Primary central nervous system lymphoma (PCNSL) and
high-grade glioma (HGG) are malignant brain tumors, with annual
incidence rates of ~0.45 and 4 per 100,000 population,
respectively, in the United State (1). The diseases can mimic each other in
clinical, radiological and even pathological examinations (2,3);
however, they require completely different management.
Approximately 90% of PCNSL cases are diffuse large B-cell lymphomas
(DLBCL). High-dose methotrexate-based polychemotherapy plus
rituximab is currently the treatment of choice for CNS DLBCL
(4). Conversely, the primary
treatment modality for HGG is surgery, followed by chemotherapy and
radiotherapy (5). Furthermore,
prior to surgery, corticosteroids can be administered to decrease
the symptomatic tumor-associated edema of HGG. As the management is
completely different for these entities, a precise diagnosis is
crucial.
PCNSL and HGG can display overlapping clinical,
radiological and partial pathological features that cause
challenges in making the differential diagnosis between them.
Firstly, both entities show a predilection for males and older
patients (>65 years old) (1),
commonly involving the deep white matter and the corpus callosum
(6,7). The symptoms are associated with the
involved areas of the brain, such as focal neurological deficits,
neurocognitive impairment, seizures and signs of elevated
intracranial pressure (headaches, nausea and vomiting) (6,8).
Secondly, PCNSL and glioblastoma multiforme (GBM) can mimic each
other in both imaging and histological examinations. For example, a
case report (9) showed that CNS
DLBCL in an immunocompromised patient was misdiagnosed as GBM by
ring-like enhancement on contrast-enhanced T1-weighted imaging
(CE-T1WI) and obvious edema in T2-fluid-attenuated inversion
recovery (T2-FLAIR) images. This case was also misdiagnosed as GBM
on intraoperative frozen section examination showing coagulation
necrosis and atypical cell infiltration. DLBCL was recorded as the
final diagnosis until postoperative formalin-fixed,
paraffin-embedded (FFPE) tissue examination accompanied by
immunohistochemistry (IHC) staining was performed. Similarly,
another case report (10)
mentioned that GBM was also nearly misdiagnosed as CNS DLBCL on
magnetic resonance imaging (MRI) with and without gadolinium-based
contrast, which showed little surrounding edema and mild diffusion
restriction. Flow cytometry of the cerebrospinal fluid (CSF) also
revealed an abnormal B-cell population, providing further evidence
for the diagnosis of lymphoma. However, a stereotactic biopsy of
the brain mass confirmed the diagnosis of GBM.
Pathological changes in FFPE tissues accompanied by
IHC staining are acknowledged as the gold standard for the
diagnosis of brain tumors. Microscopically, PCNSL typically
exhibits high cellularity and a diffuse growth pattern. Large
central areas of geographical necrosis are usually accompanied by
variable to frequent perivascular lymphoma islands from central to
peripheral lesions. Astrocytic and microglial proliferation, as
well as inflammatory reactions, are often observed in the
surrounding parenchyma. GBM also presents with large central
necrotic areas surrounded by accumulated pleomorphic glial tumor
cells in the periphery. Compared with PCNSL, HGG displays
microvascular proliferation, which is usually most marked around
necrotic foci and in the peripheral zone of infiltration. In some
cases, there are isolated tumor cells or islands infiltrating the
parenchyma rather than distinct masses, or the tumor cells show
obvious heterogeneity, especially in stereotaxic needle biopsies;
thus, further IHC staining is needed to make a differential
diagnosis (11).
Stereotactic biopsy is sometimes inconclusive after
the use of corticosteroids, which can induce rapid tumor shrinkage
and false-negative results (12),
especially with limited specimens. In one study, the rate of
occurrence of false-negative biopsies after <1 week of steroid
treatment was 33.3%, increasing to 57.1% after >1 week of
steroid treatment (2). A
reasonable interval of at least 2–4 weeks after withholding steroid
usage is recognized as necessary prior to brain biopsy (2,13–15),
yet this causes a delay in the diagnosis of PCNSL. Furthermore,
stereotactic biopsy is not always feasible in patients (13). Thus, imaging, liquid analysis and
molecular tests are quite meaningful for prompt diagnosis and can
provide circumstantial evidence for pathological examinations,
especially with limited samples. Although independent diagnostic
research progress for PCNSL and HGG is abundant, the present
narrative review only includes the progress from comparative
diagnostic studies between PCNSL and HGG, with the purpose of
building a liaison between radiology and pathology, as well as to
provide more options for clinicians to improve the accuracy and
speed of the decision-making process.
DWI calculates the apparent diffusion coefficient
(ADC) values to quantify the diffusion of unbound extracellular
water molecules (19). Differences
in cellularity between PCNSL and GBM can be assessed using DWI.
High cellular density and a large nuclear to cytoplasmic ratio lead
to the reduction of water diffusion (19), which causes increased signal
shadows and a decreased ADC value (20), differentiating untreated PCNSL from
GBM (21–24). Different ADC parameters have been
discussed for the purpose of differential diagnosis, including
ADCmin, rADCmean, and ADC5% [the
lowest and mean ADC value obtained from placed regions of interest
was defined as the minimum ADC (ADCmin) and mean ADC
(ADCmean), respectively.
rADCmean=(ADCmean of the
tumor)/(ADCmean of the normal appearing white matter of
the contralateral hemisphere). Percentile values are the Nth
percentile from the ADC histograms that reflect the distribution of
ADC values in the region of interest. ADC5% is the fifth
percentile from the ADC histograms that reflect the distribution of
ADC values in the region of interest]. All ADC parameters are lower
in PCNSL compared with those in GBM. A previous study (25) demonstrated a cutoff value of
0.68×10−3 mm2/sec for ADC5% to
differentiate CNS DLBCL and GBM, with a sensitivity of 100%. In
addition, molecular types of tumors may affect ADC values. For
example, MYC proto-oncogene bHLH transcription factor (MYC) and
BCL2 apoptosis regulator (BCL2) gene rearrangements in CNS DLBCL
lead to lower rADCmin and rADCmean values,
while isocitrate dehydrogenase [NADP(+)]1 (IDH1) mutation in GBM
leads to higher values, when compared with wild-types (18). Methylation of the
O(6)-methylguanine-DNA methyltransferase (MGMT) promoter in GBM may
lead to higher ADC values with lower perfusion (26). Epidermal growth factor receptor
variant III mutant GBM showed a higher cell density, leading to
increased perfusion and a lower ADC value (27).
Combined conventional MRI and DWI can more
accurately reflect histologically related information for the
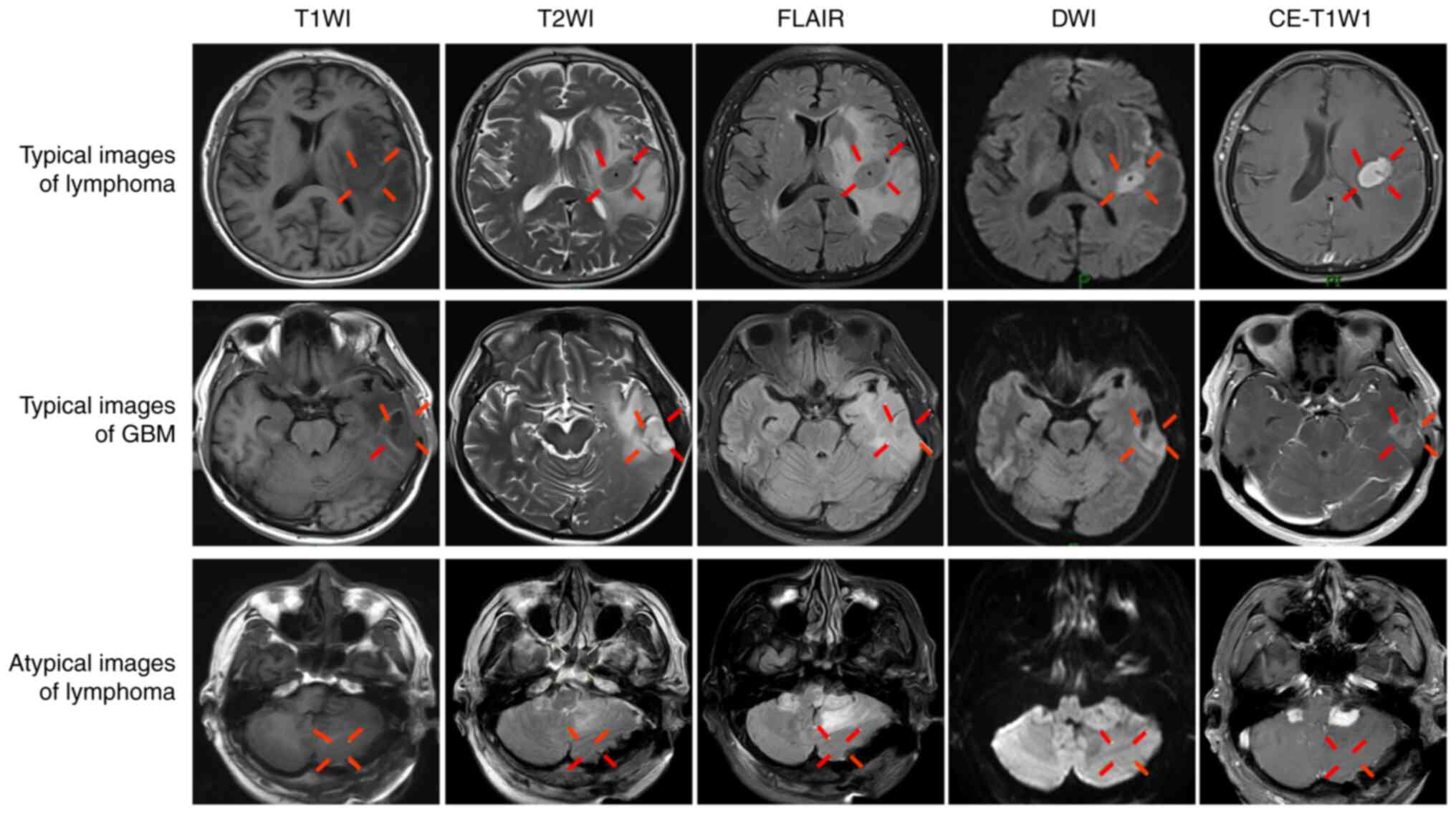
differential diagnosis. Fig. 1
presents representative combined images from the Department of
Radiology of China-Japan Union Hospital of Jilin University
(Changchun, China). For example, typical images of a representative
DLBCL case occurring in the left temporal lobe are shown in
Fig. 1. The lesions were
classified as isointense on T1WI and T2WI, as high intensity on
FLAIR, surrounded by finger-like edema limited diffusion on DWI,
and as uniform with obvious enhancement on CE-T1W1. Representative
images from a GBM case are also shown in Fig. 1. The lesions occurred in the left
temporal lobe, with a low signal on T1WI and a high signal
surrounded by finger-like edema on T2WI. The lesions were
isointense on FLAIR, and showed restricted diffusion on DWI and
garland-like enhancement on CE-T1W1. However, lymphoma often
presents with atypical images, not only in conventional MRI, but
also in DWI. Fig. 1 shows a
representative case in which the lesions, which occurred in the
posterior fossa and were finally diagnosed as DLBCL by pathological
examination, presented as isointense on T1WI and T2WI, as slightly
hyperintense on FLAIR, with no surrounding edema and no restricted
diffusion on DWI, and as obvious uniform enhancement on CE-T1WI.
Thus, more parameters were needed for the differential
diagnosis.
DSC MRI is perfusion MRI using contrast medium
injection. DSC MRI tracks the T2 weighted signal to the image and
calculates perfusion metrics (28). Differences in angiogenesis between
PCNSL and HGG can be reflected by cerebral blood flow (CBF) and
cerebral blood volume (CBV). Compared with HGG, PCNSL displays
lower CBF and CBV values due to the absence of neovascularization
(29). Neska-Matuszewska et
al (30) reported that max
rCBV [max rCBV=(CBVmax of the tumor)/(CBVmax
of the normal appearing white matter of the contralateral
hemisphere)] demonstrated a high accuracy of 98.5% in
differentiating 16 CNS B-cell lymphomas from 20 GBMs and 20
metastases. Arterial spin labeling (ASL) can be used to detect CBF
values by non-invasively labeling blood without injection of
contrast medium (31). It is
suitable for patients who cannot undergo the injection of a
contrast agent. A meta-analysis showed that DSC had a higher
diagnostic accuracy than ASL in differentiating HGG from PCNSL
(31). However, the accuracy of
CBV decreases due to damage to the blood-brain barrier (BBB) from
both HGG and PCNSL, which causes contrast agent leakage (32). A preload contrast dose is used to
minimize the effects of leakage (19). After a preload injection, Chaganti
et al (33) used a mean
rCBV of 2.68 to differentiate 11 PCNSLs from 15 HGGs, with an area
under the curve (AUC) of 1.000.
Serious BBB damage can be analyzed by percentage
signal recovery (PSR) and volume transfer constant
(Ktrans), which can compensate for the deficiency of
CBV. In the study by Cindil et al (32), both parameters were reported to be
higher in PCNSL than in HGG. The PSR was calculated from the
time-signal curve of DSC MRI. PSR represents a complex combination
of tissue microstructure and hemodynamic effects, such as blood
flow, vascular permeability and cellular geometry (34). PSR is a promising parameter that
performs better than rCBV in both a PSR-optimized protocol without
preload (AUC, 0.979) (32) and a
CBV-optimized protocol with preload (AUC, 0.830) (35). Ktrans is a parameter of
permeability calculated from T1-weighted signal curves and is
obtained from DCE MRI (28). It is
still controversial to evaluate the effect of the Ktrans
parameter for the differentiation between PCNSL and HGG. One
previous study (21) reported no
significant difference in the distribution of Ktrans
between CNS DLBCL and GBM. However, another study by Lu et
al (22) revealed that a
cutoff of 0.187 for Ktrans reached a high AUC of 0.852
for differentiating CNS DLBCL from GBM.
Differences in microhemorrhage, calcification and
neovascularity (veins) between CNS DLBCL and GBM could be reflected
by the intratumoral susceptibility signal (ITSS) on SWI (36). A higher ITSS presents more dot-like
foci of susceptibility within the tumor. In one study, the ITSS was
significantly higher in GBM than in B-cell PCNSL, and performed
well in the differentiation of PCNSL from GBM, with an AUC of 0.800
(37). In addition, a recent study
(18) reported lower ITSS and
higher rSWI values [rSWI=(mean SWI of the tumor)/(mean SWI of the
matching contralateral normal-appearing white matter)] in
IDH1-mutant GBM compared with those in wild-type GBM, suggesting
that it would be difficult to distinguish B-cell type PCNSL from
IDH1-mutant GBM by ITSS or mean rSWI value.
The alterations in metabolites such as lipid and
myo-inositol (mIns) within the tumor could be detected by MRS.
Lipid levels are associated with necrosis and the activation of
lymphocytes and macrophages, which are significantly higher in
PCNSL than in GBM (8,38). The mIns level reflects the
expression of inositol-3-phosphate synthase 1 (ISYNA1), which is
the rate-limiting enzyme of the first step in the biosynthesis of
mIns. Higher ISYNA1 expression in GBM leads to a higher mIns level
(39).
Different uptake of radiotracers between PCNSL and
HGG is presented by the tumor to normal contralateral cortex
activity (T/N) ratio and standardized uptake value (SUV). The SUV
and T/N ratio are semiquantitative indicators of radiotracer
utilization. Most clinical centers use a radiolabeled glucose
analog such as [18F] fluorodeoxyglucose (FDG) as the tracer
(19). Other tracers such as
11C-methionine also show potential value in differentiating PCNSL
from GBM; however, 11C-methionine has limited value in the clinical
routine due to its short half-life (20 min) (40). The SUV of FDG is significantly
higher in CNS lymphoma than in GBM due to the higher tumor cell
density with elevated glycolytic metabolism (41). Nakajima et al (25) reported that a cutoff
SUVmax value of 9.35 performed well in differentiating
CNS DLBCL from GBM, with an AUC of 0.933. Similarly, Zhou et
al (42) reported an AUC of
0.910 for differentiating 40 PCNSLs from 52 GBMs, with a higher
SUVmax cutoff value of 13.77. However, some atypical
PCNSLs show low FDG uptake, which is closely associated with the
negative expression of mutated melanoma-associated antigen 1
protein (43).
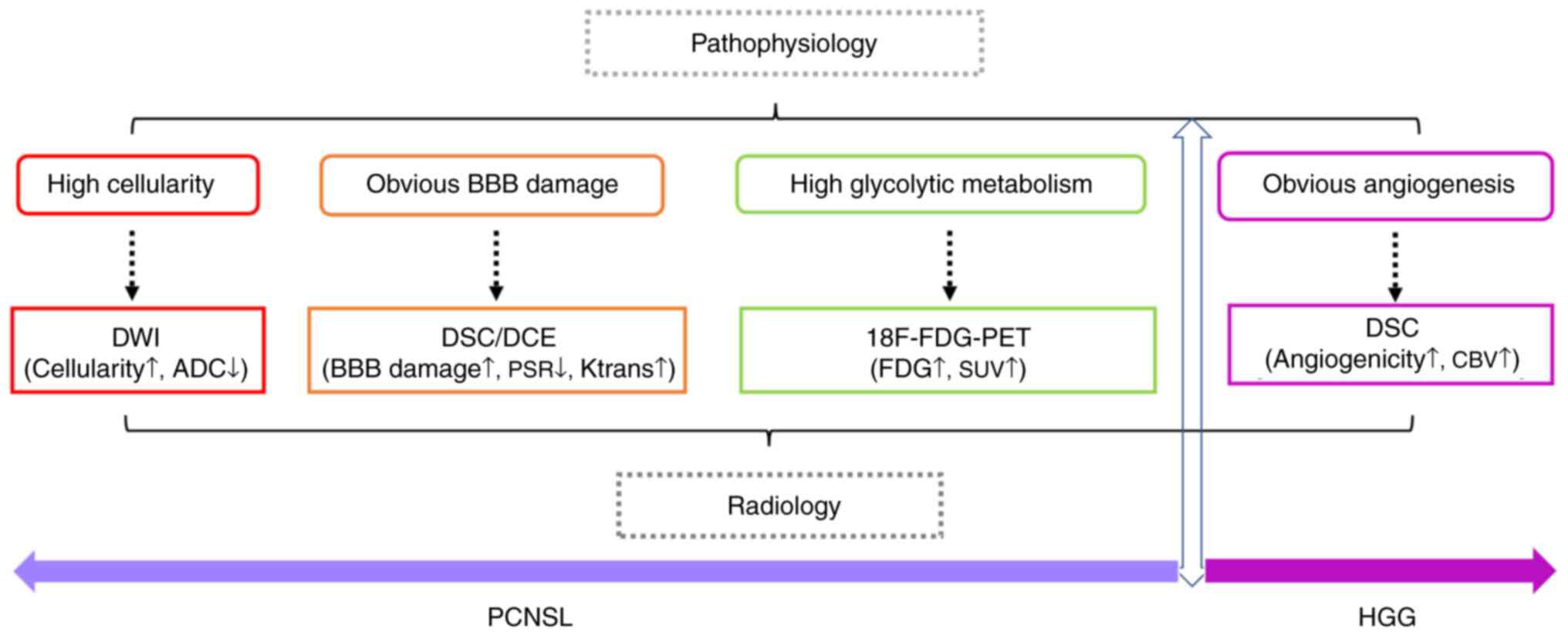
Details of all radiological parameters and case
numbers of PCNSL and GBM involved in a series of studies are
summarized in Table SI. Marked
differences between PCNSL and HGG were observed in DWI, DSC/DCE and
18F-FDG-PET/CT, which corresponded to the pathophysiological
features of the two entities. As shown in Fig. 2, PCNSL presents with a diffuse
growth pattern, obvious BBB damage and high cellularity associated
with elevated glycolytic metabolism, which correspond to low ADC
values in DWI, increased PSR values in DSC MRI, increased
Ktrans values in DCE MRI and high FDG uptake on PET/CT.
Furthermore, the feature of microvascular proliferation of HGG can
be reflected by an increased CBV value in DSC.
Radiomics analyses provide promising evidence for a
differential diagnosis, especially in tumors with high
heterogeneity (44). Radiomics
includes feature-based and deep learning-based components.
Feature-based radiomics uses a series of mathematically predefined
features that are typically extracted from a segmented region
(45). Deep learning-based
radiomics utilizes artificial neural networks that imitate the
vision of humans and automatically extract high-dimensional
features from the input images at different levels of scaling and
abstraction (45).
The diagnostic value of image features extracted
from different MRI types was researched for feature-based
radiomics. Priya et al (46) found that features extracted from
CE-T1WI could distinguish between 46 PCNSLs and 97 GBMs, with an
AUC of 0.924. Different combinations of conventional MRI such as
T1WI, T2WI, CE-T1WI and FLAIR also performed well using
feature-based radiomics (47–49).
MacIver et al (50)
reported the high performance of an ADC map for diagnosing 48
PCNSLs and 42 GBMs (AUC, 0.880). Mehrnahad et al (51) also concluded the usefulness of
feature-based radiomics derived from an ADC map in differentiating
57 GBMs from 25 PCNSLs. The diffusion condition of tumors in the
ADC map was combined with CE-T1WI and analyzed by feature-based
radiomics. The combination was as effective as radiologists, with
an AUC of 0.946 when differentiating 14 PCNSLs from 28 GBMs
(52). In one study, a radiomics
model combining CE-T1WI and ADC maps (AUC, 0.935) performed better
than veteran radiologists (AUC, 0.923-0.945) for distinguishing
between 26 PCNSLs and 22 GBMs (53). Kim et al (54) used multi-parametric MRI (T2WI,
CE-T1WI and ADC) for differentiating PCNSL and GBM, with an AUC of
0.956. Combining CE-T1WI, ADC and FLAIR also showed high accuracy
(AUC, 0.977) (55). Conventional
MRI could also be combined with perfusion-weighted MRI. In the
study by Nakagawa et al (56), compared with the results of two
radiologists, a model that extracted features from T2WI, CE-T1WI,
an ADC map and an rCBV map provided a better performance for
diagnosing 25 PCNSLs and 45 GBMs.
Deep learning-based radiomics also perform well in
the differential diagnosis. McAvoy et al (57) identified the high accuracy of
convolutional neural networks (CNNs) based on CE-T1WI for the
differential diagnosis between 24 CNS-DLBCLs and 35 GBMs. In a
larger population (92 PCNSLs and 97 GBMs), deep learning radiomics
with data enhancement performed better than two neuroradiologists
(58). Another group also reported
CE-T1W1-based multiparametric CNNs and showed that it had a similar
accuracy to radiologists (accuracy, 0.899; P=0.886) for
differentiating between 136 PCNSLs and 153 GBMs (59). For differentiating 14 atypical GBMs
from 11 PCNSLs, the CE-T1WI-based deep learning model also
performed well (60).
In summary, radiomics remains a potential tool for
the discrimination between PCNSL and HGG. Details of all radiomics
models and case numbers of PCNSL and GBM involved in a series of
studies are summarized in Table
SII.
CSF sampling is an additional choice other than
biopsy if it is safe and does not delay the diagnostic process or
treatment (7). CSF analysis
includes flow cytometry and cytology, and may consider gene
rearrangements (16). In cytology,
GBM cells have hyperchromatic nuclei and a variable amount of
cytoplasm, appearing singly or arranged in small cohesive groups,
while PCNSL usually consists of a monotonous population of
dyshesive large lymphoid cells (63). One study mentioned that flow
cytometry of CSF improves the sensitivity up to 2–3 times more than
cytology analysis (64).
Furthermore, another research group (65) defined the sensitivity of cytology
and flow cytometry as 13.3 and 23.3%, respectively, by analyzing
the CSF of 30 patients with PCNSL. However, studies confirmed
considerable diagnostic delays of PCNSL that may take up to several
weeks due to lengthy cytology or flow cytometry analysis of CSF,
especially under conditions of limited or rare tumor cells in CSF
specimens where more procedures will be required, and detection
time will be further increased (13,66).
The detection of biomarkers in CSF and blood may improve the
diagnosis. Diagnostic biomarkers include proteins, RNA, DNA and
extracellular vesicles (EVs).
Proteins in CSF can be analyzed by latex
agglutination turbidimetric immunoassay (LATIA), enzyme-linked
immunosorbent assay (ELISA) and targeted proteomics assays such as
selected reaction monitoring (SRM). Some proteins, such as C-X-C
motif chemokine ligand 13 (CXCL13), interleukin-10 (IL-10),
β2-microglobulin (B2M), soluble IL-2 receptor (sIL-2R),
apolipoprotein C-II (APOC2), glycoprotein non-metastatic melanoma
protein B (GPNMB), and V-set and immunoglobulin domain-containing
protein 4 (VSIG4), are considered promising protein biomarkers for
PCNSL in the CSF (67). CXCL13 and
IL-10 are mediators of the migration and growth of B cells,
respectively. B2M is a component of the major histocompatibility
complex class I molecules. sIL-2R is a receptor that is mostly
released by regulatory T cells (68). APOC2 is an exchangeable
apolipoprotein that activates lipoprotein lipase (69). GPNMB is an endogenous type 1
transmembrane glycoprotein (70).
VSIG4 is a phagocytic receptor that negatively regulates T-cell
proliferation and IL-2 production (71). All these proteins may be
upregulated in the CSF of patients with PCNSL compared with
patients with GBM (71–75). The combination of different
biomarkers can improve diagnostic performance. Maeyama et al
(72) proposed a multi-marker
prediction algorithm that was effective (AUC, 0.994) in
distinguishing 32 PCNSLs from 21 GBMs and 51 other brain lesions by
incorporating the detection of CXCL13, IL-10, B2M and sIL-2R in the
CSF. In the study, only B2M was measured by LATIA, while the
remaining biomarkers were analyzed by ELISA. Combined analysis of
APOC2, GPNMB and VSIG4 proteins in CSF was effective (AUC, 0.953)
in distinguishing 28 PCNSLs from 7 GBMs 3 astrocytomas using the
SRM method (71).
Alterations in tumor-specific genes and methylation
of circulating tumor DNA (ctDNA) can be detected by gene-targeted
PCR for the purpose of differential diagnosis, with high
sensitivity (76). Cell-free DNA
(cfDNA) is DNA shed from the cell into the body fluids. A portion
of cfDNA is derived from tumor cells, which is referred to as ctDNA
(71). The CSF sampling serves as
a better choice for the detection of mutations compared with
plasma, which contains less cfDNA or ctDNA than CSF due to the BBB
(76–78). Myeloid differentiation primary
response gene 88 (MYD88) encodes a cytosolic adapter protein that
plays a central role in innate and adaptive immunity. CD79B encodes
the B-cell antigen receptor complex-associated protein β chain. The
alterations of L265 in MYD88 and Y196 in CD79B are detected in
recurrent lymphoid tumors. A large cohort study (79) detected mutations in MYD88 L265 and
CD79B Y196 in 71.7% and 64.2% of CNS DLBCL cases, respectively, but
no MYD88 or CD79B mutations were detected in GBM. Hiemcke-Jiwa
et al (80) used the
droplet digital PCR method to detect MYD88 mutation in cfDNA and
found that 8 out of 11 CSF specimens from PCNSL were positive,
including 2 unknown PCNSL samples and 6 CNS DLBCL samples, while
all CSF samples from 3 GBMs were negative for the mutation.
Combining gene mutations with protein markers can improve
diagnostic performance. Ferreri et al (81) combined MYD88 mutational status
(assessed by TaqMan-based PCR) with IL-10 levels in CSF and
differentiated 36 PCNSLs from 106 other CNS diseases (10 GBMs),
with 94% sensitivity and 98% specificity. Furthermore, certain
methylated DNA markers can provide valuable diagnostic clues for
differentiation. Wang et al (77) detected the methylation of MGMT
promoter in ctDNA in 18 out of 28 CSF samples from HGG using
methylation-specific PCR. Recently, Downs et al (82) identified methylation markers cg054
and SCG3 in plasma via a novel method of tailed amplicon
multiplexed-methylation-specific PCR, with a high level of accuracy
to distinguish PCNSLs from other CNS tumors.
Different physiological and pathological processes
between PCNSL and GBM can also be reflected by extracellular RNAs
(exRNAs), such as small non-coding RNA (ncRNA) (83), which freely exist in body fluids or
concentrated in carriers such as EVs (84). MicroRNAs (miRNAs/miRs) are snRNAs
of 18 to 24 nucleotides in length (85). EVs are small lipid bilayer-enclosed
vesicles containing proteins, lipids and nucleic acids that release
from cells (86). A number of
exRNAs, such as free miRNA (miR-15b and miR-21) and RNA in EVs
(RNU6-1) have been used to differentiate between PCNSL and GBM in
previous studies (87). miR-15b is
important in glioma carcinogenesis for regulating cell cycle
progression (88). One study
reported higher miR-15b levels in the CSF of 10 gliomas compared
with those in 23 PCNSLs and 17 other CNS diseases, with an AUC of
0.960 (89). miR-21 is one of the
most highly expressed miRNAs and mainly targets the phosphatase and
tensin homologue (PTEN) gene (90). A previous study showed that PCNSL
cases displayed relatively higher miR-21 levels compared with those
in patients with GBM and healthy individuals. miR-21 levels are
higher in the CSF (89) and serum
(87) of B-cell type PCNSL than
those of GBM. RNU6-1 is an snRNA that is negatively regulated by
the PTEN pathway (91). More
alterations of the PTEN pathway in GBM than in PCNSL leads to
higher RNU6-1 expression (92). A
study showed that high levels of RNU6-1 in EVs derived from serum
helped differentiate between 18 GBMs and 12 PCNSLs, with an AUC of
0.700 (92). Overall, the limited
studies on miRNAs with regard to distinguishing PCNSL from HGG have
presented heterogeneous results without consistent expression
variation across cohorts due to the lack of standards for liquid
collection, RNA extraction, RNA sequencing and statistical
analysis. A large cohort with comparative research of the
aforementioned miRNAs will be meaningful for potential clinical
application.
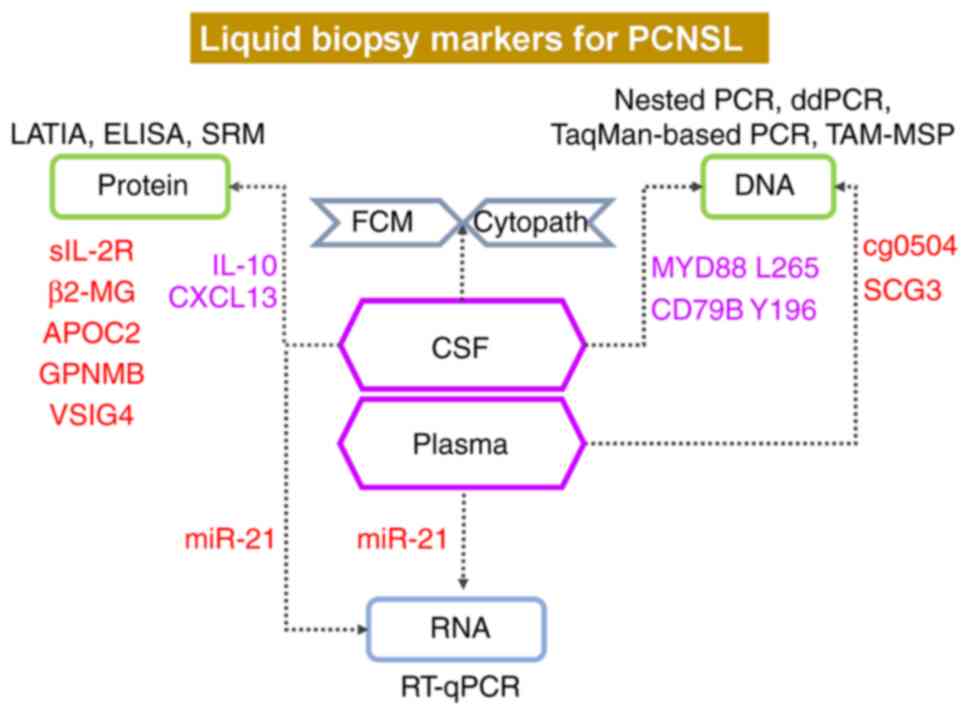
Details of all of the liquid parameters and case
numbers of PCNSL and GBM involved in a series of studies are
summarized in Table SIII. Among
these data, as shown in Fig. 3,
examinations of CXCL13 and IL-10 showed more supportive evidence.
Since DLBCL accounts for most PCNSL cases, MYD88 L265 and CD79B
Y196 in the CSF are meaningful for the diagnosis of PCNSL. Other
promising evidence, such as an increased miR-21 level in the CSF
and plasma, increased cg0504 and SCG3 levels in the plasma, and
increased sIL-2R, β2-MG, APOC2, GPNMB and VSIG4 levels in the CSF,
are also suggestive of PCNSL, yet further evidence is required.
Combining different detection methods or parameters
may provide more accurate and robust support for diagnosis
(Table SIV). Combined
technologies, including MRI and DWI, have most commonly been used
(22,23,93).
A previous study (22) combined
rADC with Ktrans to distinguish 18 CNS DLBCLs from 42
GBMs, and this method performed better than using each parameter
alone. Makino et al (23)
designed a two-step decision tree with an rCBVmax of 4
and ADCmin of 1. The combination facilitated
differentiation between 33 PCNSLs and 54 GBMs. Another study
(93) showed that the combination
of mean and maximum CBV, mean and maximum selective ADC, and
rCBVmean could obtain 100% accuracy for discrimination
between 37 PCNSLs and 37 GBMs. A corporation analysis of diffusion
and susceptibility also showed a good performance (18). Ozturk et al (18) combined rSWI with
rADCmean to differentiate 31 B-cell type PCNSLs with
BCL2 and MYC rearrangements from 57 atypical GBMs without visible
necrosis. The combination improved the diagnostic performance to an
AUC of 0.936. Saini et al (37) used ADC, corrected rCBV, back flux
exchange rate and ITSS scores to perform a multiparametric
assessment of 30 B-cell PCNSLs and 70 GBMs. The study reported the
good performance of this model, with an AUC of 0.920. A combination
of ADC values and biomarker analysis has also been used in research
(94), including the average ADC,
and CXCL13 and IL-10 levels in the CSF, with a better performance
than any single variable model. Recently, a combination of
18F-FDG-PET and ASL was also introduced to differentiate PCNSL from
GBM, which achieved a better performance than either technique
alone (95). Yet, to the best of
our knowledge, there is no standard multiparametric model that has
been assessed prospectively in a large population. Further studies
are needed.
Accurate diagnosis is a prerequisite for the precise
treatment of PCNSL and GBM. Multidisciplinary participation,
including clinicians, radiologists and pathologists, is meaningful
for improving the accuracy and speed of the decision-making
process. However, most of the radiological and pathological markers
discussed within the present review are from retrospective
small-scale and heterogeneous cohorts. Thus, current evidence from
these studies shows the limited quality of radiological and liquid
biopsy, which is unable to replace the need for histology
examination.
Although stereotactic biopsy is an optimal choice
for diagnosis, it is not always conclusive. Otherwise, surgical
resection may be another option, not only for providing enough
pathological specimens but also for significantly improving the
overall survival and progression-free survival of certain patients
with PCNSL (96,97). A recent systematic review
highlighted the possible benefit of cytoreductive surgery in PCNSL
(98). Another recent study
reported that resection significantly prolonged the survival of
patients <70 years old with superficial solitary lymphoma
lesions (99). However, when
lymphoma lesions are deeply seated, it is frequently not amenable
to resect them, and biopsy will be a safer option (99,100).
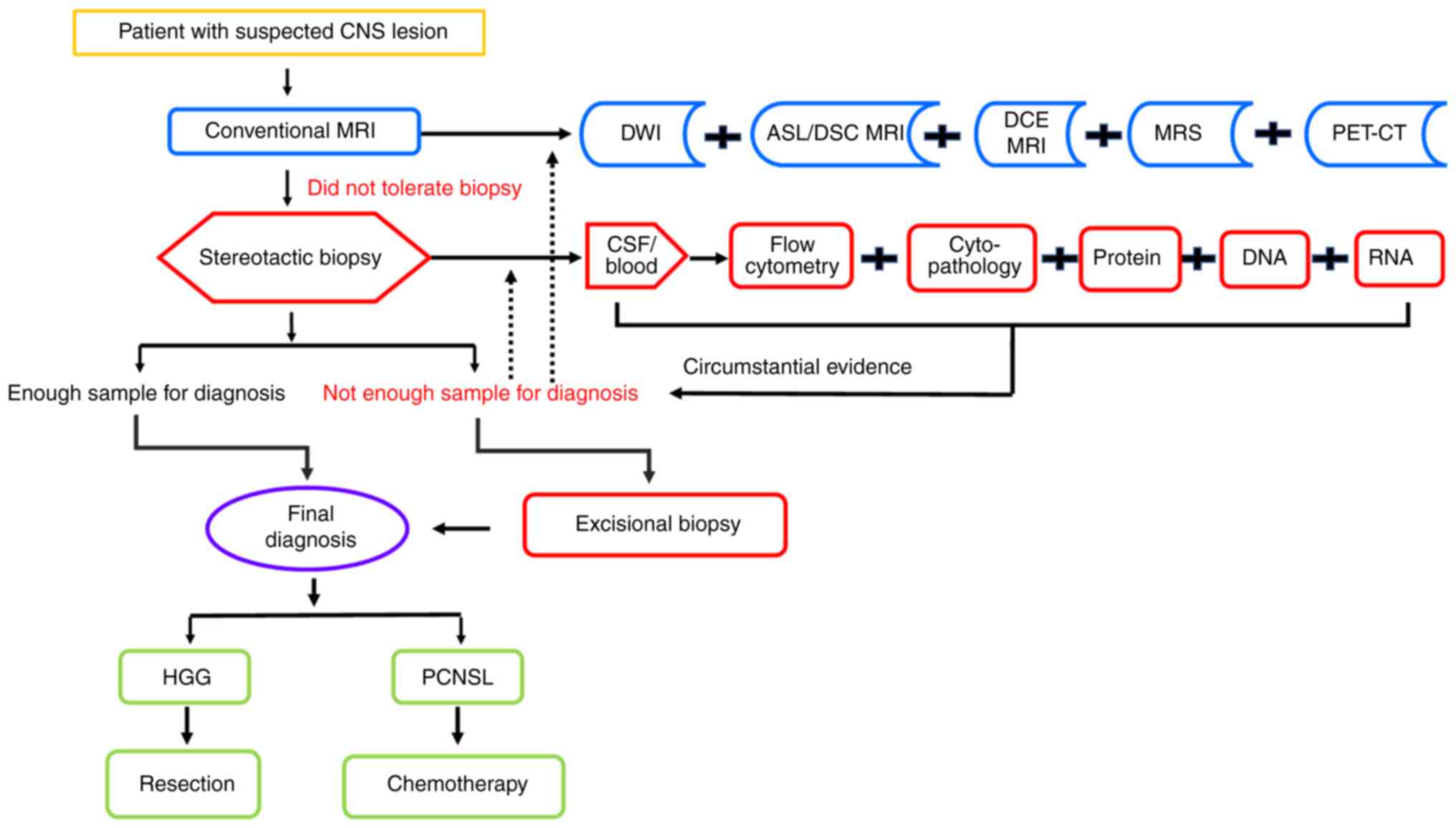
Although radiological examination, and CSF and blood
analysis are useful for the diagnosis of PCNSL, it is not always
practical in clinical due to the economic and staff/equipment time
aspects. Thus, as shown in Fig. 4,
based on the literature, we propose an optional procedure using
multiparametric imaging technologies and liquid biopsies to improve
the diagnostic performance and management of CNS neoplasms,
especially PCNSL. More evidence should be collected in the future
for the clinical management of PCNSL and in order to make the
current diagnostic workflow more reasonable and practical.
Not applicable.
This study was supported by the Young Scientists Fund of the
National Natural Science Foundation of China (grant no. 81700198),
the Science and Technology Development Project of Jilin Province
(grant no. 20190701064GH), the Open Project of Key Laboratory of
Tumor Immunology and Pathology (Army Medical University), Ministry
of Education (grant no. 2018jsz101) and the College Student
Innovation and Entrepreneurship Training Program of Jilin
University (grant no. 202010183X453).
Not applicable.
XJW developed the concept, designed the study and
revised the manuscript. LMC collected the literature and wrote the
draft. MCZ and YZ respectively reorganized the context in radiology
and liquid biopsy. LMC, MCZ and YZ contributed equally. BJ and XMW
respectively contributed suggestions on PET-CT and pathology. MCZ,
YZ, BJ and LMC participated in writing or revising the review. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Not applicable.
Written informed consent was obtained from the
individuals for the publication of any potentially identifiable
images or data included in this article.
The authors declare that they have no competing
interests.
|
1
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2015–2019. Neuro Oncol. 24 (Suppl
5):v1–v95. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox CP, Phillips EH, Smith J, Linton K,
Gallop-Evans E, Hemmaway C, Auer DP, Fuller C, Davies AJ, McKay P,
et al: Guidelines for the diagnosis and management of primary
central nervous system diffuse large B-cell lymphoma. Br J
Haematol. 184:348–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugita Y, Muta H, Ohshima K, Morioka M,
Tsukamoto Y, Takahashi H and Kakita A: Primary central nervous
system lymphomas and related diseases: Pathological characteristics
and discussion of the differential diagnosis. Neuropathology.
36:313–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grommes C, Rubenstein JL, DeAngelis LM,
Ferreri AJM and Batchelor TT: Comprehensive approach to diagnosis
and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol.
21:296–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKinnon C, Nandhabalan M, Murray SA and
Plaha P: Glioblastoma: Clinical presentation, diagnosis, and
management. BMJ. 374:n15602021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexander BM and Cloughesy TF: Adult
Glioblastoma. J Clin Oncol. 35:2402–2409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiavazza C, Pellerino A, Ferrio F,
Cistaro A, Soffietti R and Ruda R: Primary CNS lymphomas:
Challenges in diagnosis and monitoring. Biomed Res Int.
2018:36069702018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grommes C and DeAngelis LM: Primary CNS
Lymphoma. J Clin Oncol. 35:2410–2418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su L, Ding M, Chen L, Li C and Lao M:
Primary central nervous system lymphoma in a patient with systemic
lupus erythematosus mimicking high-grade glioma: A case report and
review of literature. Medicine (Baltimore). 97:e110722018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhatt VR, Shrestha R, Shonka N and Bociek
RG: Near misdiagnosis of glioblastoma as primary central nervous
system lymphoma. J Clin Med Res. 6:299–301. 2014.PubMed/NCBI
|
|
11
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannini C, Dogan A and Salomão DR: CNS
Lymphoma: A practical diagnostic approach. J Neuropathol Exp
Neurol. 73:478–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morell AA, Shah AH, Cavallo C, Eichberg
DG, Sarkiss CA, Benveniste R, Ivan ME and Komotar RJ: Diagnosis of
primary central nervous system lymphoma: A systematic review of the
utility of CSF screening and the role of early brain biopsy.
Neurooncol Pract. 6:415–423. 2019.PubMed/NCBI
|
|
14
|
Patrick LB and Mohile NA: Advances in
primary central nervous system lymphoma. Curr Oncol Rep. 17:602015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Velasco R, Mercadal S, Vidal N, Alañá M,
Barceló MI, Ibáñez-Juliá MJ, Bobillo S, Caldú Agud R, García Molina
E, Martínez P, et al: Diagnostic delay and outcome in
immunocompetent patients with primary central nervous system
lymphoma in Spain: A multicentric study. J Neurooncol. 148:545–554.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nabors LB, Portnow J, Baehring J, Bloch O,
Brem S, Butowski N, Cannon DM, Chao S, Chheda MG, Clark SW, et al:
NCCN Clinical Practice Guidelines in Oncology Central Nervous
System Cancers (Version 2.2022 - September 29, 2022). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425
|
|
17
|
Chukwueke UN and Nayak L: Central nervous
system lymphoma. Hematol Oncol Clin North Am. 33:597–611. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozturk K, Soylu E and Cayci Z:
Differentiation between primary CNS lymphoma and atypical
glioblastoma according to major genomic alterations using diffusion
and susceptibility-weighted MR imaging. Eur J Radiol.
141:1097842021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barajas RF, Politi LS, Anzalone N, Schöder
H, Fox CP, Boxerman JL, Kaufmann TJ, Quarles CC, Ellingson BM, Auer
D, et al: Consensus recommendations for MRI and PET imaging of
primary central nervous system lymphoma: Guideline statement from
the International primary CNS lymphoma collaborative group (IPCG).
Neuro Oncol. 23:1056–1071. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villanueva-Meyer JE, Mabray MC and Cha S:
Current clinical brain tumor imaging. Neurosurgery. 81:397–415.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Lee M, Buck O, Woo KM, Zhang Z,
Hatzoglou V, Omuro A, Arevalo-Perez J, Thomas AA, Huse J, et al:
Diagnostic accuracy of T1-Weighted dynamic contrast-enhanced-MRI
and DWI-ADC for differentiation of glioblastoma and primary CNS
Lymphoma. AJNR Am J Neuroradiol. 38:485–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu S, Wang S, Gao Q, Zhou M, Li Y, Cao P,
Hong X and Shi H: Quantitative evaluation of diffusion and dynamic
contrast-enhanced magnetic resonance imaging for differentiation
between primary central nervous system lymphoma and glioblastoma. J
Comput Assist Tomogr. 41:898–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makino K, Hirai T, Nakamura H, Kuroda JI,
Shinojima N, Uetani H, Kitajima M and Yano S: Differentiating
between primary central nervous system lymphomas and glioblastomas:
Combined use of perfusion-weighted and diffusion-weighted magnetic
resonance imaging. World Neurosurg. 112:e1–e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn SJ, Shin HJ, Chang JH and Lee SK:
Differentiation between primary cerebral lymphoma and glioblastoma
using the apparent diffusion coefficient: Comparison of three
different ROI methods. PLoS One. 9:e1129482014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakajima S, Okada T, Yamamoto A, Kanagaki
M, Fushimi Y, Okada T, Arakawa Y, Takagi Y, Miyamoto S and Togashi
K: Primary central nervous system lymphoma and glioblastoma:
Differentiation using dynamic susceptibility-contrast
perfusion-weighted imaging, diffusion-weighted imaging, and
(18)F-fluorodeoxyglucose positron emission tomography. Clin
Imaging. 39:390–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suh CH, Kim HS, Jung SC, Choi CG and Kim
SJ: Clinically relevant imaging features for MGMT promoter
methylation in multiple glioblastoma studies: A systematic review
and meta-analysis. AJNR Am J Neuroradiol. 39:1439–1445.
2018.PubMed/NCBI
|
|
27
|
Akbari H, Bakas S, Pisapia JM, Nasrallah
MP, Rozycki M, Martinez-Lage M, Morrissette JJD, Dahmane N,
O'Rourke DM and Davatzikos C: In vivo evaluation of EGFRvIII
mutation in primary glioblastoma patients via complex
multiparametric MRI signature. Neuro Oncol. 20:1068–1079. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quarles CC, Bell LC and Stokes AM: Imaging
vascular and hemodynamic features of the brain using dynamic
susceptibility contrast and dynamic contrast enhanced MRI.
Neuroimage. 187:32–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suh CH, Kim HS, Jung SC, Park JE, Choi CG
and Kim SJ: MRI as a diagnostic biomarker for differentiating
primary central nervous system lymphoma from glioblastoma: A
systematic review and meta-analysis. J Magn Reson Imaging.
50:560–572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neska-Matuszewska M, Bladowska J, Sasiadek
M and Zimny A: Differentiation of glioblastoma multiforme,
metastases and primary central nervous system lymphomas using
multiparametric perfusion and diffusion MR imaging of a tumor core
and a peritumoral zone-Searching for a practical approach. PLoS
One. 13:e01913412018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu W, Wang Q, Shao A, Xu B and Zhang J:
The performance of MR perfusion-weighted imaging for the
differentiation of high-grade glioma from primary central nervous
system lymphoma: A systematic review and meta-analysis. PLoS One.
12:e01734302017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cindil E, Sendur HN, Cerit MN, Dag N,
Erdogan N, Celebi FE, Oner Y and Tali T: Validation of combined use
of DWI and percentage signal recovery-optimized protocol of DSC-MRI
in differentiation of high-grade glioma, metastasis, and lymphoma.
Neuroradiology. 63:331–342. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaganti J, Taylor M, Woodford H and Steel
T: Differentiation of primary central nervous system lymphoma and
high-grade glioma with dynamic susceptibility contrast-derived
metrics: Pilot study. World Neurosurg. 151:e979–e987. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Semmineh NB, Xu J, Skinner JT, Xie J, Li
H, Ayers G and Quarles CC: Assessing tumor cytoarchitecture using
multiecho DSC-MRI derived measures of the transverse relaxivity at
tracer equilibrium (TRATE). Magn Reson Med. 74:772–784. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee MD, Baird GL, Bell LC, Quarles CC and
Boxerman JL: Utility of percentage signal recovery and baseline
signal in DSC-MRI optimized for relative CBV measurement for
differentiating glioblastoma, lymphoma, metastasis, and meningioma.
AJNR Am J Neuroradiol. 40:1445–1450. 2019.PubMed/NCBI
|
|
36
|
Hsu CC, Watkins TW, Kwan GN and Haacke EM:
Susceptibility-Weighted imaging of glioma: Update on current
imaging status and future directions. J Neuroimaging. 26:383–390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saini J, Kumar Gupta P, Awasthi A, Pandey
CM, Singh A, Patir R, Ahlawat S, Sadashiva N, Mahadevan A and Kumar
Gupta R: Multiparametric imaging-based differentiation of lymphoma
and glioblastoma: Using T1-perfusion, diffusion, and
susceptibility-weighted MRI. Clin Radiol. 73:986e7–986e15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki F, Takayasu T, Nosaka R, Amatya
VJ, Doskaliyev A, Akiyama Y, Tominaga A, Takeshima Y, Sugiyama K
and Kurisu K: Magnetic resonance spectroscopy detection of high
lipid levels in intraaxial tumors without central necrosis: A
characteristic of malignant lymphoma. J Neurosurg. 122:1370–1379.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagashima H, Sasayama T, Tanaka K, Kyotani
K, Sato N, Maeyama M, Kohta M, Sakata J, Yamamoto Y, Hosoda K, et
al: Myo-inositol concentration in MR spectroscopy for
differentiating high grade glioma from primary central nervous
system lymphoma. J Neurooncol. 136:317–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nomura Y, Asano Y, Shinoda J, Yano H,
Ikegame Y, Kawasaki T, Nakayama N, Maruyama T, Muragaki Y and Iwama
T: Characteristics of time-activity curves obtained from dynamic
11C-methionine PET in common primary brain tumors. J
Neurooncol. 138:649–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong Z, Jiang C, Zhu R, Feng S, Wang Y, Li
J, Chen W, Liu P, Zhao D, Ma W, et al: 18F-FDG-PET-based
radiomics features to distinguish primary central nervous system
lymphoma from glioblastoma. Neuroimage Clin. 23:1019122019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou W, Wen J, Hua F, Xu W, Lu X, Yin B,
Geng D and Guan Y: 18F-FDG PET/CT in immunocompetent
patients with primary central nervous system lymphoma:
Differentiation from glioblastoma and correlation with DWI. Eur J
Radiol. 104:26–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim HO, Kim JS, Kim SO, Chae SY, Oh SJ,
Seo M, Lee SH, Oh JS, Ryu JS, Huh JR and Kim JH:
Clinicopathological characteristics of primary central nervous
system lymphoma with low 18F-fludeoxyglucose uptake on brain
positron emission tomography. Medicine (Baltimore). 99:e201402020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Zhou Y, Li L, Hou W, Ma X and Tian
R: Current status and quality of radiomics studies in lymphoma: A
systematic review. Eur Radiol. 30:6228–6240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lohmann P, Galldiks N, Kocher M, Heinzel
A, Filss CP, Stegmayr C, Mottaghy FM, Fink GR, Jon Shah N and
Langen KJ: Radiomics in neuro-oncology: Basics, workflow, and
applications. Methods. 188:112–121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Priya S, Ward C, Locke T, Soni N,
Maheshwarappa RP, Monga V, Agarwal A and Bathla G: Glioblastoma and
primary central nervous system lymphoma: Differentiation using MRI
derived first-order texture analysis-a machine learning study.
Neuroradiol J. 34:320–328. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han Y, Wang ZJ, Li WH, Yang Y, Zhang J,
Yang XB, Zuo L, Xiao G, Wang SZ, Yan LF and Cui GB: Differentiation
between primary central nervous system lymphoma and atypical
glioblastoma based on MRI morphological feature and signal
intensity ratio: A retrospective multicenter study. Front Oncol.
12:8111972022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Suh HB, Choi YS, Bae S, Ahn SS, Chang JH,
Kang SG, Kim EH, Kim SH and Lee SK: Primary central nervous system
lymphoma and atypical glioblastoma: Differentiation using radiomics
approach. Eur Radiol. 28:3832–3839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen C, Zheng A, Ou X, Wang J and Ma X:
Comparison of radiomics-based machine-learning classifiers in
diagnosis of glioblastoma from primary central nervous system
lymphoma. Front Oncol. 10:11512020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
MacIver CL, Busaidi AA, Ganeshan B,
Maynard JA, Wastling S, Hyare H, Brandner S, Markus JE, Lewis MA,
Groves AM, et al: Filtration-Histogram based magnetic resonance
texture analysis (MRTA) for the distinction of primary central
nervous system lymphoma and glioblastoma. J Pers Med. 11:8762021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mehrnahad M, Rostami S, Kimia F, Kord R,
Taheri MS, Rad HS, Haghighatkhah H, Moradi A and Kord A:
Differentiating glioblastoma multiforme from cerebral lymphoma:
Application of advanced texture analysis of quantitative apparent
diffusion coefficients. Neuroradiol J. 33:428–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang D, Park JE, Kim YH, Kim JH, Oh JY,
Kim J, Kim Y, Kim ST and Kim HS: Diffusion radiomics as a
diagnostic model for atypical manifestation of primary central
nervous system lymphoma: Development and multicenter external
validation. Neuro Oncol. 20:1251–1261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xia W, Hu B, Li H, Geng C, Wu Q, Yang L,
Yin B, Gao X, Li Y and Geng D: Multiparametric-MRI-Based radiomics
model for differentiating primary central nervous system lymphoma
from glioblastoma: Development and cross-vendor validation. J Magn
Reson Imaging. 53:242–250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim Y, Cho HH, Kim ST, Park H, Nam D and
Kong DS: Radiomics features to distinguish glioblastoma from
primary central nervous system lymphoma on multi-parametric MRI.
Neuroradiology. 60:1297–1305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bathla G, Priya S, Liu Y, Ward C, Le NH,
Soni N, Maheshwarappa RP, Monga V, Zhang H and Sonka M:
Radiomics-based differentiation between glioblastoma and primary
central nervous system lymphoma: A comparison of diagnostic
performance across different MRI sequences and machine learning
techniques. Eur Radiol. 31:8703–8713. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nakagawa M, Nakaura T, Namimoto T,
Kitajima M, Uetani H, Tateishi M, Oda S, Utsunomiya D, Makino K,
Nakamura H, et al: Machine learning based on multi-parametric
magnetic resonance imaging to differentiate glioblastoma multiforme
from primary cerebral nervous system lymphoma. Eur J Radiol.
108:147–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McAvoy M, Prieto PC, Kaczmarzyk JR,
Fernández IS, McNulty J, Smith T, Yu KH, Gormley WB and Arnaout O:
Classification of glioblastoma versus primary central nervous
system lymphoma using convolutional neural networks. Sci Rep.
11:152192021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Y, Liang K, He J, Ma H, Chen H,
Zheng F, Zhang L, Wang X, Ma X and Chen X: Deep learning with data
enhancement for the differentiation of solitary and multiple
cerebral glioblastoma, lymphoma, and tumefactive demyelinating
lesion. Front Oncol. 11:6658912021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xia W, Hu B, Li H, Shi W, Tang Y, Yu Y,
Geng C, Wu Q, Yang L, Yu Z, et al: Deep learning for automatic
differential diagnosis of primary central nervous system lymphoma
and glioblastoma: Multi-Parametric magnetic resonance imaging based
convolutional neural network model. J Magn Reson Imaging.
54:880–887. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tariciotti L, Caccavella VM, Fiore G,
Schisano L, Carrabba G, Borsa S, Giordano M, Palmisciano P, Remoli
G, Remore LG, et al: A deep learning model for preoperative
differentiation of glioblastoma, brain metastasis and primary
central nervous system lymphoma: A pilot study. Front Oncol.
12:8166382022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yun J, Park JE, Lee H, Ham S, Kim N and
Kim HS: Radiomic features and multilayer perceptron network
classifier: A robust MRI classification strategy for distinguishing
glioblastoma from primary central nervous system lymphoma. Sci Rep.
9:57462019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park JE, Kim HS, Lee J, Cheong EN, Shin I,
Ahn SS and Shim WH: Deep-learned time-signal intensity pattern
analysis using an autoencoder captures magnetic resonance perfusion
heterogeneity for brain tumor differentiation. Sci Rep.
10:214852020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chhieng DC, Elgert P, Cohen JM, Jhala NC
and Cangiarella JF: Cytology of primary central nervous system
neoplasms in cerebrospinal fluid specimens. Diagn Cytopathol.
26:209–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bromberg JE, Breems DA, Kraan J, Bikker G,
van der Holt B, Smitt PS, van den Bent MJ, van't Veer M and Gratama
JW: CSF flow cytometry greatly improves diagnostic accuracy in CNS
hematologic malignancies. Neurology. 68:1674–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schroers R, Baraniskin A, Heute C, Vorgerd
M, Brunn A, Kuhnhenn J, Kowoll A, Alekseyev A, Schmiegel W,
Schlegel U, et al: Diagnosis of leptomeningeal disease in diffuse
large B-cell lymphomas of the central nervous system by flow
cytometry and cytopathology. Eur J Haematol. 85:520–528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Baraniskin A, Deckert M,
Schulte-Altedorneburg G, Schlegel U and Schroers R: Current
strategies in the diagnosis of diffuse large B-cell lymphoma of the
central nervous system. Br J Haematol. 156:421–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
van Westrhenen A, Smidt LCA, Seute T,
Nierkens S, Stork ACJ, Minnema MC and Snijders TJ: Diagnostic
markers for CNS lymphoma in blood and cerebrospinal fluid: A
systematic review. Br J Haematol. 182:384–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang ZZ, Grote DM, Ziesmer SC, Manske MK,
Witzig TE, Novak AJ and Ansell SM: Soluble IL-2Rα facilitates
IL-2-mediated immune responses and predicts reduced survival in
follicular B-cell non-Hodgkin lymphoma. Blood. 118:2809–2820. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wolska A, Reimund M and Remaley AT:
Apolipoprotein C-II: The re-emergence of a forgotten factor. Curr
Opin Lipidol. 31:147–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Saade M, Araujo de Souza G, Scavone C and
Kinoshita PF: The Role of GPNMB in inflammation. Front Immunol.
12:6747392021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Waldera-Lupa DM, Poschmann G,
Kirchgaessler N, Etemad-Parishanzadeh O, Baberg F, Brocksieper M,
Seidel S, Kowalski T, Brunn A, Haghikia A, et al: A multiplex assay
for the stratification of patients with primary central nervous
system lymphoma using targeted mass spectrometry. Cancers (Basel).
12:17322020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Maeyama M, Sasayama T, Tanaka K, Nakamizo
S, Tanaka H, Nishihara M, Fujita Y, Sekiguchi K, Kohta M, Mizukawa
K, et al: Multi-marker algorithms based on CXCL13, IL-10, sIL-2
receptor, and β2-microglobulin in cerebrospinal fluid to diagnose
CNS lymphoma. Cancer Med. 9:4114–4125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rubenstein JL, Wong VS, Kadoch C, Gao HX,
Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, et
al: CXCL13 plus interleukin 10 is highly specific for the diagnosis
of CNS lymphoma. Blood. 121:4740–4748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shao J, Chen K, Li Q, Ma J, Ma Y, Lin Z,
Kang H and Chen B: High level of IL-10 in cerebrospinal fluid is
specific for diagnosis of primary central nervous system lymphoma.
Cancer Manag Res. 12:6261–6268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Masouris I, Manz K, Pfirrmann M, Dreyling
M, Angele B, Straube A, Langer S, Huber M, Koedel U and Von
Baumgarten L: CXCL13 and CXCL9 CSF levels in central nervous system
lymphoma-diagnostic, therapeutic, and prognostic relevance. Front
Neurol. 12:6545432021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
McEwen AE, Leary SES and Lockwood CM:
Beyond the blood: CSF-Derived cfDNA for diagnosis and
characterization of CNS tumors. Front Cell Dev Biol. 8:452020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang Z, Jiang W, Wang Y, Guo Y, Cong Z, Du
F and Song B: MGMT promoter methylation in serum and cerebrospinal
fluid as a tumor-specific biomarker of glioma. Biomed Rep.
3:543–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Juratli TA, Stasik S, Zolal A, Schuster C,
Richter S, Daubner D, Juratli MA, Thowe R, Hennig S, Makina M, et
al: TERT promoter mutation detection in cell-free tumor-derived DNA
in patients with IDH wild-type glioblastomas: A pilot prospective
study. Clin Cancer Res. 24:5282–5291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nakamura T, Tateishi K, Niwa T, Matsushita
Y, Tamura K, Kinoshita M, Tanaka K, Fukushima S, Takami H, Arita H,
et al: Recurrent mutations of CD79B and MYD88 are the hallmark of
primary central nervous system lymphomas. Neuropathol Appl
Neurobiol. 42:279–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ,
Bromberg JEC, Nierkens S, Jiwa NM, Minnema MC and Huibers MMH:
MYD88 p.(L265P) detection on cell-free DNA in liquid biopsies of
patients with primary central nervous system lymphoma. Br J
Haematol. 185:974–977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ferreri AJM, Calimeri T, Lopedote P,
Francaviglia I, Daverio R, Iacona C, Belloni C, Steffanoni S,
Gulino A, Anghileri E, et al: MYD88 L265P mutation and
interleukin-10 detection in cerebrospinal fluid are highly specific
discriminating markers in patients with primary central nervous
system lymphoma: Results from a prospective study. Br J Haematol.
193:497–505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Downs BM, Ding W, Cope LM, Umbricht CB, Li
W, He H, Ke X, Holdhoff M, Bettegowda C, Tao W and Sukumar S:
Methylated markers accurately distinguish primary central nervous
system lymphomas (PCNSL) from other CNS tumors. Clin Epigenetics.
13:1042021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jelski W and Mroczko B: Molecular and
circulating biomarkers of brain tumors. Int J Mol Sci. 22:70392021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kim S, Jeon OH and Jeon YJ: Extracellular
RNA: Emerging roles in cancer cell communication and biomarkers.
Cancer Lett. 495:33–40. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Birkó Z, Nagy B, Klekner Á and Virga J:
Novel molecular markers in glioblastoma-benefits of liquid biopsy.
Int J Mol Sci. 21:75222020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang K, Wang S, Cheng Y, Tian Y and Hou J:
Role of miRNA-21 in the diagnosis and prediction of treatment
efficacy of primary central nervous system lymphoma. Oncol Lett.
17:3475–3481. 2019.PubMed/NCBI
|
|
88
|
Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang
M, Li D, Zhao Y, Ge R, Li G, et al: MicroRNA-15b regulates cell
cycle progression by targeting cyclins in glioma cells. Biochem
Biophys Res Commun. 380:205–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Baraniskin A, Kuhnhenn J, Schlegel U,
Maghnouj A, Zollner H, Schmiegel W, Hahn S and Schroers R:
Identification of microRNAs in the cerebrospinal fluid as biomarker
for the diagnosis of glioma. Neuro Oncol. 14:29–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cabarcas S, Watabe K and Schramm L:
Inhibition of U6 snRNA transcription by PTEN. Online J Biol Sci.
10:114–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Puigdelloses M, Gonzalez-Huarriz M,
Garcia-Moure M, Martinez-Velez N, Esparragosa Vazquez I, Bruna J,
Zandio B, Agirre A, Marigil M, Petrirena G, et al: RNU6-1 in
circulating exosomes differentiates GBM from non-neoplastic brain
lesions and PCNSL but not from brain metastases. Neurooncol Adv.
2:vdaa0102020.PubMed/NCBI
|
|
93
|
Eisenhut F, Schmidt MA, Putz F, Lettmaier
S, Fröhlich K, Arinrad S, Coras R, Luecking H, Lang S, Fietkau R
and Doerfler A: Classification of primary cerebral lymphoma and
glioblastoma featuring dynamic susceptibility contrast and apparent
diffusion coefficient. Brain Sci. 10:8862020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mabray MC, Barajas RF, Villanueva-Meyer
JE, Zhang CA, Valles FE, Rubenstein JL and Cha S: The combined
performance of ADC, CSF CXC Chemokine Ligand 13, and CSF
Interleukin 10 in the diagnosis of central nervous system lymphoma.
AJNR Am J Neuroradiol. 37:74–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hatakeyama J, Ono T, Takahashi M, Oda M
and Shimizu H: Differentiating between primary central nervous
system lymphoma and glioblastoma: The diagnostic value of combining
18F-fluorodeoxyglucose positron emission tomography with
arterial spin labeling. Neurol Med Chir (Tokyo). 61:367–375. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Weller M, Martus P, Roth P, Thiel E and
Korfel A; German PCNSL Study Group, : Surgery for primary CNS
lymphoma? Challenging a paradigm. Neuro Oncol. 14:1481–1484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Deng X, Xu X, Lin D, Zhang X, Yu L, Sheng
H, Yin B, Zhang N and Lin J: Real-World impact of surgical excision
on overall survival in primary central nervous system lymphoma.
Front Oncol. 10:1312020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Labak CM, Holdhoff M, Bettegowda C, Gallia
GL, Lim M, Weingart JD and Mukherjee D: Surgical resection for
primary central nervous system lymphoma: A systematic review. World
Neurosurg. 126:e1436–e1448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schellekes N, Barbotti A, Abramov Y, Sitt
R, Di Meco F, Ram Z and Grossman R: Resection of primary central
nervous system lymphoma: Impact of patient selection on overall
survival. J Neurosurg. Feb 26–2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bierman PJ: Surgery for primary central
nervous system lymphoma: Is it time for reevaluation? Oncology
(Williston Park). 28:632–637. 2014.PubMed/NCBI
|