Introduction
The human body contains microbiota, which plays a
vital role in health and disease; the number of microorganisms is
estimated to be ~1013, and constitutes 1–3% of the body
mass (1). Microbiota is in a
symbiotic equilibrium with the host. Environmental factors
including age, diet, disease and drug metabolism can lead to
microbial imbalance, which can induce inflammatory responses or
lead to drug resistance (2,3). Disruption of this equilibrium also has
an impact on cancer; the microbiota can influence every stage of
cancer as well as the therapeutic process through direct and
indirect actions, the main mechanisms of which may be associated
with the metabolites produced by microorganisms and the
inflammatory state they cause (4,5). There
are also beneficial effects of microbiota on cancer treatment, as
confirmed by recent clinical trials on fecal microbiota
transplantation (FMT) in combination with immunotherapy for cancer
treatment, potentially opening up new targets for cancer treatment
(6).
Prostate cancer (Pca) is the second most common
cancer type globally, with nearly 1.4 million new cases and ~0.4
million Pca-associated mortalities worldwide in 2020 (7). Radical surgery and radiotherapy
continue to be the options for treating localized diseases.
Androgen deprivation therapy (ADT), hormone therapy and
chemotherapy are also effective in male patients with Pca (8). However, certain patients progress to
castration-resistant prostate cancer (CRPC) within 2–3 years after
starting ADT treatment, resulting in a poor prognosis (9). However, there is still a lack of
effective tests to distinguish between indolent and resistant Pca
at an early stage. Thus, there is an urgent need for improved risk
stratification tools to avoid overtreatment and under-treatment of
aggressive Pca (10).
Next-generation sequencing (NGS) and metagenomics
are expected further to establish the link between microbiota and
Pca, opening up new areas of Pca research (11). Microbiota may not only be a
stratification factor to predict risk, but may also provide new
options for treating Pca by clarifying the interaction between
cancer and microbiota (12).
Therefore, understanding the link between the microbiome and Pca is
critical.
It has been proposed that the microbiota may have a
direct or indirect effect on Pca tumorigenesis and progression.
However, further research is needed to provide definitive evidence
in this area (13). In the present
review, focus was addressed on the effect of prostate cancer
treatment on the microbiome and the scope of the microbiome was
expanded to include the entire body, not just the intestinal
microbiome. The present review provides a detailed overview of the
current research on the role of human microorganisms in the risk,
progression and treatment of Pca from the aspects of direct and
indirect mechanisms.
Microbiota and Pca
Since 2015, studies on Pca and microbiota have
mainly focused on the association between prostate microbiota and
Pca. As gut microbiota research has further developed, studies
focusing on the indirect effects of microbiota and Pca have
increased in the last 5 years, which have explored the effects of
gastrointestinal microbiota and urinary microbiota on Pca, and
evaluated the association of prostatic fluid or semen with Pca.
As shown in Table I,
Table II, Table III, the present study
systematically searched for studies related to the microbiome and
Pca based on PubMed and Web of Science databases. The search
included articles published up to March 11, 2023 in English
language. Search keywords included ‘prostate cancer OR prostatic
carcinoma OR prostatic tumor’ AND ‘microbiome OR microbiota’. After
excluding review articles (n=21), original studies related to Pca
and prostate tissue, urine, semen, prostatic fluid and gut
microbiota analysis were selected (n=36).
 | Table I.Selected studies up to 2023
investigating on the prostate microbiome and Pca. |
Table I.
Selected studies up to 2023
investigating on the prostate microbiome and Pca.
| Reference | Tissue | Samples | Main findings |
|---|
| Cohen et al,
2005 (14) | Fresh tissue | 34 patients with
Pca |
Propionibacterium acnes was the
predominant microbiome in Pca tissue and positive association with
prostatic inflammation |
| Sfanos et
al, 2008 (15) | Fresh Prostatectomy
tissues | 30 patients with
Pca | Pca tissue presence
of 83 distinct microorganisms, there was no significant association
between the presence of particular species of bacteria and
histologic evidence of acute or chronic inflammation |
| Alexeyev et
al, 2006 (16) | Formalin-fixed and
embedded in paraffin | 352 patients with
BPH of which 171 progressed to Pca |
Propionibacterium acnes was the
most common bacterium in BPH and associated with Pca
development |
| Yow et al,
2017 (17) | Fresh-frozen
tissue | 10 patients with
Pca |
Enterobacteriaceae and
Propionibacterium acnes was the most common species |
| Cavarretta et
al, 2017 (18) | Formalin-fixed and
embedded in paraffin | Tumor, Peri-tumor,
and non-tumor tissues in 16 patients with Pca |
Propionibacterium spp. was the most
abundant in all regions of the tumor, Staphylococcus spp.
were more represented in the tumor/peri-tumor tissue |
| Feng et al,
2019 (22) | Fresh-frozen
tissue | 6 African and 16
Australian with Pca |
Proteobacteria was the predominance
bacterial genera. Compared with Australian and Chinese, African
samples had significantly increased bacterial abundance |
| Feng et al,
2019 (23) | Fresh-frozen
tissue | 65 patients with
Pca | Escherichia,
Propionibacterium, Acinetobacter and Pseudomonas were
most abundant in the prostate and constituting the core of the
prostate microbiome |
| Banerjee et
al, 2019 (24) | Formalin-fixed and
embedded in paraffin | 50 patients with
Pca | Some viral genomic
sequences were inserted into the host Pca sample |
| Miyake et
al, 2019 (25) | Formalin-fixed and
embedded in paraffin | 45 patients with
Pca and 33 BPH | Mycoplasma
genitalium infection was significantly different and associated
with Pca and with high Gleason scores |
| Ma et al,
2020 (26) | Fresh-frozen
tissue | 242 Pca sequencing
data from The Cancer Genome Atlas | Listeria
monocytogenes, Methylobacterium radiotolerans JCM 2831, Xanthomonas
albilineans GPE PC73, and Bradyrhizobium japonicum were
overrepresented in tumor samples |
| Salachan et
al, 2022 (27) | Fresh-frozen
tissue | 23 benign and 83
malignant |
Under-representation of Staphylococcus
saprophyticus and Vibrio parahaemolyticus,
over-abundance of Shewanella in Pca tissue |
 | Table II.Selected studies up to 2023
investigating on the urinary microbiome and Pca. |
Table II.
Selected studies up to 2023
investigating on the urinary microbiome and Pca.
| Reference | Tissue | Samples | Main findings |
|---|
| Shrestha et
al, 2018 (36) | Fresh urine samples
handled using sterile technique | 63 benign and 66
with Pca | No significant
difference in alpha/beta-diversity of urine microbiome between
benign/tumor patients |
| Ma et al,
2019 (37) | Fresh-frozen
prostatic fluid | 32 Pca and 27
non-Pca | The diversity of
microbiota in prostatic fluid of Pca patients is reduced, the
abundance of Alkaliphilus, Enterobacter, Lactococcus,
Cronobacter, Carnobacterium, and Streptococcus were
significantly different |
| Yu et al,
2015 (38) | Urinary, EPS and
seminal fluid samples without contamination | 13 patients with
Pca and 21 BPH | Escherichia
coli was decrease in urine sample and Escherichia coli was
increase in EPS and seminal fluid, Enterococci increased in
semen in the Pca group |
| Alanee et
al, 2019 (39) | First voided urine
samples | 16 benign and 14
with Pca | Increased abundance
of clostridium XVIII & IV, lachnospira,
acetanaerobacterium, and faecalibacterium in Pca
patients |
| Tsai et al,
2022 (40) | Fresh-frozen urine
samples | 62 BPH and 62 Pca
and benign | Compared with the
control group, Faecalibacterium, Staphylococcus,
Ruminococcaceae_UCG_002, Neisseria, and Agathobacter had
significant abundance differences in the Pca group |
 | Table III.Selected studies up to 2023
investigating on the gut microbiome and Pca. |
Table III.
Selected studies up to 2023
investigating on the gut microbiome and Pca.
| Reference | Tissue | Samples | Main findings |
|---|
| Zhong et al,
2022 (47) | Frozen fecal
samples | 15 patients with
Pca and 20 BPH | Antibiotic exposure
leads to elevated relative abundance of Proteobacteria and
increased LPS affects Pca progression through the NF-κB-IL6-STAT3
axis in mice |
| Amirian et
al, 2013 (48) | N/A | N/A | There may be
significant differences in the composition of the gut microbiome
among individuals at higher risk of Pca |
| Liss et al,
2018 (49) | Frozen rectal swab
samples | 64 with Pca and 41
without cancer | The abundant of
Bacteroides and Streptococcus was significantly
increased in Pca, with no significant differences in microbiome
diversity |
| Golombos et
al, 2018 (50) | Frozen fecal
samples | 8 benign and 12
with Pca | Higher relative
abundance of Bacteriodes massiliensis was in Pca
patients |
| Matsushita et
al, 2021 (51) | Frozen fecal
samples | 96 with Pca and 56
without cancer | High-risk Pca
patients had an increased relative abundance of SCFAs-producing
bacteria, including Rikenellaceae, Alistipes and
Lachnospira |
| Liu et al,
2019 (54) | Frozen fecal
samples | N/A | 11 phylotypes were
decreased in abundance in HFD-fed Pca-mcie, including
equol-producing bacterium Adlercreutzia |
| Liu et al,
2019 (55) | Frozen fecal
samples | N/A | Higher abundance of
Lachonospiraceae, Roseburia and Amycolatopsis,
increased serum L-methionine decreased α-linolenic acid in combined
maternal and post-weaning HFD-fed Pca mice |
| Matsushita et
al, 2021 (57) | Frozen fecal
samples | N/A | Gut microbiome
affects IGF-1 levels in serum and prostate tissue via SCFAs, which
promote Pca cell proliferation |
| Matsushita et
al, 2022 (57) | Frozen fecal
samples | N/A | Dysbiosis of gut
microbiota leads to elevated LPS and promotes Pca progression
through histamine H1 receptor signaling in HFD-fed mice |
| Sato et al,
2022 (59) | Fresh-frozen
tissue | 203 Pca, 150 Pca
and 50 Pca sequencing data from GEO | LD-fed Pca mice
abundance of Clostridiales and Lactobacillales. The
proportion of the order Lactobacillales was negatively related with
PCa progression. |
| Liu et al,
2020 (66) | Frozen fecal
samples | 21 matched patients
with HSPC and CRPC | The abundance of
Phascolarctobacterium and Ruminococcus increased in
CRPC, and there was no significant difference in microbiota
diversity |
| Li et al,
2021 (67) | Frozen fecal
samples | 56 patients on ADT
and 30 patients underwent RRP | There are
significant differences in the diversity of the microbiota,
Ruminococcus Gnavus and Bacteroides spp. were
enriched in the ADT group |
| Kure et al,
2022 (68) | Frozen fecal
samples | 23 Pca patients
under going ADT | The abundance of
Proteobacteria changed significantly after ADT and was
positively correlated with lactate concentration |
| Pernigoni et
al, 2021 (69) | Frozen fecal
samples | 19 patients with
HSPC and 55 patients with CRPC | The gut microbiota
of CRPC patients or castrated mice, including Ruminococcus
gnavus, can convert androgen precursors into active
androgens |
| Liu et al,
2021 (70) | Frozen fecal
samples | 5 patients with
Pca | FMT from CRPC
patients to prostate mice increased the abundant of
Ruminococcus, resulting in Pca growth |
| Huang et al,
2021 (71) | Frozen fecal
samples | N/A |
Akkermansiaceae was elevated in the
first three weeks of the cancer-bearing Pca mice |
| Sfanos et
al, 2018 (75) | Frozen fecal
samples | 6 control, 3 benign
and 21 Pca patients | The relative
abundance was high in Pca patients taking ATT, Akkermansia
muciniphila and Ruminococcaceae spp. |
| Daisley et
al, 2020 (76) | Frozen fecal
samples | 33 Pca patients
without treatment, 21 with ADT alone and 14 with ADT + AA | Compared with
patients without treatment, Corynebacterium spp. abundance
was reduced in patients with ADT or ADT+AA, and Akkermansia
muciniphila abundance was increased in patients receiving oral
AA |
| Terrisse et
al, 2022 (79) | Frozen fecal
samples | 10 patients with
HSPC and 32 patients with CRPC | PC reduced the
relative abundance of Akkermansia muciniphila, ADT reversed
the effects of Pca on thymic cortical areas and increased
circulating recent thymic emigrant cells |
| Peiffer et
al, 2022 (80) | Frozen fecal and
oral swish samples | 23 with mCRPC of
which 12 patients classified as responder | Decrease the
abundance of Akkermansia muciniphila in the responded to ITT
treatment samples |
Pca and prostate microbiome (direct):
Alteration of prostate microbiota in patients with Pca
Several studies have confirmed the existence of
pathogens related to the risk of disease in cancerous prostate
tissue, including bacteria, viruses or fungi. However, direct
evidence that microorganisms contribute to the development of Pca
and how microorganisms influence the progression of Pca is still
lacking. In 2005, using bacterial cultures of prostate tissue,
Cohen et al (14) found that
Propionibacterium acnes spp. was the predominant
microorganism detected in 35% of the Pca samples. In 2008, Sfanos
et al (15) analyzed
prostate tissue from 30 post-operative patients with Pca for
bacterial culture and 16S ribosomal RNA (rRNA) gene sequencing. The
majority of individual bacterial cultures were negative, while 16S
rRNA gene sequencing showed the presence of 83 distinct
microorganisms, and the authors suggested that the species present
in the prostate may be ‘unculturable’.
Certain studies using 16S rRNA have found that
Propionibacterium acnes may be associated with the
occurrence and progression of Pca (16–18).
Cavarretta et al (18)
assessed microbiome profiles in prostate tumors, peri-tumor and
non-tumor tissues. It was found that Propionibacterium spp.
was the predominant genera in tumor tissues, but no significant
differences were found in peri-tumor and non-tumor tissues.
However, considering that Propionibacterium acnes is one of
the common sequencing contaminants and should be treated with
skepticism, further studies are still needed to clarify the role of
Propionibacterium acnes in Pca (19,20).
Geographical and ethnic diversity exists in all
parts of the human microbiome (21). Feng et al (22) used metagenomic analysis of microbial
content within prostate tumor tissue from different regions. A
significant increase was identified in α-diversity from the African
sample compared with the European-derived sample (P=0.004), and
high-risk Pca tissues in Africa contained an abundance of anaerobic
bacteria. In another study, Feng et al (23) analyzed the microbial content of
postoperative prostate tissue from 65 Chinese patients with Pca,
and it was revealed that Escherichia, Propionibacterium,
Acinetobacter and Pseudomonas were the most abundant
microbiome, which forms the core of the prostate tissue, which was
also consistent with previous studies (16–18).
Such study may be the first to investigate the association between
Pca and prostatic microbiota in a Chinese population (23).
Pca and prostate microbiome (direct):
Resulting chronic inflammation produced by prostate microbiota
Several studies identified viruses or infection
factors from Pca tissues such as Human papillomaviruses and
Mycoplasma genitalium (24,25).
However, these studies were limited by the factor of sample size,
and lacked supporting evidence. Another study utilized large-scale
RNA-sequencing data along with matched clinical data from 242
patients with Pca from The Cancer Genome Atlas, and found that the
microbiome from prostate tissue played a major anticancer role in
Pca by recruiting immune cells, which were negatively correlated
with Pca Gleason score, Tumor-Node-Metastasis stage,
prostate-specific antigen level, and androgen receptor (AR)
expression (26). Further in
vitro and in vivo experiments are necessary to validate
these results. A recent study compared the microbiota profile from
94 patients with Pca with tumor and benign tissue sequencing by
meta-transcriptomic analysis (27).
It was demonstrated that Shewanella was enriched in
malignant prostate tissues, while Staphylococcus
saprophyticus and Vibrio parahaemolyticus were decreased
in malignant prostate tissues. In addition, the researchers also
observed that Microbacterium was significantly enriched in
pathologically advanced T3 (P<0.01) (27).
These studies suggested that Pca tissue contained
different microbial species, which may be related to prostatic
inflammation and carcinogenesis (24–27).
It is generally accepted that healthy prostate tissue appears
unlikely to have commensal microbiota, prostatic fluid containing
high levels of zinc and antimicrobial immune proteins or prostatic
epithelial cells expressing pathogen pattern recognition receptors
such as Toll-like receptor 4, which block the entry and
colonization of the microbiota in prostate tissue (28,29).
In the pathological state, the prostate epithelial cell barrier is
disrupted, and the reduction of antibacterial components in the
prostatic fluid may lead to microbial infiltration and the
development/progression of Pca (30). Future research is needed to
investigate further the reasons for colonization of the prostate by
the microbiota and the direct mechanisms of their interaction.
However, ethical concerns make it difficult to truly analyze the
prostate tissue of healthy individuals. The emerging organoid
method may help to clarify the mechanism of prostate microbiota and
Pca (31).
Pca and urinary microbiome (indirect):
Differences in urethral microbiota between patients with Pca and
healthy individuals are inconclusive
Compared with prostate microbiota, urinary
microbiota samples are easy to obtain, non-invasive and have the
potential to be used as screening biomarkers that can improve
prediction of Pca risk (32).
Previous studies have suggested that healthy urine is sterile,
while thanks to the advancement of NGS methods, recent studies have
revealed that urine microbiota has unique structures that are
different from gut microbiota and show diversity in different sex,
ages and disease states (33–35).
Urinary microbiota was associated with various female urinary
diseases, including emergency urinary incontinence and overactive
bladder (33). There are
differences in the abundance of urinary microbiota between males
and females (34). However, few
studies have investigated the association between urinary
microbiota and Pca.
Shrestha et al (36) collected urine samples from 135
patients assessed by 16S rRNA sequencing analysis and found that
the abundance of Propionibacterium lymphophilum was
significantly increased in patients with Pca; however, there was no
significant difference in diversity. In the study by Guest et
al (32), there was no
significant difference in the microbiota diversity in urine samples
from patients with Pca compared with controls. Previous studies
have demonstrated differences in certain urinary microbiota in
patients with Pca; however, the exact role of these microorganisms
in Pca tumorigenesis and progression remains unclear. Further
research is needed to establish a concrete connection between
urinary microbiota and Pca.
The microbiota in semen and prostatic fluid appears
to be more associated with prostate tissue than urine. Few studies
have evaluated the association between seminal and prostatic fluid
microbiota and Pca. In a study by Ma et al (37), 16S rRNA sequencing was used to
analyze the prostatic fluid microbiota of patients with Pca. It was
identified that the diversity of prostatic fluid microbiota in
patients with Pca was reduced compared with the non-cancer group.
No specific microbial species existed in the Pca or non-cancer
groups. It was suggested that the prostatic fluid microbiome may
contribute to maintaining the stability of the prostatic
microenvironment.
In another Chinese cohort study, prostate/seminal
fluid and urine samples from patients with Pca/benign prostatic
hyperplasia (BPH) were collected for analysis. In seminal fluid,
the abundance of Enterococcus was decreased in the Pca
group, but there was little change in prostatic fluid and urine
(38). Notably, in the study of
prostate microbiota, several studies found that
Enterobacteriaceae was one of the most abundant microbiotas
in prostate tissue (17,27). The limitations of the study were the
small sample size and the control of contamination during sampling
of the urinary microbiome, which remains a problem to be
solved.
In 2019, Alanee et al (39) found that patients with Pca had
similar bacterial communities within their urinary microbiome
profile, and increased abundance of Veillonella,
Streptococcus and Bacteroides, as well as decreased
abundance of Faecalibacterium, Lactobaccili and
Acinetobacter in patients with Pca compared with patients
with BPH. The researchers also collected the fecal microbiota of
the patients for analysis, and no clustering was found in the fecal
microbiota with benign or malignant tumor. In a similar study,
researchers also found significant differences in the abundance of
Faecalibacterium in the urine flora of the Pca group
(40). The articles did not
investigate whether the gut microbiome affected the urinary
microbiome, although some studies have found that gut microbiota
was associated with recurrent urinary tract infections (41), and FMT could reduce urinary tract
infections (42). The association
between gut and urine microbiota needs to be further explored.
Pca and gut microbiome (indirect):
Differences in the composition of gut microbiota in patients with
Pca
Although all body sites were colonized, the highest
microbial counts were found in the colon (43). Gut microbiota can regulate numerous
functions of the tumor-bearing organism, and thus influence tumor
development and treatment (43,44).
The known mechanisms include modulating the intestinal epithelial
barrier, regulating the functional activity of lymphoid organs,
regulating the tumor microenvironment and influencing the function
of anticancer drugs (44). certain
studies have been conducted to investigate if the composition of
gut microbiota may be involved in the progression of Pca,
particularly CRPC. However, numerous mechanisms by which gut
microbiota affects Pca remain unclear, and further research is
needed to support the translation of these current results into
clinical practice.
Antibiotics not only act on the bacteria that cause
infections but also affect the microbiome in the body. Treatment
with antibiotics affected the abundance of 1/3 of the gut
microbiota and there were individual differences in recovery time
(45). The use of penicillin,
quinolones and sulfonamides increased the risk of Pca, which the
investigators hypothesized may be associated with the human
microbiota (46). In a study by Mao
et al (47) in 2022, it was
found that disruption of the intestinal barrier and dysbiosis of
the gut microbiota due to antibiotic exposure exacerbated the
progression of Pca. Previous studies have also found that
antibiotic-induced gut microbiota disturbances affect the efficacy
of docetaxel in Pca (47).
Amirian et al (48) hypothesized in 2013 that the
composition of the gastrointestinal microbiome may be significantly
different in individuals with a higher risk of Pca. This hypothesis
was completed in 2018 by Liss et al (49), who developed a microbiome-derived
risk factor to predict the future risk of Pca. The aforementioned
study assessed the gut microbiota composition, and found that
Bacteroides and Streptococcus were enriched in the
Pca group. A previous study also illustrated an increased relative
abundance of Bacteroides in the gut microbiome of patients
with Pca compared with patients with BPH (50). Similarly, Matsushita et al
(51) identified that patients with
high-risk Pca had an increased relative abundance of short-chain
fatty acids (SCFAs)-producing bacteria. These studies have
suggested a correlation between gut microbiota composition and Pca
risk, potentially due to microbiota metabolites, which has
implications for identifying high-risk patients and may provide
additional insights into the development of Pca.
Pca and gut microbiome (indirect): Lifestyle
habits affect Pca through the gut microbiome
Lifestyle, particularly dietary patterns, also have
a significant impact on the occurrence and development of Pca
(8). Previous studies have shown
that obesity due to a high-fat diet (HFD) induces chronic systemic
inflammation and participates in the progression of Pca, and one of
the possible mechanisms involved may be the gut microbiota
(52,53). One study found that the abundance of
21 bacterial phylotypes in the gut microbiota of HFD-fed mice with
Pca was increased, and the abundance of equol-producing bacteria
Adlercreutzia was decreased compared with the control group,
and the serum equol of HFD-mice was significantly decreased
(54). Another study by the same
group found that post-weaning HFD significantly promoted Pca in the
offspring, yet combined maternal HFD and post-weaning HFD decreased
Pca progression in the offspring (55). The gut microbiota compositions are
predominantly of vertical inheritance (56), and additional studies may be needed
to clarify the correlation between vertically transmitted
microbiota profile and Pca.
To investigate the effect of HFD on Pca progression,
Matsushita et al (57) found
that, in HFD-fed Pca mice, oral administration of an antibiotic
mixture significantly altered the abundance of gut microbiota,
inhibited the proliferation of Pca cells, and reduced circulating
insulin-like growth factor-1 (IGF-1) levels and prostate IGF-1
expression. The results suggested the existence of a gut
microbiota-IGF-1-prostate axis. The authors found that the gut
microbiota may be associated with tumor progression through the
generation of SCFAs, leading to elevated IGF-1 levels (57). In another study, HFD-fed mice
prostate tissue expression of Hdc and gene levels of histamine
receptors were upregulated. Fexofenadine, a histamine H1 receptor
antagonist, significantly reduced the proliferation of Pca cells in
HFD-fed mice by suppressing the IL6/STAT3 signaling pathway
(58). There were also differences
in the composition and diversity of gut microbiota in mice fed with
different components of HFD, such as lard and fish oil diets, and
it was revealed that a diet rich in saturated fatty acids could
lead to the progression of Pca and change the abundance of gut
microbiota (59). The gut
microbiota dysbiosis caused by HFD leads to Pca progression through
multiple pathways, which further supports the existence of the gut
microbiota-prostate axis. Modifying dietary patterns and
intervention of gut microbiota may reduce the risk of Pca
development (52–55,57–59).
Microbiota and Pca therapy: Interaction of
microbiota with Pca treatment
As shown in Fig. 1,
antitumor treatment could potentially result in changes in the
microbiota profile, and the microbiota could also affect the
efficacy of the treatment or the absorption and metabolism of the
drug (44,60). The mechanisms linking the human gut
microbiome with treatment could be an important basis for a new
generation of therapies. In 2018, Routy et al (61) found that patients treated with
antibiotics developed resistance to anti-PD-1 immunotherapy drugs.
By comparing the gut microbiome of patients with cancer who
responded to this immunotherapy with that of patients with cancer
who did not respond, it was found that the relative abundance of
Akkermansia muciniphila correlated with clinical response to
this immunotherapy in patients with cancer. It has also been found
that patients with high levels of Bifidobacterium longum,
Collinsella aerofaciens and Enterococcus faecium have a
higher propensity to respond to anti-PD-L1 therapy, and FMT from
responding patients to germ-free mice may improve the efficacy of
anti-PD-L1 therapy (62). Patients
treated with chemotherapy/radiotherapy also showed significant
changes in the composition of the microbiota. A recent study
revealed significant differences in gut microbiota abundance at the
end of chemo-radiation therapy for rectal cancer, and these changes
were associated with ethnic or regional factors (63). Individual differences in microbiota
resulted by regional, ethnic, dietary and genetic factors affect
the efficacy of oncology treatment, and treatment also changes the
composition of the microbiota. The interaction between cancer
management and microbiota may be one of the key factors
contributing to the individualized differences in oncology
treatment (64).
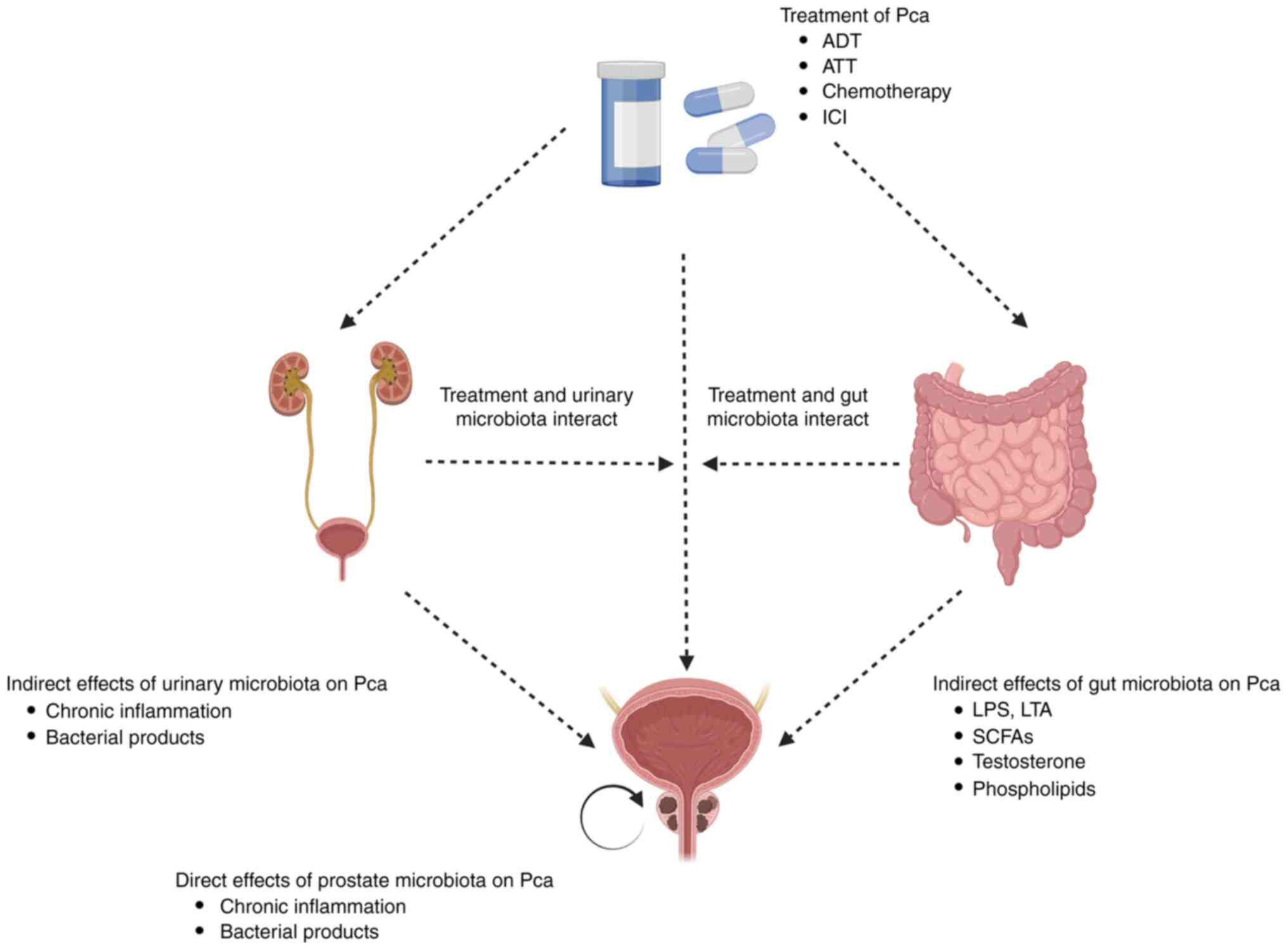 | Figure 1.The direct/indirect connection of
microbiota in Pca progression and treatment. Studies have indicated
that prostate, urethral, and intestinal microbiota may be directly
or indirectly involved in the progression of Pca, potentially
through the induction of chronic tissue inflammation or the
stimulation of microbiota products. There is a close relationship
between the microbiota and Pca treatment, as the administration of
treatment can alter the microbiota structure of patients, and
microbiota may also affect the efficacy of Pca treatment. Pca,
prostate cancer; ADT, androgen deprivation therapy; ATT, androgen
receptor axis-targeting therapeutics; ICI, immune checkpoint
inhibitor; LPS, lipopolysaccharide; SCFAs, short-chain fatty acid;
LTA, lymphotoxin alpha. |
For Pca, radiotherapy/chemotherapy as well as ADT
treatment have the potential to influence the gut/urine/prostate
microbiota composition; however, few studies have precisely
elucidated the effect of specific microbial species or microbial
profiles on the efficacy or toxicity of Pca treatment. FMT can
alter the composition of the intestinal microbiota. A clinical
trial (trial registration number NCT03341143) in 2021 found that
treatment of PD-1-refractory melanoma with FMT in combination with
anti-PD-1 drugs was effective in enhancing the efficacy of PD-1
drugs, and the results of the study showed that the use of FMT
could alter the gut microbiome of patients and affect the tumor
microenvironment to overcome anti-PD-1 therapy resistance in
patients with melanoma (6). In
addition, certain probiotics have been found to be effective in
promoting cancer cell apoptosis and combating oxidative stress, and
probiotic supplementation has also been shown to reduce the adverse
effects of chemotherapy/radiotherapy and immunotherapy (60). In summary, recent studies have shown
that microbiota composition is crucial to the efficacy of oncology
treatment. On one hand, knowing the microbiota composition of a
patient before treatment can assist physicians in determining the
most appropriate treatment protocol for the patient; on the other
hand, targeting therapy to the microbiota can also enhance the
patient's response to other oncological treatments (62). It is necessary to gain an improved
understanding of the correlation between different profiles of
microbiota and treatment in Pca, so that the role of microbiota in
the treatment of Pca can be comprehensively appreciated.
ADT remains the primary treatment for patients with
advanced Pca. Although nearly all patients respond to ADT, which is
termed hormone-sensitive prostate cancer (HSPC), the duration of
ADT treatment sensitivity varies from months to years, and patients
often develop resistance to ADT, which is termed CRPC (65). Several studies have hypothesized
that intestinal microbiota may play a role in the resistance of Pca
to ADT. Liu and Jiang (66) used
16S rRNA to compare the microbiota of patients with Pca (n=21)
before and after ADT treatment, and 12 microbial phylotypes
including Phascolarctobacterium and Ruminococcus were
found to have increased abundance in the gut microbiota of patients
with CRPC after ADT treatment. Another study similarly found
significant differences in microbiota diversity among patients with
Pca treated with ADT (n=56), with an increased abundance of the
pro-inflammatory bacteria Ruminococcus gnavus and
Bacteroides spp. (67). It
was identified that the gut microbiota changed over time not only
before and after ADT, but also after ADT. Kure et al
(68) revealed that microbiota
diversity decreased significantly at 24 weeks after ADT, and the
abundance of Proteobacteria changed significantly after ADT,
and was positively correlated with lactate concentration. Previous
studies have found that ADT can lead to significant changes in the
microbiota of patients with CRPC. These findings suggest a possible
link between ADT and alterations in the microbiota, but there is a
lack of evidence on how gut microbiota affects Pca progression
after ADT.
In 2021, Pernigoni et al (69) showed that the expansion of
androgen-synthesizing gut microbiota may mediate resistance to ADT.
The study used two mouse models of Pca that initially showed tumor
shrinkage after surgical castration and subsequent progression to
CRPC, and Ruminococcus gnavus was significantly enriched in
CRPC mice. In patients with CRPC, Ruminococcus was also
shown to be significantly enriched and associated with phospholipid
metabolism (70). Next, the
investigators performed FMT from CRPC mice and healthy mice on
recipient mice in the castration-sensitive phase. The results
revealed that FMT from healthy mice inhibited tumor growth, while
FMT from CRPC mice led to tumor progress. Metabolomic analysis
showed that the dehydroepiandrosterone (DHEA) and testosterone
levels were significantly elevated in FMT-CRPC mice. Similarly,
functional analysis in a previous study showed an increase in the
taxa of bacteria with steroid biosynthesis functions in Pca mice
(71). In vivo and in
vitro, Pernigoni et al (69) demonstrated the ability of
Ruminococcus gnavus to convert pregnenolone into active DHEA
and testosterone. The aforementioned studies observed increased
abundance of certain gut microbiota, including Ruminococcus
(66,69,70),
Bacteroides (67,69) and Phascolarctobacterium
(66), which act as antagonists to
ADT, potentially due to the additional androgens provided by
specific genus. While most studies have primarily focused on
increasing the fraction of microbiota after ADT treatment, few have
investigated which microbiota exhibit decreased in abundance
post-treatment, which may be a potential target for probiotic
supplementation in CRPC. For instance, Prevotella enrichment
in HSPC has been found to inhibit Pca progression in mice (69).
There is an interactive association between gut
microbiota and drug therapy. On one hand, it has been shown that
gut microbiota impacts drug absorption, disposal, metabolism,
pharmacology or toxicity. On the other hand, drug treatment can
also lead to changes in gut microbiota and affect the host
(47,72). However, few studies have clarified
the effect of specific microbial species on the efficacy/toxicity
of drug therapy for Pca.
In patients with CRPC, there were still small
concentrations of extragonadal androgens present even after the end
of ADT treatment, part of which was synthesized by the gut
microbiota (69), and systemic
upregulation of androgen synthesis could also activate the AR
pathway in Pca cells (73). Based
on these observations, oral AR axis-targeting therapeutics (ATT)
have become one of the prominent treatment options for patients
with advanced CRPC, including abiraterone acetate and enzalutamide
(74). Sfanos et al
(75) found that the abundance of
Akkermansia muciniphila and Ruminococcaceae spp. was
higher in patients with Pca receiving ATT (including bicalutamide,
enzalutamide and abiraterone acetate), and functional enrichment
analysis suggested a significant enrichment in the oral ATT group
for functions involved in steroid/hormone biosynthesis. A previous
study has shown a correlation between the relative abundance of
Akkermansia muciniphila and clinical responses to immune
checkpoint inhibitors (ICIs), and it was observed that oral
supplementation with Akkermansia muciniphila restored the
efficacy of PD-1 blockers (61).
Following treatment with ATT in patients with Pca, a study found
that treatment with abiraterone acetate resulted in enrichment in
Akkermansia muciniphila, which, through a specific
interaction with abiraterone acetate, increased the ability to
synthesize vitamin K2 and influenced treatment response in patients
with Pca (76). These current
results suggested that the role of gut microbiota in ATT treatment
was inconsistent. Part of genera may attenuate the effect of ATT
treatment by synthesizing extra-tumoral androgens, while part of
genera may play a synergistic role with ATT through other pathways,
and different genera could play different roles.
Significant variability in the response of ICIs such
as anti-CTLA-4 and anti-PD-L1 in different individuals and tumor
types could also be attributed to the effect of gut microbiota
(61,77). The gut microbiota composition was
found to predict response to anti-PD-1 therapy (78), and FMT from immunotherapy-responsive
patients into tumor xenograft mouse models enhanced antitumor
immunity (61,77). Terrisse et al (79) found that the immune system and gut
microbiota determined the efficacy of ADT in Pca, and that ADT
reversed the effects of Pca on thymic cortical areas and increased
circulating thymic emigrant cells. However, treatment with ICI in
patients with Pca did not enhance the efficacy of ADT.
To investigate whether different gut microbiota
composition affects the efficacy of ITT therapy in Pca, Peiffer
et al (80) performed 16S
rRNA gene sequencing of the profile of gut microbiota of patients
with CRPC before and after anti-PD-1 treatment, and inconsistent
with previous results from other oncology studies (61), the study found a decrease in the
relative abundance of Akkermansia muciniphila in the
responding samples. Shaikh et al (78) combined sequencing data from multiple
ICI-related microbiome studies to develop an integrated microbiome
prediction index to identify whether or not there was a response to
ICI therapy. However, the study found no significant difference in
the index results between responders and non-responders (80). In the area of Pca, the interactive
effect of microbiota and ICI needs further investigation.
Conclusions and future directions
The present review aimed to clarify the direction of
subsequent research by providing the current state of research in
Pca and microbiota research. An increasing number of studies have
been conducted to analyze the correlation between microbiota and
Pca. Microbiota is recognized as one of the potentially critical
factors influencing Pca development/progression. However, there is
still a lack of adequate understanding of the mechanisms of
microbiota at different locations in the development/progression
and treatment of Pca. Recent studies have demonstrated that,
compared with healthy individuals, there may be differences in the
abundance of microbiota in patients with Pca, whether in the
urethra, prostate tissue or intestine. Nevertheless, the research
provided in the present review often presents conflicting
information, emphasizing the need for further study in this area
using a standardized approach.
Research on the association between urinary and
prostate microbiota and Pca has progressed slowly. Previous studies
suggest that epithelial structural disruption and inflammatory
states leading to colonization may be potential mechanisms for Pca
progression. However, this mechanism is not yet understood. Gut
microbiota may act indirectly through different microbiota
metabolites and sex hormone levels, and influence Pca progression
and treatment (Fig. 2).
Understanding how gut microbiota affects Pca will help to stratify
in an improved manner the risk of Pca progression and develop new
treatments. The impact of the microbiome on cancer (including Pca)
treatment is bilateral. On one hand, the microbiome can
significantly influence the treatment of cancer, while cancer
treatment can in turn shape the composition of the microbiome. As
therapeutic tools continue to evolve, particularly ATT treatment
for Pca, it is critical to explore and understand the complex
underlying links between Pca and the microbiome.
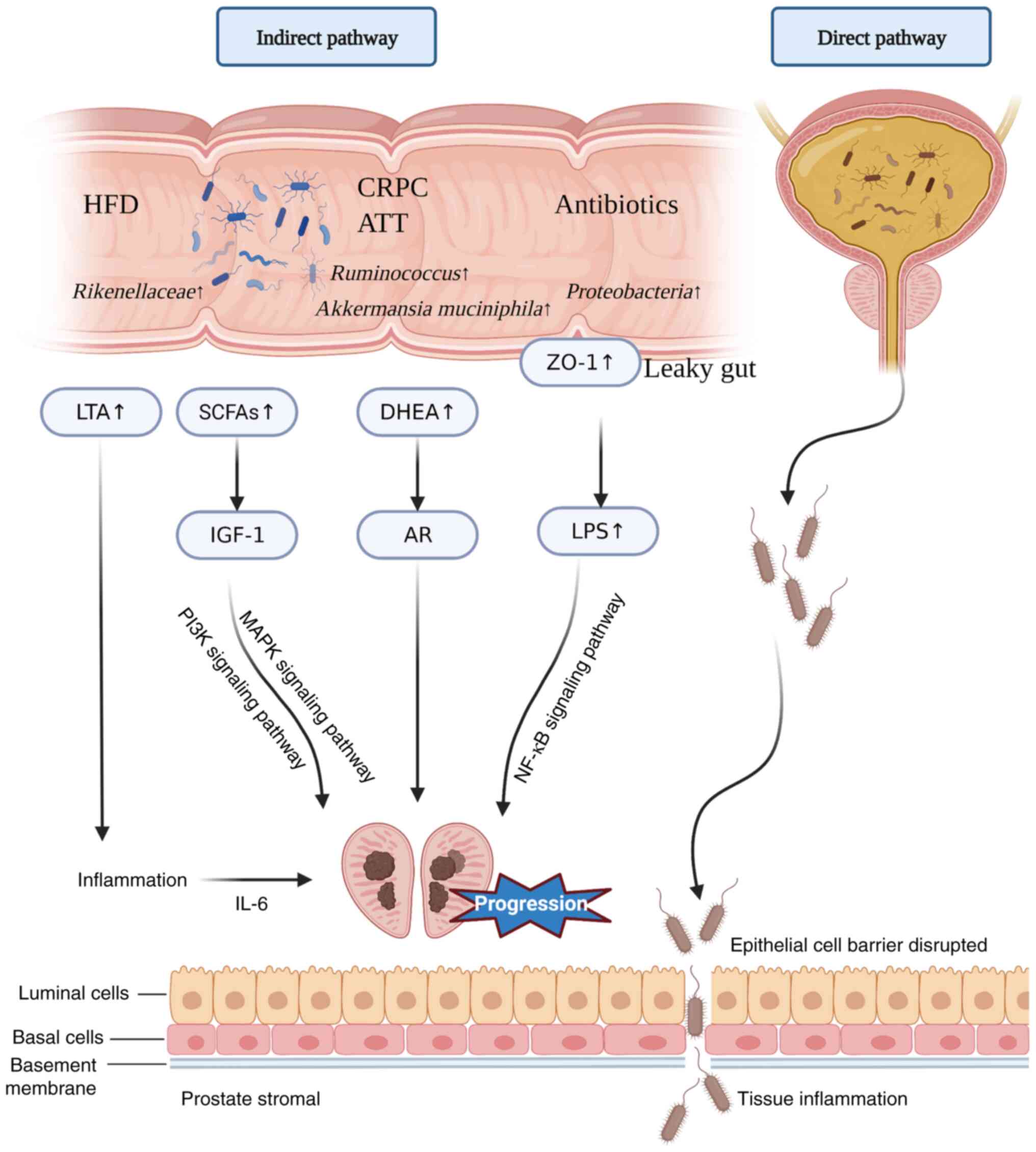 | Figure 2.Mechanisms of impact of gut and
urinary microbiota on Pca. Antibiotic use leads to increased
intestinal permeability and infiltration of the bacterial component
LPS into the body circulation, promoting Pca progression. HFDs
promote Pca progression by upregulating IL-6 and promoting local
inflammation in the prostate. SCFAs have been found to play an
important role in the promotion of Pca growth. The ATT or CRPC
cause an increase of Ruminococcus in the gut, and the
production of DHEA promotes Pca growth. Disruption of the prostate
epithelial cell barrier in pathological states may contribute to
microbial infiltration and the progression of Pca. Pca, prostate
cancer; LPS, lipopolysaccharide; HFD, high-fat diet; SCFA,
short-chain fatty acid; ATT, androgen receptor axis-targeting
therapeutics; CRPC, castration-resistant prostate cancer; DHEA,
dehydroepiandrosterone; IGF-1, insulin-like growth factor-1; AR,
androgen receptor; LTA, lymphotoxin alpha; ZO-1, zonula
occludens-1. |
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
BWX and JWW wrote the manuscript. BWX was a major
contributor in writing the manuscript. DXZ made substantial
contributions to conception and design. XPH designed this review
and critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
AR
|
androgen receptor
|
|
ATT
|
androgen receptor axis-targeting
therapeutics
|
|
BPH
|
benign prostatic hyperplasia
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
DHEA
|
dehydroepiandrosterone
|
|
FMT
|
fecal microbiota transplantation
|
|
HSPC
|
hormone-sensitive prostate cancer
|
|
ICI
|
immune checkpoint inhibitor
|
|
IGF-1
|
insulin-like growth factor-1
|
|
NGS
|
next-generation sequencing
|
|
Pca
|
prostate cancer
|
|
SCFAs
|
short-chain fatty acids
|
References
|
1
|
Sender R, Fuchs S and Milo R: Revised
estimates for the number of human and bacteria cells in the body.
PLoS Biol. 14:e10025332016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch SV and Pedersen O: The human
intestinal microbiome in health and disease. N Engl J Med.
375:2369–2379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marchesi JR, Adams DH, Fava F, Hermes GD,
Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM,
et al: The gut microbiota and host health: A new clinical frontier.
Gut. 65:330–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xavier JB, Young VB, Skufca J, Ginty F,
Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie
L, et al: The cancer microbiome: Distinguishing direct and indirect
effects requires a systemic view. Trends Cancer. 6:192–204. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whisner CM and Aktipis CA: The role of the
microbiome in cancer initiation and progression: how microbes and
cancer cells utilize excess energy and promote one another's
growth. Curr Nutr Rep. 8:42–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davar D, Dzutsev AK, McCulloch JA,
Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding
Q, Pagliano O, et al: Fecal microbiota transplant overcomes
resistance to anti-PD-1 therapy in melanoma patients. Science.
371:595–602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. JAMA. 317:2532–2542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chandrasekar T, Yang JC, Gao AC and Evans
CP: Mechanisms of resistance in castration-resistant prostate
cancer (CRPC). Transl Androl Urol. 4:365–380. 2015.PubMed/NCBI
|
|
10
|
Bratt O, Folkvaljon Y, Eriksson MH, Akre
O, Carlsson S, Drevin L, Lissbrant IF, Makarov D, Loeb S and
Stattin P: Undertreatment of men in their seventies with high-risk
nonmetastatic prostate cancer. Eur Urol. 68:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salachan PV and Sørensen KD: Dysbiotic
microbes and how to find them: A review of microbiome profiling in
prostate cancer. J Exp Clin Cancer Res. 41:312022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alexander JL, Wilson ID, Teare J, Marchesi
JR, Nicholson JK and Kinross JM: Gut microbiota modulation of
chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol.
14:356–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katongole P, Sande OJ, Joloba M, Reynolds
SJ and Niyonzima N: The human microbiome and its link in prostate
cancer risk and pathogenesis. Infect Agent Cancer. 15:532020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen RJ, Shannon BA, McNeal JE, Shannon T
and Garrett KL: Propionibacterium acnes associated with
inflammation in radical prostatectomy specimens: A possible link to
cancer evolution? J Urol. 173:1969–1974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sfanos KS, Sauvageot J, Fedor HL, Dick JD,
De Marzo AM and Isaacs WB: A molecular analysis of prokaryotic and
viral DNA sequences in prostate tissue from patients with prostate
cancer indicates the presence of multiple and diverse
microorganisms. Prostate. 68:306–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexeyev O, Bergh J, Marklund I,
Thellenberg-Karlsson C, Wiklund F, Grönberg H, Bergh A and Elgh F:
Association between the presence of bacterial 16S RNA in prostate
specimens taken during transurethral resection of prostate and
subsequent risk of prostate cancer (Sweden). Cancer Causes Control.
17:1127–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yow MA, Tabrizi SN, Severi G, Bolton DM,
Pedersen J, Giles GG and Southey MC: Australian prostate cancer
bioresource: Characterisation of microbial communities within
aggressive prostate cancer tissues. Infect Agent Cancer. 12:42017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cavarretta I, Ferrarese R, Cazzaniga W,
Saita D, Lucianò R, Ceresola ER, Locatelli I, Visconti L, Lavorgna
G, Briganti A, et al: The microbiome of the prostate tumor
microenvironment. Eur Urol. 72:625–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhofer R, Minich JJ, Marotz C, Cooper
A, Knight R and Weyrich LS: Contamination in low microbial biomass
microbiome studies: Issues and recommendations. Trends Microbiol.
27:105–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Achermann Y, Goldstein EJ, Coenye T and
Shirtliff ME: Propionibacterium acnes: From commensal to
opportunistic biofilm-associated implant pathogen. Clin Microbiol
Rev. 27:419–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lloyd-Price J, Abu-Ali G and Huttenhower
C: The healthy human microbiome. Genome Med. 8:512016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Y, Jaratlerdsiri W, Patrick SM, Lyons
RJ, Haynes AM, Collins CC, Stricker PD, Bornman MSR and Hayes VM:
Metagenomic analysis reveals a rich bacterial content in high-risk
prostate tumors from African men. Prostate. 79:1731–1738. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Y, Ramnarine VR, Bell R, Volik S,
Davicioni E, Hayes VM, Ren S and Collins CC: Metagenomic and
metatranscriptomic analysis of human prostate microbiota from
patients with prostate cancer. BMC Genomics. 20:1462019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banerjee S, Alwine JC, Wei Z, Tian T, Shih
N, Sperling C, Guzzo T, Feldman MD and Robertson ES: Microbiome
signatures in prostate cancer. Carcinogenesis. 40:749–764. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyake M, Ohnishi K, Hori S, Nakano A,
Nakano R, Yano H, Ohnishi S, Owari T, Morizawa Y, Itami Y, et al:
Mycoplasma genitalium infection and chronic inflammation in human
prostate cancer: Detection using prostatectomy and needle biopsy
specimens. Cells. 8:2122019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma J, Gnanasekar A, Lee A, Li WT, Haas M,
Wang-Rodriguez J, Chang EY, Rajasekaran M and Ongkeko WM: Influence
of intratumor microbiome on clinical outcome and immune processes
in prostate cancer. Cancers (Basel). 12:25242020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salachan PV, Rasmussen M, Fredsøe J, Ulhøi
B, Borre M and Sørensen KD: Microbiota of the prostate tumor
environment investigated by whole-transcriptome profiling. Genome
Med. 14:92022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stamey TA, Fair WR, Timothy MM and Chung
HK: Antibacterial nature of prostatic fluid. Nature. 218:444–447.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gatti G, Quintar AA, Andreani V, Nicola
JP, Maldonado CA, Masini-Repiso AM, Rivero VE and Maccioni M:
Expression of toll-like receptor 4 in the prostate gland and its
association with the severity of prostate cancer. Prostate.
69:1387–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sfanos KS, Yegnasubramanian S, Nelson WG
and De Marzo AM: The inflammatory microenvironment and microbiome
in prostate cancer development. Nat Rev Urol. 15:11–24. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weeber F, Ooft SN, Dijkstra KK and Voest
EE: Tumor organoids as a pre-clinical cancer model for drug
discovery. Cell Chem Biol. 24:1092–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guest C, Harris R, Sfanos KS, Shrestha E,
Partin AW, Trock B, Mangold L, Bader R, Kozak A, Mclean S, et al:
Feasibility of integrating canine olfaction with chemical and
microbial profiling of urine to detect lethal prostate cancer. PLoS
One. 16:e02455302021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hilt EE, Putonti C, Thomas-White K, Lewis
AL, Visick KL, Gilbert NM and Wolfe AJ: Aerococcus urinae isolated
from women with lower urinary tract symptoms: In Vitro aggregation
and genome analysis. J Bacteriol. 202:e00170–e20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lewis DA, Brown R, Williams J, White P,
Jacobson SK, Marchesi JR and Drake MJ: The human urinary
microbiome; bacterial DNA in voided urine of asymptomatic adults.
Front Cell Infect Microbiol. 3:412013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adebayo AS, Ackermann G, Bowyer RCE, Wells
PM, Humphreys G, Knight R, Spector TD and Steves CJ: The urinary
tract microbiome in older women exhibits host genetic and
environmental influences. Cell Host Microbe. 28:298–305.e3. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shrestha E, White JR, Yu SH, Kulac I,
Ertunc O, De Marzo AM, Yegnasubramanian S, Mangold LA, Partin AW
and Sfanos KS: Profiling the urinary microbiome in men with
positive versus negative biopsies for prostate cancer. J Urol.
199:161–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma X, Chi C, Fan L, Dong B, Shao X, Xie S,
Li M and Xue W: The microbiome of prostate fluid is associated with
prostate cancer. Front Microbiol. 10:16642019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu H, Meng H, Zhou F, Ni X, Shen S and Das
UN: Urinary microbiota in patients with prostate cancer and benign
prostatic hyperplasia. Arch Med Sci. 11:385–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alanee S, El-Zawahry A, Dynda D, Dabaja A,
McVary K, Karr M and Braundmeier-Fleming A: A prospective study to
examine the association of the urinary and fecal microbiota with
prostate cancer diagnosis after transrectal biopsy of the prostate
using 16S RNA gene analysis. Prostate. 79:81–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai KY, Wu DC, Wu WJ, Wang JW, Juan YS,
Li CC, Liu CJ and Lee HY: Exploring the association between gut and
urine microbiota and prostatic disease including benign prostatic
hyperplasia and prostate cancer using 16S rRNA sequencing.
Biomedicines. 10:26762022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Worby CJ, Schreiber HL IV, Straub TJ, van
Dijk LR, Bronson RA, Olson BS, Pinkner JS, Obernuefemann CLP, Muñoz
VL, Paharik AE, et al: Longitudinal multi-omics analyses link gut
microbiome dysbiosis with recurrent urinary tract infections in
women. Nat Microbiol. 7:630–639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeney SES, Lane F, Oliver A, Whiteson K
and Dutta S: Fecal microbiota transplantation for the treatment of
refractory recurrent urinary tract infection. Obstet Gynecol.
136:771–773. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Vos WM, Tilg H, Van Hul M and Cani PD:
Gut microbiome and health: Mechanistic insights. Gut. 71:1020–1032.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sepich-Poore GD, Zitvogel L, Straussman R,
Hasty J, Wargo JA and Knight R: The microbiome and human cancer.
Science. 371:eabc45522021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dethlefsen L, Huse S, Sogin ML and Relman
DA: The pervasive effects of an antibiotic on the human gut
microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol.
6:e2802008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boursi B, Mamtani R, Haynes K and Yang YX:
Recurrent antibiotic exposure may promote cancer formation-another
step in understanding the role of the human microbiota? Eur J
Cancer. 51:2655–2664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhong W, Wu K, Long Z, Zhou X, Zhong C,
Wang S, Lai H, Guo Y, Lv D, Lu J and Mao X: Gut dysbiosis promotes
prostate cancer progression and docetaxel resistance via activating
NF-κB-IL6-STAT3 axis. Microbiome. 10:942022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amirian ES, Petrosino JF, Ajami NJ, Liu Y,
Mims MP and Scheurer ME: Potential role of gastrointestinal
microbiota composition in prostate cancer risk. Infect Agent
Cancer. 8:422013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liss MA, White JR, Goros M, Gelfond J,
Leach R, Johnson-Pais T, Lai Z, Rourke E, Basler J, Ankerst D and
Shah DP: Metabolic biosynthesis pathways identified from fecal
microbiome associated with prostate cancer. Eur Urol. 74:575–582.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Golombos DM, Ayangbesan A, O'Malley P,
Lewicki P, Barlow L, Barbieri CE, Chan C, DuLong C, Abu-Ali G,
Huttenhower C and Scherr DS: The role of gut microbiome in the
pathogenesis of prostate cancer: A prospective, pilot study.
Urology. 111:122–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsushita M, Fujita K, Motooka D, Hatano
K, Fukae S, Kawamura N, Tomiyama E, Hayashi Y, Banno E, Takao T, et
al: The gut microbiota associated with high-Gleason prostate
cancer. Cancer Sci. 112:3125–3135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hayashi T, Fujita K, Nojima S, Hayashi Y,
Nakano K, Ishizuya Y, Wang C, Yamamoto Y, Kinouchi T, Matsuzaki K,
et al: High-fat diet-induced inflammation accelerates prostate
cancer growth via IL6 signaling. Clin Cancer Res. 24:4309–4318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Smith KS, Frugé AD, van der Pol W, Caston
NE, Morrow CD, Demark-Wahnefried W and Carson TL: Gut microbial
differences in breast and prostate cancer cases from two randomised
controlled trials compared to matched cancer-free controls. Benef
Microbes. 12:239–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Wu X and Jiang H: High dietary fat
intake lowers serum equol concentration and promotes prostate
carcinogenesis in a transgenic mouse prostate model. Nutr Metab
(Lond). 16:242019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Y, Wu X and Jiang H: Combined maternal
and post-weaning high fat diet inhibits male offspring's prostate
cancer tumorigenesis in transgenic adenocarcinoma of mouse prostate
model. Prostate. 79:544–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moeller AH, Suzuki TA, Phifer-Rixey M and
Nachman MW: Transmission modes of the mammalian gut microbiota.
Science. 362:453–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsushita M, Fujita K, Hayashi T, Kayama
H, Motooka D, Hase H, Jingushi K, Yamamichi G, Yumiba S, Tomiyama
E, et al: Gut microbiota-derived short-chain fatty acids promote
prostate cancer growth via IGF1 signaling. Cancer Res.
81:4014–4026. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Matsushita M, Fujita K, Hatano K, Hayashi
T, Kayama H, Motooka D, Hase H, Yamamoto A, Uemura T, Yamamichi G,
et al: High-fat diet promotes prostate cancer growth through
histamine signaling. Int J Cancer. 151:623–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sato H, Narita S, Ishida M, Takahashi Y,
Mingguo H, Kashima S, Yamamoto R, Koizumi A, Nara T, Numakura K, et
al: Specific gut microbial environment in lard diet-induced
prostate cancer development and progression. Int J Mol Sci.
23:22142022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Badgeley A, Anwar H, Modi K, Murphy P and
Lakshmikuttyamma A: Effect of probiotics and gut microbiota on
anti-cancer drugs: Mechanistic perspectives. Biochim Biophys Acta
Rev Cancer. 1875:1884942021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immuno-therapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
González- Mercado VJ, Lim J, Berk L, Esele
M, Rodríguez CS and Colón-Otero G: Gut microbiota differences in
Island Hispanic Puerto Ricans and mainland non-Hispanic whites
during chemoradiation for rectal cancer: A pilot study. Curr Probl
Cancer. 44:1005512020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dai Z, Fu J, Peng X, Tang D and Song J:
Intestinal microbiota: The driving force behind advances in cancer
immunotherapy. Cancers (Basel). 14:47962022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Watson PA, Arora VK and Sawyers CL:
Emerging mechanisms of resistance to androgen receptor inhibitors
in prostate cancer. Nat Rev Cancer. 15:701–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Y and Jiang H: Compositional
differences of gut microbiome in matched hormone-sensitive and
castration-resistant prostate cancer. Transl Androl Urol.
9:1937–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li JKM, Wang LL, Wong CYP, Chiu PKF, Teoh
JYC, Kwok HSW, Leung SCH, Wong SH, Tsui SKW and Ng CF: A
cross-sectional study on gut microbiota in prostate cancer patients
with prostatectomy or androgen deprivation therapy. Prostate Cancer
Prostatic Dis. 24:1063–1072. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kure A, Tsukimi T, Ishii C, Aw W, Obana N,
Nakato G, Hirayama A, Kawano H, China T, Shimizu F, et al: Gut
environment changes due to androgen deprivation therapy in patients
with prostate cancer. Prostate Cancer Prostatic Dis.
13:10.1038/s41391–022-00536-3. 2022.
|
|
69
|
Pernigoni N, Zagato E, Calcinotto A,
Troiani M, Mestre RP, Calì B, Attanasio G, Troisi J, Minini M,
Mosole S, et al: Commensal bacteria promote endocrine resistance in
prostate cancer through androgen biosynthesis. Science.
374:216–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Y, Yang C, Zhang Z and Jiang H: Gut
microbiota dysbiosis accelerates prostate cancer progression
through increased LPCAT1 expression and enhanced DNA repair
pathways. Front Oncol. 11:6797122021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang PY, Yang YC, Wang CI, Hsiao PW,
Chiang HI and Chen TW: Increase in Akkermansiaceae in gut
microbiota of prostate cancer-bearing mice. Int J Mol Sci.
22:96262021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wilson ID and Nicholson JK: Gut microbiome
interactions with drug metabolism, efficacy, and toxicity. Transl
Res. 179:204–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mohler JL, Gregory CW, Ford OH III, Kim D,
Weaver CM, Petrusz P, Wilson EM and French FS: The androgen axis in
recurrent prostate cancer. Clin Cancer Res. 10:440–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Buttigliero C, Tucci M, Bertaglia V,
Vignani F, Bironzo P, Di Maio M and Scagliotti GV: Understanding
and overcoming the mechanisms of primary and acquired resistance to
abiraterone and enzalutamide in castration resistant prostate
cancer. Cancer Treat Rev. 41:884–892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sfanos KS, Markowski MC, Peiffer LB, Ernst
SE, White JR, Pienta KJ, Antonarakis ES and Ross AE: Compositional
differences in gastrointestinal microbiota in prostate cancer
patients treated with androgen axis-targeted therapies. Prostate
Cancer Prostatic Dis. 21:539–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Daisley BA, Chanyi RM, Abdur-Rashid K, Al
KF, Gibbons S, Chmiel JA, Wilcox H, Reid G, Anderson A, Dewar M, et
al: Abiraterone acetate preferentially enriches for the gut
commensal Akkermansia muciniphila in castrate-resistant prostate
cancer patients. Nat Commun. 11:48222020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shaikh FY, White JR, Gills JJ, Hakozaki T,
Richard C, Routy B, Okuma Y, Usyk M, Pandey A, Weber JS, et al: A
uniform computational approach improved on existing pipelines to
reveal microbiome biomarkers of nonresponse to immune checkpoint
inhibitors. Clin Cancer Res. 27:2571–2583. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Terrisse S, Goubet AG, Ueda K, Thomas AM,
Quiniou V, Thelemaque C, Dunsmore G, Clave E, Gamat-Huber M,
Yonekura S, et al: Immune system and intestinal microbiota
determine efficacy of androgen deprivation therapy against prostate
cancer. J Immunother Cancer. 10:e0041912022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Peiffer LB, White JR, Jones CB, Slottke
RE, Ernst SE, Moran AE, Graff JN and Sfanos KS: Composition of
gastrointestinal microbiota in association with treatment response
in individuals with metastatic castrate resistant prostate cancer
progressing on enzalutamide and initiating treatment with anti-PD-1
(pembrolizumab). Neoplasia. 32:1008222022. View Article : Google Scholar : PubMed/NCBI
|
















