gefitinib-resistant NSCLC (63). Kitajima and Barbie classified NF1 or
RASA1 mutations in small-cell lung carcinoma and proposed the
clinical evaluation of MAPK inhibition in an analysis of large
genomic datasets of NSCLC [MSK-IMPACT dataset at MSKCC (n=2,004)
and TCGA combined lung cancer dataset (n=1,144)] (64). RASA1 and NF1 mutations are strong
drivers of NSCLC (65). Fibroblast
growth factor receptor-2 (FGFR-2) is a tyrosine kinase receptor,
which can selectively bind to fibroblast growth factor (FGF) to
promote autophosphorylation and mediate cell response through
downstream MAPK and Akt signalling. A previous study has revealed
that FGFR-2 mutation is an important driver of lung cancer, which
has become a key target of lung cancer drug development. In an
FGFR2-mutant resistant model, RASA1 was found to be inactivated
(66).
The incidence of colorectal cancer (CRC) is rising
and is predicted to increase to 2.5 million new cases by 2035 and
is ranked third among malignant tumours across the world and second
in some developing areas (67,68).
Colorectal specimens including 468 colorectal tumour samples from a
large personalised medicine initiative and 17 paired
primary-metastatic and 2 metastatic-metastatic specimens from 18
CRC patients were analysed by next-generation sequencing to reveal
the presence of RASA1 mutations (69). RASA1 expression has been linked to
multiple miRNAs in colorectal cancer. In vivo, miR-31 has
been revealed to be negatively correlated with RASA1 protein level
and miR-31 was a key player in RAS signalling activation through
the inhibition of RASA1 translation, resulting in accelerated
growth of colorectal cancer cells and stimulation of tumour
formation (70). In rectal cancer
cells, expression of miR-21 reduced the expression level of RASA1
resulting in the promotion of cell proliferation, anti-apoptosis,
and tumour cell formation (71).
RASA1 encoding RAS GTPase is one of the target genes that is
continuously downregulated in cells overexpressing miR-21 and
upregulated in cells exposed to miR-21 inhibitors (72). These results coincide with a study
by Sun et al in which upregulation of miR-223 can be
detected in colon cancer cells and promotes tumour growth.
Conversely, miR-223 inhibition may reduce or halt the growth of
solid tumours (73). miR-335 has
been revealed to inhibit the expression of the RASA1 gene by
targeting a specific sequence of the 3′UTR of RASA1. Low expression
of RASA1 has been revealed to promote tumour cell development and
miR-335 expression was increased in patients with CRC compared with
normal mucosa, whereas high expression of miR-335 was significantly
associated with tumour size and CRC differentiation. miR-335
overexpression in CRC cells was revealed to promote cell
proliferation and tumour growth in vivo (74). Antoine-Bertrand et al
observed an interaction between p120RasGAP and cancer cells in CRC,
which regulates axonal growth mediated by netrin-1 and guides the
development of cortical neurons in embryos (75). KRAS mutations are found in 40% of
patients with CRC and the effective treatment of CRC with late KRAS
mutation is limited at present (76). RASA1 is as an effector of KRAS
mutation and may play an important role as a drug treatment target
(77). However, studies using
CRISPR technology have described that only loss of NF1 promotes
resistance to EGFR inhibition (78).
Primary liver cancer has the seventh-highest
incidence (0.84 million new cases in 2018 year) and second highest
mortality (0.78 million deaths in 2018) worldwide (79). China has the highest prevalence
owing to an increased prevalence of 18.3 per 100,000 and its
population of 1.4 billion (80).
Mutations of RASA1 have been found by detecting RASA1 and other
members of the RasGAP family through sequencing of liver cancer
tissue genes (81). The analysis of
RASA1 expression and the prognosis of patients revealed that the
expression of RASA1 was closely related to tumour size and
differentiation and that the prognosis was poor with low RASA1
expression, indicating that RASA1 could be a predictive factor for
the prognosis of patients with liver cancer (82). RASA1 is also regulated by miRNA
although other potential oncogenes also contribute to the
development of liver cancer. Increased miR-31 expression and low
RASA1 expression have been detected in cells and tissues from liver
cancer patients. Furthermore, it has been confirmed that miR-31
promotes cellular proliferation and inhibits liver cancer cells
apoptosis by downregulating RASA1 expression (83). When liver cancer cells are exposed
to a hypoxic environment, a significant increase in miR-182
expression occurs, which then promotes angiogenesis via RASA1 and
leads to proliferation of cancer cells (84). Thus, RASA1 levels are related to
liver cancer cell proliferation. In addition, RASA1 is regulated by
pituitary homeobox 1 (PITX1) and protein tyrosine phosphatase 1B
(PTP1B). PITX1 has a tumour suppressing function through the
regulation of RasGAP expression. PITX1 is downregulated by PTP1B,
which drives proliferation and inhibits apoptosis of tumour cells
by acting on RASA1 (85). In cells
exposed to mild stress, RASA1 is cleaved into an N-terminal
fragment (fragment N) by caspase-3, which potently inhibits the
activity of NF-κB by augmenting its translocation to the cytoplasm
(26). Studies have revealed that
liver cancer incidence is reduced by the caspase-3/p120 RasGAP
stress-sensing module. However, the overall survival is not
affected (26,86).
Breast cancer is the second leading cause of cancer
related-deaths in women (2.08 million new cases and 0.62 million
deaths in 2018) and although the genotyping of breast cancers and
the subsequent targeted treatment significantly improve the
curative rate to more than 65%, it remains necessary to discover
more tumour markers for further treatment optimisation (79,87).
Some studies have shown that the low expression of RASA1 is related
to the occurrence of breast cancer (88,89).
This is consistent with a study that demonstrated the presence of 7
genetic mutations in breast cancer, which included RASA1 mutations
(90). The expression of RASA1 is
prevalent in triple-negative breast cancer (TNBC) tumours and
tumour cells with low estrogenic receptor expression (91). Both docking protein 2 (Dok2) and
RASA1 function as tumour suppressors and have been detected in
several types of solid tumours. Our previous study revealed that a
weak Dok2/RASA1 expression was associated with poorly
differentiated breast adenocarcinoma. Moreover, a decrease in Dok2
and RASA1 expression was linked to larger tumour size and a
heightened chance of metastasis to the axillary lymph node
(92). RASA1 is also regulated by
miRNA and other potential oncogenes contribute to the development
of breast cancer. miR-206/21 has been revealed to promote the
growth of hepatoma cells by inhibiting the translation of RASA1 and
promoting Ras/Erk signal transduction and cell differentiation
(92). miR-421 which targets RASA1,
has been revealed to inhibit TNBC tumour growth and metastasis
(93). As C2 domains of RASA1
participate in the regulation of calcium signalling, L-type
voltage-gated calcium channel gamma subunit 4 (CACNG4) can promote
the stability of the internal environment of breast cancer cells
and improve the survival and metastasis ability of tumour cells
through alternating calcium signalling events and invading key
survival and metallic pathway genes including RASA1 (94).
RASA1 mutation and abnormal expression is also
regarded as a contributing factor to the development of
gynaecologic tumours such as cervical and ovarian cancer (95,96).
The incidence (98,900 new cases in 2015) of cervical cancer in
China has been ranked second in the world after Chile with a clear
trend towards younger age of onset (97). However, research examining the
relationship between cervical cancer and RASA1 is limited. Zhang
et al revealed that miR-21 was highly expressed in the serum
of cervical cancer patients compared with healthy control subjects.
In addition, the cervical cancer cell lines HeLa and HT-3 were used
and it was revealed that miR-21 decreased the expression of RASA1,
which led to increased cell proliferation and migration via
Ras-induced epithelial-mesenchymal transition (95). In the development of ovarian cancer,
the circular RNA circ-ITCH was revealed to extensively inhibit the
viability and motility of ovarian cancer cell lines SK-OV-3 and
Caov-3 and dampens tumorigenesis in xenografted NOD mice by
upregulating RASA1 expression (96).
Leukaemia (0.43 million new cases and 0.31 million
deaths in 2018) is the most common type of cancer in children and
young adults, highlighting the importance of discovering novel
tumour markers for early diagnosis (79). Scans at DNA and RNA levels using
microarray technology in HL-60 cells have demonstrated that RASA1
is a candidate cancer-related gene (53). Lubeck et al confirmed that a
lack of RasGAP alone in T cells in RASA1 and NF1 double-deficient
mice leads to the development of T cell acute lymphoblastic
leukaemia/lymphoma (98). Falconi
et al revealed that RASA1 was downregulated and inhibited
MSC clone formation in acute myeloid leukaemia patients (51). RASA1 has been revealed to be
regulated by miR-223 although the mechanism in leukaemia requires
further research (99).
OSCCs are a group of aggressive tumours known for
their rapid spread to the lymph nodes and a high treatment failure
rate. In addition, the incidence rate of OSCC is increasing
(incidence increase ranging from 0.4 to 3.3% per year) among young
people below the age of 50 (100).
By sequencing the entire genome of 50 paired primary tumours of the
tongue and 120 OSCC from male individuals in Taiwan, it has been
revealed that RASA1 variants are related to cigarette smoking,
betel nut chewing, human papillomavirus infection, and tumour stage
(101,102). The development of betel quid
chewing-associated tongue carcinomas has been revealed to be
related to mutations in RASA1 and CpG islands (103). The expression of miR-182 in OTSCC
was revealed to be significantly upregulated, and negatively
correlated with RASA1 levels, suggesting that the miR-182/RASA1
axis is a potential target for the treatment of OSCC (104).
While pancreatic cancer is not as common as other
tumours, the outcome is markedly poor (105). In pancreatic cancer, several gene
mutations including RASA1 are more pronounced (106). Kent et al reported that the
expression of RASA1 was decreased in pancreatic cancer cells and
further study revealed that RNAi knockdown of RASA1 significantly
enhanced the progression of pancreatic cancer for both Capan-1 and
MiaPaCa2 cell lines consistent with the miR-31 overexpression
phenotype (107).
Prostate cancer incidence is high in Europe and
North America with ~0.45 million and 0.23 million new cases in
2018, respectively (108). RASA1
has been discovered to be a potential target gene for advanced
drug-resistant recurrent prostate cancer by whole transcriptome
sequencing (109). In a
genome-wide association study that included 12,518 cases of
prostate cancer, RASA1 was revealed to be associated with
aggressive prostate cancer while it exhibited no association with
nonaggressive prostate cancer (110).
Melanoma samples in patients with metastatic disease
often exhibit a loss of RASA1 expression and low RASA1 mRNA has
been linked to a decrease in overall survival in patients with
BRAF-mutated melanoma (111,112).
In thyroid or gastroenteropancreatic neuroendocrine
tumours, the relationship between BRAF and multiple gene mutations
including RASA1 has been documented (113,114). Kidney cancer has many subtypes
with a clear increasing trend in the incidence in the United
States. A similar rise in incidence is also seen in Chinese men. In
renal clear cell carcinoma, QKI-5-stable RASA1 mRNA has been found
to directly bind to the reactive elemental region of RASA1 QKI and
subsequently prevents the activation of the RAS-MAPK signalling,
leading to inhibition of cell growth and inducing cell cycle arrest
(115). Another study revealed
that RASA1 may play a key role in the progression of RCC by
decreasing miR-223-3p and subsequently increasing F-box/WD
repeat-containing protein 7 (FBXW7) expression (116).
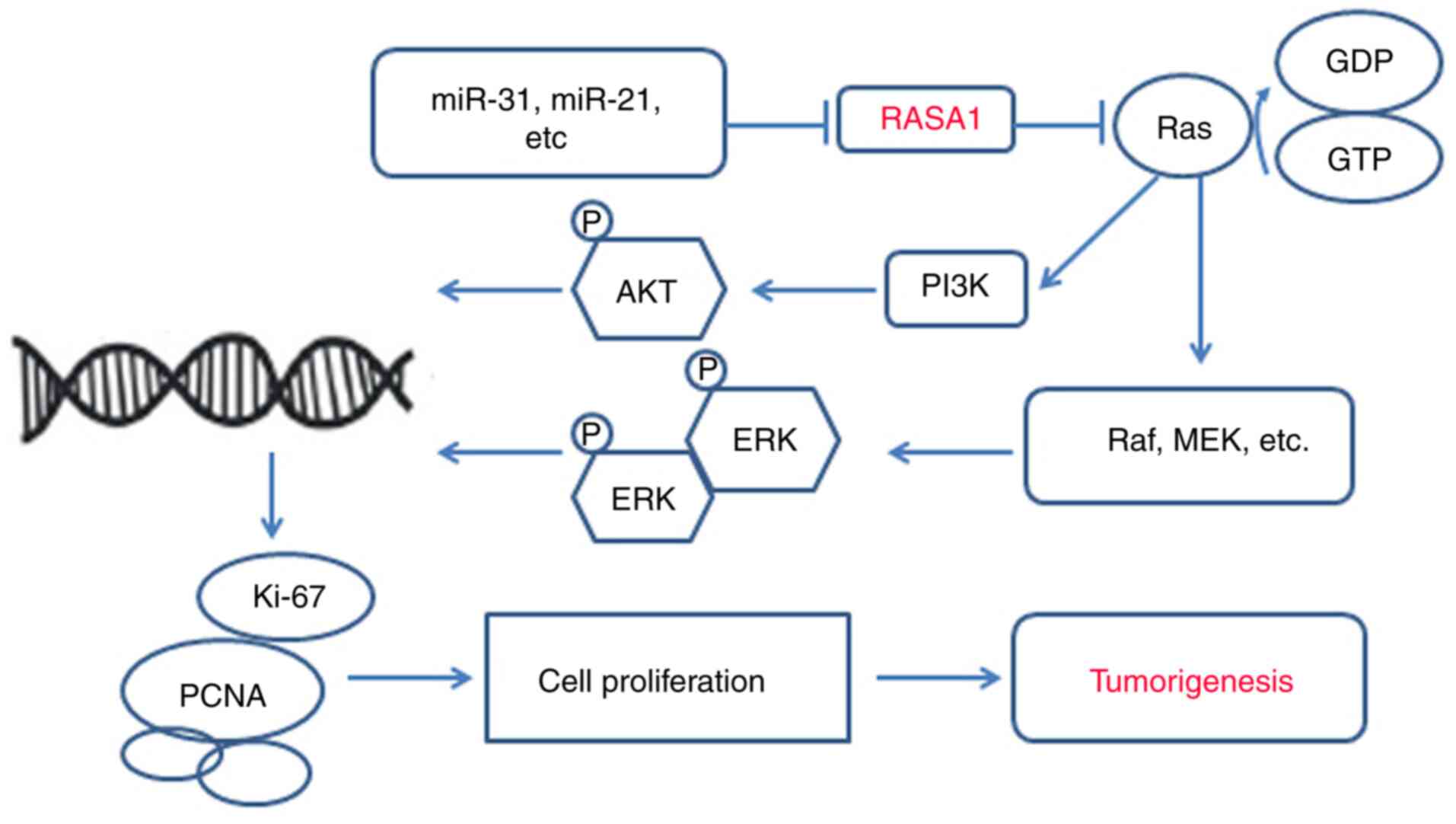
Ras can be activated by inhibiting RasGAPs and
increases tumour development risk (122). In this review, we revealed that
mutations or aberrant expression of RASA1 are present in almost all
cancers and tumour cells and that during the development of
tumours, Ras/Raf/MEK/ERK play a central role (59,78,104).
This signalling pathway is connected by small GTP protein-membrane
binding ligands that initiate a cascade of receptor tyrosine
kinases and cytoplasmic proteins. The first protein kinase RAF in
the RAS recruitment cascade is activated after GTP binding on the
cell membrane through a series of complex processes including
changing the phosphorylation state of the protein and binding with
the skeleton protein and other kinases. The activated Raf kinase
continues to phosphorylate and activate MAPKK protein kinase MEK1/2
and finally phosphorylates and activates ERK1/2, inducing abnormal
proliferation, invasion, growth, and distant metastasis of
malignant tumours (75,123). In addition, loss of RASA1 function
can enhance RAS-ERK signal amplification (66,78).
PI3K/AKT have been described as key regulators of cell
proliferation and differentiation and are involved in tumour cell
proliferation, invasion, and metastasis (124). Some studies have revealed that
aberrant RASA1 expression activates PI3K/AKT signalling (51,63,64).
Moreover, numerous miRNAs have been revealed to regulate RASA1
expression in several cancers such as lung, colorectal,
hepatocellular, and triple-negative breast cancers (60,70,83,91).
The miRNA/RASA1 axis is an attractive target for tumour treatment
and its effectiveness has been demonstrated in numerous studies
(60,70,71,83,92).
miRNAs are capable of regulating RASA1 and could be a novel
targeted treatment strategy for tumours. Numerous miRNAs have been
revealed to be involved in different cancers, such as miR-21 in
lung cancer, and thus these miRNAs should be focused on as targeted
treatments (125). Various genes
or proteins such as RASA1 have fundamental roles at normal levels,
however an imbalance of these genes or proteins has been revealed
to result in disease or tumors, therefore, targeted therapies such
as oncolytic viruses can correct local discordance and treat tumors
without affecting overall function (Fig. 2).
Although RASA1 primarily participates in
angiogenesis and vascular-related diseases, whole-genome sequencing
confirmed the importance of RASA1 in tumorigenesis, invasion,
metastasis, and drug resistance (120). RASA1 was first reported in blood
vessel development or angiogensis and its other functions including
antitumor have been gradually revealed, RASA1 play the similar role
in physiological process such as angiogenesis, vascular-related
diseases or cancers, only the outcome are not same. Normal or
balanced expression levels of RASA1 promote normal blood vessel
development; aberrant or imbalanced RASA1 expression levels result
vascular-related diseases such as CM-AVM or KTWS (40,126).
RASA1 has more than one mechanism of action in the development of
cancers. In some cancers, the role of RASA1 is more profound and
precise (127). From these
pathways, we can conduct genetic screening or testing, including
RASA1 testing in serum, before the cancer fully develops to achieve
early detection and prevention of cancer. Furthermore, these
findings may lead to the development of novel drugs that halt
cancer progression by blocking the pathways it uses for growth and
dissemination. In addition, RASA1 also serves as a good reference
for the prognosis of cancer after surgery and treatment. Therefore,
for some cancers with a less clear mechanism of action, the
aberrant expression of RASA1 may be used to advance cancer
treatment strategies.
Not applicable.
The present study was supported by the Nature
Science Foundation of Hubei Province (grant no. 2017CFB786), the
Hubei Province Health and Family Planning Scientific Research
Project (grant no. WJ2016Y10), the Jingzhou Science and Technology
Bureau Project (grant no. 2017-93), and the National innovation and
entrepreneurship training program for College Students (grant no.
202010489017). All funding was provided to XP.
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the present
study.
XP designed and supervised the study. YZ, YL, BS, HX
and YS reviewed the references. YZ, YL and QW performed the
research. YZ, XP and JC wrote the manuscript. YZ, PS and RL
contributed to tables and figures. XP and JC acquired funding.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Harrell Stewart DR and Clark GJ: Pumping
the brakes on RAS-negative regulators and death effectors of RAS. J
Cell Sci. 133:jcs2388652020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai Y, Walker SA, de Vet E, Cook S, Welch
HC and Lockyer PJ: Ca2+-dependent monomer and dimer formation
switches CAPRI Protein between Ras GTPase-activating protein (GAP)
and RapGAP activities. J Biol Chem. 286:19905–19916. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sot B, Behrmann E, Raunser S and
Wittinghofer A: Ras GTPase activating (RasGAP) activity of the dual
specificity GAP protein Rasal requires colocalization and C2 domain
binding to lipid membranes. Proc Natl Acad Sci USA. 110:111–116.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaber Chehayeb R, Stiegler AL and Boggon
TJ: Crystal structures of p120RasGAP N-terminal SH2 domain in its
apo form and in complex with a p190RhoGAP phosphotyrosine peptide.
PLoS One. 14:e02261132019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henkemeyer M, Rossi DJ, Holmyard DP, Puri
MC, Mbamalu G, Harpal K, Shih TS, Jacks T and Pawson T: Vascular
system defects and neuronal apoptosis in mice lacking ras
GTPase-activating protein. Nature. 377:695–701. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawasaki J, Aegerter S, Fevurly RD,
Mammoto A, Mammoto T, Sahin M, Mably JD, Fishman SJ and Chan J:
RASA1 functions in EPHB4 signaling pathway to suppress endothelial
mTORC1 activity. J Clin Invest. 124:2774–2784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Wang Z, Wu H, Jiang W and Hu R: Ras
ssDNA aptamer inhibits vascular smooth muscle cell proliferation
and migration through MAPK and PI3K pathways. Int J Mol Med.
35:1355–1361. 2015.PubMed/NCBI
|
|
8
|
Lei Z, van Mil A, Brandt MM, Grundmann S,
Hoefer I, Smits M, El Azzouzi H, Fukao T, Cheng C, Doevendans PA,
et al: MicroRNA-132/212 family enhances arteriogenesis after
hindlimb ischaemia through modulation of the Ras-MAPK pathway. J
Cell Mol Med. 19:1994–2005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anand S, Majeti BK, Acevedo LM, Murphy EA,
Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN,
Lapinski PE, et al: MicroRNA-132-mediated loss of p120RasGAP
activates the endothelium to facilitate pathological angiogenesis.
Nat Med. 16:909–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Norden PR, Kim DJ, Barry DM, Cleaver OB
and Davis GE: Cdc42 and k-ras control endothelial tubulogenesis
through apical membrane and cytoskeletal polarization: Novel
stimulatory roles for GTPase effectors, the small GTPases, Rac2 and
Rap1b, and inhibitory influence of Arhgap31 and Rasa1. PLoS One.
11:e01477582016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Teng JM, North PE, Lapinski PE and
King PD: RASA1-dependent cellular export of collagen IV controls
blood and lymphatic vascular development. J Clin Invest.
130:3545–3561. 2019. View Article : Google Scholar
|
|
12
|
Jing L, Li H, Zhang T, Lu J and Zhong L:
MicroRNA-4530 suppresses cell proliferation and induces apoptosis
by targeting RASA1 in human umbilical vein endothelial cells. Mol
Med Rep. 19:3393–3402. 2019.PubMed/NCBI
|
|
13
|
Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao
L, Yu Y, Huang H, Hu Y, Yang Z, et al: MicroRNA-132, delivered by
mesenchymal stem cell-derived exosomes, promote angiogenesis in
myocardial infarction. Stem Cells Int. 2018:32903722018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapinski PE, Kwon S, Lubeck BA, Wilkinson
JE, Srinivasan RS, Sevick-Muraca E and King PD: RASA1 maintains the
lymphatic vasculature in a quiescent functional state in mice. J
Clin Invest. 122:733–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth Flach RJ, Guo CA, Danai LV, Yawe JC,
Gujja S, Edwards YJ and Czech MP: Endothelial Mitogen-activated
protein kinase kinase kinase kinase 4 is critical for lymphatic
vascular development and function. Mol Cell Biol. 36:1740–1749.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lapinski PE, Lubeck BA, Chen D, Doosti A,
Zawieja SD, Davis MJ and King PD: RASA1 regulates the function of
lymphatic vessel valves in mice. J Clin Invest. 127:2569–2585.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castorena-Gonzalez JA, Srinivasan RS, King
PD, Simon AM and Davis MJ: Simplified method to quantify valve
Back-leak uncovers severe mesenteric lymphatic valve dysfunction in
mice deficient in connexins 43 and 37. J Physiol. 598:2297–23102.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lapinski PE, Qiao Y, Chang CH and King PD:
A role for p120 RasGAP in thymocyte positive selection and survival
of naive T cells. J Immunol. 187:151–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pixley FJ, Xiong Y, Yu RY, Sahai EA,
Stanley ER and Ye BH: BCL6 suppresses RhoA activity to alter
macrophage morphology and motility. J Cell Sci. 118:1873–1883.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hancock ML, Preitner N, Quan J and
Flanagan JG: MicroRNA-132 is enriched in developing axons, locally
regulates Rasa1 mRNA, and promotes axon extension. J Neurosci.
34:66–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bros M, Youns M, Kollek V, Buchmüller D,
Bollmann F, Seo EJ, Schupp J, Montermann E, Usanova S, Kleinert H,
et al: Differentially tolerized mouse antigen presenting cells
share a common miRNA signature including enhanced mmu-miR-223-3p
expression which is sufficient to imprint a protolerogenic state.
Front Pharmacol. 9:9152018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schauer SN, Sontakke SD, Watson ED,
Esteves CL and Donadeu FX: Involvement of miRNAs in equine follicle
development. Reproduction. 146:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J and Ruan K: miR-335 is involved in
the rat epididymal development by targeting the mRNA of RASA1.
Biochem Biophys Res Commun. 402:222–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Li D, Wikstrom JD, Pivarcsi A,
Sonkoly E, Ståhle M and Landén NX: MicroRNA-132 promotes fibroblast
migration via regulating RAS p21 protein activator 1 in skin wound
healing. Sci Rep. 7:77972017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi J, Ma X, Su Y, Song Y, Tian Y, Yuan S,
Zhang X, Yang D, Zhang H, Shuai J, et al: MiR-31 mediates
inflammatory signaling to promote re-epithelialization during skin
wound healing. J Invest Dermatol. 138:2253–2263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khalil H, Loukili N, Regamey A,
Cuesta-Marban A, Santori E, Huber M and Widmann C: The
caspase-3-p120-RasGAP module generates a NF-κB repressor in
response to cellular stress. J Cell Sci. 128:3502–3513. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wooderchak-Donahue WL, Johnson P, McDonald
J, Blei F, Berenstein A, Sorscher M, Mayer J, Scheuerle AE, Lewis
T, Grimmer JF, et al: Expanding the clinical and molecular findings
in RASA1 capillary malformation-arteriovenous malformation. Eur J
Hum Genet. 26:1521–1536. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Revencu N, Boon LM, Mendola A, Cordisco
MR, Dubois J, Clapuyt P, Hammer F, Amor DJ, Irvine AD, Baselga E,
et al: RASA1 mutations and associated phenotypes in 68 families
with capillary malformation-arteriovenous malformation. Hum Mutat.
34:1632–1641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J, Streicher JL, Medne L, Krantz ID and
Yan AC: EPHB4 Mutation implicated in capillary
malformation-arteriovenous malformation syndrome: A case report.
Pediatr Dermatol. 34:e227–e230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amyere M, Revencu N, Helaers R, Pairet E,
Baselga E, Cordisco M, Chung W, Dubois J, Lacour JP, Martorell L,
et al: Germline Loss-of-function mutations in EPHB4 cause a second
form of capillary Malformation-arteriovenous malformation (CM-AVM2)
Deregulating RAS-MAPK signaling. Circulation. 136:1037–1048. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pérez-Alfayate R, Martínez-Moreno N,
Rosati SD, Moreu-Gamazo M, Pérez-García C and Martínez-Alvarez R:
Klippel-Trenaunay-Weber syndrome associated with multiple cerebral
arteriovenous malformations: Usefulness of Gamma Knife stereotactic
radiosurgery in this syndrome. World Neurosurg. 141:425–429. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boutarbouch M, Ben Salem D, Giré L, Giroud
M, Béjot Y and Ricolfi F: Multiple cerebral and spinal cord
cavernomas in Klippel-Trenaunay-Weber syndrome. J Clin Neurosci.
17:1073–1075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karadag A, Senoglu M, Sayhan S,
Okromelidze L and Middlebrooks EH: Klippel-Trenaunay-Weber syndrome
with atypical presentation of cerebral cavernous angioma: A case
report and literature review. World Neurosurg. 126:354–358. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Q, Zheng JW, Yang XJ, Wang HJ, Ma D
and Qin ZP: Detection of RASA1 mutations in patients with sporadic
Sturge-Weber syndrome. Childs Nerv Syst. 27:603–607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kadam SD, Gucek M, Cole RN, Watkins PA and
Comi AM: Cell proliferation and oxidative stress pathways are
modified in fibroblasts from Sturge-Weber syndrome patients. Arch
Dermatol Res. 304:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chida A, Shintani M, Wakamatsu H, Tsutsumi
Y, Iizuka Y, Kawaguchi N, Furutani Y, Inai K, Nonoyama S and
Nakanishi T: ACVRL1 gene variant in a patient with vein of Galen
aneurysmal malformation. J Pediatr Genet. 2:181–189.
2013.PubMed/NCBI
|
|
37
|
Komiyama M, Miyatake S, Terada A, Ishiguro
T, Ichiba H and Matsumoto N: Vein of Galen aneurysmal malformation
in monozygotic Twin. World Neurosurg. 91:672.e11–e15. 2016.
View Article : Google Scholar
|
|
38
|
Zweier M and Rauch A: The MEF2C-related
and 5q14.3q15 microdeletion syndrome. Mol Syndromol. 2:164–170.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burrows PE, Gonzalez-Garay ML, Rasmussen
JC, Aldrich MB, Guilliod R, Maus EA, Fife CE, Kwon S, Lapinski PE,
King PD and Sevick-Muraca EM: Lymphatic abnormalities are
associated with RASA1 gene mutations in mouse and man. Proc Natl
Acad Sci USA. 110:8621–8626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Revencu N, Boon LM, Dompmartin A, Rieu P,
Busch WL, Dubois J, Forzano F, van Hagen JM, Halbach S, Kuechler A,
et al: Germline mutations in RASA1 are not found in patients with
Klippel-trenaunay syndrome or capillary malformation with limb
overgrowth. Mol Syndromol. 4:173–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wooderchak-Donahue WL, McDonald J,
O'Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fülöp GT,
Langa C, et al: BMP9 mutations cause a Vascular-anomaly syndrome
with phenotypic overlap with hereditary hemorrhagic telangiectasia.
Am J Hum Genet. 93:530–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hernandez F, Huether R, Carter L, Johnston
T, Thompson J, Gossage JR, Chao E and Elliott AM: Mutations in
RASA1 and GDF2 identified in patients with clinical features of
hereditary hemorrhagic telangiectasia. Hum Genome Var. 2:150402015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin W, Reddy MA, Chen Z, Putta S, Lanting
L, Kato M, Park JT, Chandra M, Wang C, Tangirala RK and Natarajan
R: Small RNA sequencing reveals microRNAs that modulate angiotensin
II effects in vascular smooth muscle cells. J Biol Chem.
287:15672–15683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diao X, Shen E, Wang X and Hu B:
Differentially expressed microRNAs and their target genes in the
hearts of streptozotocin-induced diabetic mice. Mol Med Rep.
4:633–640. 2011.PubMed/NCBI
|
|
45
|
Queirós AM, Eschen C, Fliegner D,
Kararigas G, Dworatzek E, Westphal C, Sanchez Ruderisch H and
Regitz-Zagrosek V: Sex- and estrogen-dependent regulation of a
miRNA network in the healthy and hypertrophied heart. Int J
Cardiol. 169:331–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ying W, Tseng A, Chang RC, Morin A, Brehm
T, Triff K, Nair V, Zhuang G, Song H, Kanameni S, et al:
MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative
macrophage activation. J Clin Invest. 125:4149–4159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nozari A, Aghaei-Moghadam E, Zeinaloo A,
Alavi A, Ghasemi Firouzabdi S, Minaee S, Eskandari Hesari M and
Behjati F: A pathogenic homozygous mutation in the pleckstrin
homology domain of RASA1 is responsible for familial tricuspid
atresia in an Iranian consanguineous family. Cell J. 21:70–77.
2019.PubMed/NCBI
|
|
48
|
Dai X, Yi M, Wang D, Chen Y and Xu X:
Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5
expression in a traumatic brain injury mice model. Biol Chem.
400:753–763. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bijkerk R, de Bruin RG, van Solingen C,
van Gils JM, Duijs JM, van der Veer EP, Rabelink TJ, Humphreys BD
and van Zonneveld AJ: Silencing of microRNA-132 reduces renal
fibrosis by selectively inhibiting myofibroblast proliferation.
Kidney Int. 89:1268–1280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Telegina DV, Korbolina EE, Ershov NI,
Kolosova NG and Kozhevnikova OS: Identification of functional
networks associated with cell death in the retina of OXYS rats
during the development of retinopathy. Cell Cycle. 14:3544–3556.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Falconi G, Fabiani E, Fianchi L, Criscuolo
M, Raffaelli CS, Bellesi S, Hohaus S, Voso MT, D'Alò F and Leone G:
Impairment of PI3K/AKT and WNT/β-catenin pathways in bone marrow
mesenchymal stem cells isolated from patients with myelodysplastic
syndromes. Exp Hematol. 44:75–83.e1-e14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang X, Guo H, Xie A, Liao O, Ju F and
Zhou Y: MicroRNA-144 relieves chronic constriction injury-induced
neuropathic pain via targeting RASA1. Biotechnol Appl Biochem.
67:294–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ulger C, Toruner GA, Alkan M, Mohammed M,
Damani S, Kang J, Galante A, Aviv H, Soteropoulos P, Tolias PP, et
al: Comprehensive genome-wide comparison of DNA and RNA level scan
using microarray technology for identification of candidate
Cancer-related genes in the HL-60 cell line. Cancer Genet
Cytogenet. 147:28–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang XY, Guan M, Vigil D, Der CJ, Lowy DR
and Popescu NC: p120Ras-GAP binds the DLC1 Rho-GAP tumor suppressor
protein and inhibits its RhoA GTPase and growth-suppressing
activities. Oncogene. 28:1401–1409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kamburov A, Lawrence MS, Polak P,
Leshchiner I, Lage K, Golub TR, Lander ES and Getz G: Comprehensive
assessment of cancer missense mutation clustering in protein
structures. Proc Natl Acad Sci USA. 112:E5486–E5495. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li L, Fan Y, Huang X, Luo J, Zhong L, Shu
XS, Lu L, Xiang T, Chan ATC, Yeo W, et al: Tumor suppression of Ras
GTPase-activating protein RASA5 through antagonizing Ras signaling
perturbation in carcinomas. Science. 21:1–18. 2019.
|
|
57
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, Etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu X, Jia Y, Stoopler MB, Shen Y, Cheng
H, Chen J, Mansukhani M, Koul S, Halmos B and Borczuk AC:
Next-generation sequencing of pulmonary sarcomatoid carcinoma
reveals high frequency of actionable MET gene mutations. J Clin
Oncol. 34:794–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Campbell JD, Alexandrov A, Kim J, Wala J,
Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et
al: Distinct patterns of somatic genome alterations in lung
adenocarcinomas and squamous cell carcinomas. Nat Genet.
48:607–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu YJ, Xu B and Xia W: Hsa-mir-182
downregulates RASA1 and suppresses lung squamous cell carcinoma
cell proliferation. Clin Lab. 60:155–159. 2014.PubMed/NCBI
|
|
61
|
Shi L, Middleton J, Jeon YJ, Magee P,
Veneziano D, Laganà A, Leong HS, Sahoo S5, Fassan M, Booton R, et
al: KRAS induces lung tumorigenesis through microRNAs modulation.
Cell Death Dis. 9:2192018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H,
Kim SW, Yang JC, Lee JS, Su WC, Kowalski D, et al: Osimertinib plus
savolitinib in patients with EGFR mutation-positive, MET-amplified,
Non-small-cell lung cancer after progression on EGFR tyrosine
kinase inhibitors: Interim results from a multicentre, open-label,
phase 1b study. Lancet Oncol. 21:373–386. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He J, Jin S, Zhang W, Wu D, Li J, Xu J and
Gao W: Long Non-coding RNA LOC554202 promotes acquired gefitinib
resistance in Non-small cell lung cancer through upregulating
miR-31 expression. J Cancer. 10:6003–6013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hayashi T, Desmeules P, Smith RS, Drilon
A, Somwar R and Ladanyi M: RASA1 and NF1 are preferentially
co-mutated and define a distinct genetic subset of
smoking-associated non-small cell lung carcinomas sensitive to MEK
inhibition. Clin Cancer Res. 24:1436–1447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kitajima S and Barbie DA: RASA1/NF1-Mutant
lung cancer: Racing to the Clinic? Clin Cancer Res. 24:1243–1245.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kas SM, de Ruiter JR, Schipper K, Schut E,
Bombardelli L, Wientjens E, Drenth AP, de Korte-Grimmerink R,
Mahakena S, Phillips C, et al: Transcriptomics and transposon
mutagenesis identify multiple mechanisms of resistance to the FGFR
Inhibitor AZD4547. Cancer Res. 78:5668–5679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Crockett SD and Nagtegaal I: Terminology,
molecular features, epidemiology, and management of serrated
colorectal neoplasia. Gastroenterology. 157:949–966. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim R, Schell MJ, Teer JK, Greenawalt DM,
Yang M and Yeatman TJ: Co-evolution of somatic variation in primary
and metastatic colorectal cancer may expand biopsy indications in
the molecular era. PLoS One. 10:e01266702015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J,
Zhang CY, Chen J and Zhang J: MicroRNA-31 activates the RAS pathway
and functions as an oncogenic MicroRNA in human colorectal cancer
by repressing RAS p21 GTPase activating protein 1 (RASA1). J Biol
Chem. 288:9508–9518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: MiR-21 RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Y, Weng W, Peng J, Hong L, Yang L,
Toiyama Y, Gao R, Liu M, Yin M, Pan C, et al: Fusobacterium
nucleatum increases proliferation of colorectal cancer cells and
tumor development in mice by activating Toll-like receptor 4
signaling to nuclear factor-κB, and Up-regulating expression of
MicroRNA-21. Gastroenterology. 152:851–866.e24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sun D, Wang C, Long S, Ma Y, Guo Y, Huang
Z, Chen X, Zhang C, Chen J and Zhang J: C/EBP-β-activated
microRNA-223 promotes tumour growth through targeting RASA1 in
human colorectal cancer. Br J Cancer. 112:1491–1500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lu Y, Yang H, Yuan L, Liu G, Zhang C, Hong
M, Liu Y, Zhou M, Chen F and Li X: Overexpression of miR-335
confers cell proliferation and tumour growth to colorectal
carcinoma cells. Mol Cell Biochem. 412:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Antoine-Bertrand J, Duquette PM, Alchini
R, Kennedy TE, Fournier AE and Lamarche-Vane N: p120RasGAP protein
mediates Netrin-1 protein-induced cortical axon outgrowth and
guidance. J Biol Chem. 291:4589–4602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yarom N, Gresham G, Boame N and Jonker D:
KRAS status as a predictor of chemotherapy activity in patients
with metastatic colorectal cancer. Clin Colorectal Cancer.
18:e309–e315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Organ SL, Hai J, Radulovich N, Marshall
CB, Leung L, Sasazuki T, Shirasawa S, Zhu CQ, Navab R, Ikura M, et
al: p120RasGAP is a mediator of rho pathway activation and
tumorigenicity in the DLD1 colorectal cancer cell line. PLoS One.
9:e861032014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Post JB, Hami N, Mertens AEE, Elfrink S,
Bos JL and Snippert HJG: CRISPR-induced RASGAP deficiencies in
colorectal cancer organoids reveal that only loss of NF1 promotes
resistance to EGFR inhibition. Oncotarget. 10:1440–1457. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. Apr
22–2020.(Epub ahead of print). View Article : Google Scholar
|
|
81
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM and Thorgeirsson SS: Inactivation of Ras
GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen YL, Huang WC, Yao HL, Chen PM, Lin
PY, Feng FY and Chu PY: Down-regulation of RASA1 is associated with
poor prognosis in human hepatocellular carcinoma. Anticancer Res.
37:781–785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hu C, Huang F, Deng G, Nie W, Huang W and
Zeng X: miR-31 promotes oncogenesis in intrahepatic
cholangiocarcinoma cells via the direct suppression of RASA1. Exp
Ther Med. 6:1265–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Du C, Weng X, Hu W, Lv Z, Xiao H, Ding C,
Gyabaah OA, Xie H, Zhou L, Wu J and Zheng S: Hypoxia-inducible
MiR-182 promotes angiogenesis by targeting RASA1 in hepatocellular
carcinoma. J Exp Clin Cancer Res. 34:672015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tai WT, Chen YL, Chu PY, Chen LJ, Hung MH,
Shiau CW, Huang JW, Tsai MH and Chen KF: Protein tyrosine
phosphatase 1B dephosphorylates PITX1 and regulates p120RasGAP in
hepatocellular carcinoma. Hepatology. 63:1528–1543. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Vanli G, Sempoux C and Widmann C: The
caspase-3/p120 RasGAP Stress-sensing module reduces liver cancer
incidence but does not affect overall survival in gamma-irradiated
and carcinogen-treated mice. Mol Carcinog. 56:1680–1684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sancho-Garnier H and Colonna M: Breast
cancer epidemiology. Presse Med. 48:1076–1084. 2019.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Suárez-Cabrera C, Quintana RM, Bravo A,
Casanova ML, Page A, Alameda JP, Paramio JM, Maroto A, Salamanca J,
Dupuy AJ, et al: A transposon-based analysis reveals RASA1 is
involved in triple-negative breast cancer. Cancer Res.
77:1357–1368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hu X, Stern HM, Ge L, O'Brien C, Haydu L,
Honchell CD, Haverty PM, Peters BA, Wu TD, Amler LC, et al: Genetic
alterations and oncogenic pathways associated with breast cancer
subtypes. Mol Cancer Res. 7:511–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu Y, Liu T, Sun Q, Niu M, Jiang Y and
Pang D: Downregulation of Ras GTPase activating protein 1 is
associated with poor survival of breast invasive ductal carcinoma
patients. Oncol Rep. 33:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang J, Peng X, Zhang K, Li C, Su B,
Zhang Y and Yu W: Co-expression and significance of Dok2 and Ras
p21 protein activator 1 in breast cancer. Oncol Lett. 14:5386–5392.
2017.PubMed/NCBI
|
|
92
|
Sharma SB, Lin CC, Farrugia MK, McLaughlin
SL, Ellis EJ, Brundage KM, Salkeni MA and Ruppert JM: MicroRNAs 206
and 21 cooperate to promote RAS-extracellular signal-regulated
kinase signaling by suppressing the translation of RASA1 and
SPRED1. Mol Cell Biol. 34:4143–4164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xiao W, Zheng S, Zou Y, Yang A and Xie X,
Tang H and Xie X: CircAHNAK1 inhibits proliferation and metastasis
of Triple-negative breast cancer by modulating miR-421 and RASA1.
Aging (Albany NY). 11:12043–12056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kanwar N, Carmine-Simmen K, Nair R, Wang
C, Moghadas-Jafari S, Blaser H, Tran-Thanh D, Wang D, Wang P, Wang
J, et al: Amplification of a calcium channel subunit CACNG4
increases breast cancer metastasis. EBioMedicine. 52:1026462020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang L, Zhan X, Yan D and Wang Z:
Circulating: MicroRNA-21 is involved in lymph node metastasis in
cervical cancer by targeting RASA1. Int J Gynecol Cancer.
26:810–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hu J, Wang L, Chen J, Gao H, Zhao W, Huang
Y, Jiang T, Zhou J and Chen Y: The circular RNA circ-ITCH
suppresses ovarian carcinoma progression through targeting
miR-145/RASA1 signaling. Biochem Biophys Res Commun. 505:222–228.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jiang X, Tang H and Chen T: Epidemiology
of gynecologic cancers in China. J Gynecol Oncol. 29:e72018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lubeck BA, Lapinski PE, Oliver JA, Ksionda
O, Parada LF, Zhu Y, Maillard I, Chiang M, Roose J and King PD:
GTPase-activating proteins (RasGAPs) Neurofibromin 1 and p120
RasGAP in T cells results in the development of T cell acute
lymphoblastic Leukemia. J Immunol. 195:31–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao
QL, Wu W and Wu XZ: MiR-223 suppresses cell proliferation by
targeting IGF-1R. PLoS One. 6:e270082016. View Article : Google Scholar
|
|
100
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Krishnan N, Gupta S, Palve V, Varghese L,
Pattnaik S, Jain P, Khyriem C, Hariharan A, Dhas K, Nair J, et al:
Integrated analysis of oral tongue squamous cell carcinoma
identifies key variants and pathways linked to risk habits, HPV,
clinical parameters and tumor recurrence. F1000Res. 4:12152015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Su SC, Lin CW, Liu YF, Fan WL, Chen MK, Yu
CP, Yang WE, Su CW, Chuang CY, Li WH, et al: Exome sequencing of
oral squamous cell carcinoma reveals molecular subgroups and novel
therapeutic opportunities. Theranostics. 7:1088–1099. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang W, Wang M, Wu Q, Zhu Q, Jiao Y, Zhu
Y, Yang B, Ni S, Yu J, Sun H and Zeng YX: Mutational signatures and
the genomic landscape of betel quid Chewing-associated tongue
carcinoma. Cancer Med. 8:701–711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang J, Wang W, Li J, Wu L, Song M and
Meng Q: miR182 activates the Ras-MEK-ERK pathway in human oral
cavity squamous cell carcinoma by suppressing RASA1 and SPRED1.
Onco Targets Ther. 10:667–679. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rajamani D and Bhasin MK: Identification
of key regulators of pancreatic cancer progression through
multidimensional systems-level analysis. Genome Med. 8:382016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kent OA, Mendell JT and Rottapel R:
Transcriptional regulation of miR-31 by oncogenic KRAS mediates
metastatic phenotypes by repressing RASA1. Mol Cancer Res.
14:267–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Culp MB, Soerjomataram I, Efstathiou JA,
Bray F and Jemal A: Recent global patterns in prostate cancer
incidence and mortality rates. Eur Urol. 77:38–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sowalsky AG, Xia Z, Wang L, Zhao H, Chen
S, Bubley GJ, Balk SP and Li W: Whole transcriptome sequencing
reveals extensive unspliced mRNA in metastatic castration-resistant
prostate cancer. Mol Cancer Res. 13:98–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Berndt SI, Wang Z, Yeager M, Alavanja MC,
Albanes D, Amundadottir L, Andriole G, Beane Freeman L, Campa D,
Cancel-Tassin G, et al: Two susceptibility loci identified for
prostate cancer aggressiveness. Nat Commun. 6:68892015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Laczny C, Leidinger P, Haas J, Ludwig N,
Backes C, Gerasch A, Kaufmann M, Vogel B, Katus HA, Meder B, et al:
miRTrail-a comprehensive webserver for analyzing gene and miRNA
patterns to enhance the understanding of regulatory mechanisms in
diseases. BMC Bioinformatics. 13:362012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sung H, Kanchi KL, Wang X, Hill KS,
Messina JL, Lee JH, Kim Y, Dees ND, Ding L, Teer JK, et al:
Inactivation of RASA1 promotes melanoma tumorigenesis via R-Ras
activation. Oncotarget. 7:23885–23996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rusinek D, Swierniak M, Chmielik E, Kowal
M, Kowalska M, Cyplinska R, Czarniecka A, Piglowski W, Korfanty J,
Chekan M, et al: BRAFV600E-associated gene expression profile:
Early changes in the transcriptome, based on a transgenic mouse
model of papillary thyroid carcinoma. PLoS One. 10:e01436882015.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Park C, Ha SY, Kim ST, Kim HC, Heo JS,
Park YS, Lauwers G, Lee J and Kim KM: Identification of the BRAF
V600E mutation in gastroenteropancreatic neuroendocrine tumors.
Oncotarget. 7:4024–4035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang RL, Yang JP, Peng LX, Zheng LS, Xie
P, Wang MY, Cao Y, Zhang ZL, Zhou FJ, Qian CN and Bao YX:
RNA-binding protein QKI-5 inhibits the proliferation of clear cell
renal cell carcinoma via post-transcriptional stabilization of
RASA1 mRNA. Cell Cycle. 15:3094–3104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang RL, Aimudula A, Dai JH and Bao YX:
RASA1 inhibits the progression of renal cell carcinoma by
decreasing the expression of miR-223-3p and promoting the
expression of FBXW7. Biosci Rep. 40:BSR201941432020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nie Y, Wu K, Yu J, Liang Q, Cai X, Shang
Y, Zhou J, Pan K, Sun L, Fang J, et al: A global burden of gastric
cancer: The major impact of China. Expert Rev Gastroenterol
Hepatol. 11:651–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li Z, Li D, Zhang G, Xiong J, Jie Z, Cheng
H, Cao Y, Jiang M, Lin L, Le Z, et al: Methylation-associated
silencing of MicroRNA-335 contributes tumor cell invasion and
migration by interacting with RASA1 in gastric cancer. Am J Cancer
Res. 4:648–662. 2014.PubMed/NCBI
|
|
119
|
Chen X, Cai S, Li B, Zhang X, Li W, Liang
H, Cao X, Wang L and Wu Z: MicroRNA-21 regulates the biological
behavior of esophageal squamous cell carcinoma by targeting RASA1.
Oncol Rep. 41:1627–1637. 2019.PubMed/NCBI
|
|
120
|
Pickering CR, Zhou JH, Lee JJ, Drummond
JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, et
al: Mutational landscape of aggressive cutaneous squamous cell
carcinoma. Clin Cancer Res. 20:6582–6592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jouenne F, Reger de Moura C, Lorillon G,
Meignin V, Dumaz N, Lebbe C6 Mourah S and Tazi A: RASA1 loss in a
BRAF-mutated Langerhans cell sarcoma: A mechanism of resistance to
BRAF inhibitor. Ann Oncol. 30:1170–1172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sears R and Gray JW: Epigenomic
inactivation of RasGAPs activates RAS signaling in a subset of
luminal b breast cancers. Cancer Discov. 7:131–133. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Degirmenci U, Wang M and Hu J: Targeting
Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells.
9:1982020. View Article : Google Scholar
|
|
124
|
Hoxhaj G and Manning BD: The PI3K-AKT
network at the interface of oncogenic signalling and cancer
metabolism. Nat Rev Cancer. 20:74–88. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Markou A, Zavridou M and Lianidou ES:
miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer
(Auckl). 7:19–27. 2016.PubMed/NCBI
|
|
126
|
Revencu N, Fastre E, Ravoet M, Helaers R,
Brouillard P, Bisdorff-Bresson A, Chung CWT, Gerard M, Dvorakova V,
Irvine AD, et al: RASA1 mosaic mutations in patients with capillary
malformation-arteriovenous malformation. J Med Genet. 57:48–52.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu Z, Deng M, Wu L and Zhang S: An
integrative investigation on significant mutations and their
down-stream pathways in lung squamous cell carcinoma reveals
CUL3/KEAP1/NRF2 relevant subtypes. Mol Med. 26:482020. View Article : Google Scholar : PubMed/NCBI
|